Microstructural Precipitation Evolution and In Vitro Degradation Behavior of a Novel Chill-Cast Zn-Based Absorbable Alloy for Medical Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Processing
2.2. Microstructural Characterization
2.3. Mechanical Characterization
2.4. Corrosion Behavior
3. Results and Discussion
3.1. Microstructural Characterization
3.2. XRD Characterization and Phase Identification
3.3. Mechanical Properties
3.4. Corrosion Behavior
4. Conclusions
- High solidification rates, typical of chill casting, promoted appreciable dendrite refinement and a uniform solute distribution all the way to the extrusion process.
- A solution treatment (ST) became a critical step process prior to extrusion: A ST temperature of 370 °C for 5 h enabled the dissolution of some phases while promoting more stable ones in the Zn-12.5Ag-1Mg alloy so that a good compromise between hardness and ductility was found.
- After extrusion, the development of an AgZn solid solution, combined with the precipitation of the ε-AgZn3 phase, and Mg-rich precipitates embedded in a η-Zn matrix formed a complex microstructure. This complex phase microstructure lead to the improvements exhibited in the mechanical properties of the Zn-12.5Ag-1Mg alloy.
- In the Zn-12.5Ag-1Mg alloy, two work hardening mechanisms were active as evidenced by the n exponents, that is, n ≈ 0.37 for log(ε) < −1.3 and n ≈ 0.76 for log(ε) > −1.3. Apparently, at low strains, dislocation motion is relatively easy without significant opposition, yet at increasing strain, other obstacles become active, probable intermetallic second phases. Additionally, the grain refinement observed in the Zn-12.5Ag-1Mg alloy together with the presence of a cubic phase embedded into its matrix provided an important increment on its ductility.
- Alloying additions of Ag and Mg in the right amounts can provide an optimal combination of properties for a good balance between strength and corrosion behavior. This is evident from the outcome of the present work, particularly notice the degradation rate increment exhibited by the Zn-12.5Ag-1Mg alloy (CR = 1.25 mm/year) when compared with either pure Zn (CR = 0.53 mm/year) or Zn-1Mg alloy (CR = 0.683 mm/year) in the extruded condition.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, Y.F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Peuster, M.; Wohlsein, P.; Brügmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal—Results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative in vitro study on pure metals (Fe, Mn, Mg, Zn and W) as biodegradable metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- McCall, K.A.; Huang, C.; Fierke, C.A. Zinc and health: Current status and future directions. J. Nutr. 2000, 22, 1437–1446. [Google Scholar] [CrossRef] [Green Version]
- Lastra, M.D.; Pastelin, R.; Camacho, A.; Monroy, B.; Aguilar, A.E. Zinc intervention on macrophages and lymphocytes response. J. Trace Elem. Med. Biol. 2001, 15, 5–10. [Google Scholar] [CrossRef]
- Saltman, P.D.; Strause, L.G. The role of trace minerals in osteoporosis. J. Am. Coll. Nutr. 1993, 12, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory II, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable metals for cardiovascular stents: From clinical concerns to recent Zn-alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public. Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin a, vitamin k, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Asp. Med. 2005, 26, 235–244. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, D.; Wang, F.; Taylor, J.A.; Zhang, M. The grain refinement mechanism of cast zinc through silver inoculation. Acta Mater. 2014, 79, 315–326. [Google Scholar] [CrossRef]
- Shao, W.; Zhao, Q. Influence of reducers on nanostructure and surface energy of silver coatings and bacterial adhesion. Surf. Coat. Technol. 2010, 204, 1288–1294. [Google Scholar] [CrossRef]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharm. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- King, D.E.; Mainous, A.G., III; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.-N.; Hudon, P.; Jung, I.-H.; Medraj, M.; Chartrand, P. Experimental study of the phase equilibria in the Mg-Zn-Ag ternary system at 300 °C. J. Alloy. Compd. 2015, 639, 593–601. [Google Scholar] [CrossRef]
- ASTM E8–08–16a. Test Methods for Tension Testing of Metallic Materials; Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Tolouei, R.; Harrison, J.; Paternoster, C.; Turgeon, S.; Chevallier, P.; Mantovani, D. The use of multiple pseudo-physiological solutions to simulate the degradation behaviour of pure iron as a metallic resorbable implant: A surface-characterization study. Phys. Chem. Chem. Phys. 2016, 18, 19637–19646. [Google Scholar] [CrossRef] [PubMed]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in C, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992; Volume 10.4, pp. 408–412. [Google Scholar]
- ASTM G102-89(2015) e1. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Jones, H. The status of rapid solidification of alloys in research and application. J. Mater. Sci. 1984, 19, 1043–1076. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.G.; Previtali, B.; Mantovani, D.; Bealand, R.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef]
- Shen, C.; Liu, X.; Fan, B.; Lan, P.; Zhou, F.; Li, X.; Wang, H.; Xiao, X.; Li, L.; Zhao, S.; et al. Mechanical properties, in vitro degradation behaviour, hemocompatibility and cytotoxicity evaluation of Zn-1.2 Mg alloy for biodegradable implants. RSC Adv. 2016, 6, 86410–86419. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Bealand, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behaviour of newly developed Zn-Ag alloys for degradable implant applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Hughes, D.A.; Hansen, N.; Bammann, D.J. Geometrically necessary boundaries, incidental dislocation boundaries and geometrically necessary dislocations. Scr. Mater. 2003, 48, 147–153. [Google Scholar] [CrossRef]
- Chrominski, W.; Lewandowska, M. Precipitation phenomena in ultrafine grained Al-Mg-Si alloy with heterogeneous microstructure. Acta Mater. 2016, 103, 547–557. [Google Scholar] [CrossRef]
- Sun, S.; Ren, Y.; Wang, L.; Yang, B.; Li, H.; Qin, G. Abnormal effect of Mn addition on the mechanical properties of as-extruded Zn alloys. Mater. Sci. Eng. A 2017, 701, 129–133. [Google Scholar] [CrossRef]
- Gong, H.; Wang, K.; Strich, R.; Zhou, J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn–Mg alloy. J. Biomed. Mater. Res. B 2015, 103, 1632–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L.; Zheng, Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn-1.5Mg alloy. J. Alloy. Compd. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, H.; Niu, J.; Zhang, L.; Zhang, H.; Pei, J.; Tan, J.; Yuan, G. Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater. Des. 2017, 117, 84–94. [Google Scholar] [CrossRef]
- Zhu, D.; Su, Y.; Young, M.L.; Ma, J.; Zheng, Y.; Tang, L. Biological responses and mechanisms of human bone marrow mesenchymal stem cells to Zn and Mg biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 27453–27461. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhou, F.; Yang, Y.; Chang, R.; Qiu, K.; Pu, Z.; Li, L.; Zheng, Y. Micro-alloying with Mn in Zn-Mg alloy for future biodegradable metals application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]













| Material | Composition (wt. %) | ||
|---|---|---|---|
| Zn | Ag | Mg | |
| Zn | 100 | - | - |
| Zn-1.0Mg | 99 | - | 1 |
| Zn-12.5Ag-1.0Mg | 86.5 | 12.5 | 1 |
| Heading | Condition | Found Phases | Reference SEM Pictures, EDS Spectrum | XRD Attribution |
|---|---|---|---|---|
| Zn-1Mg | As-cast | H-Zn solid solution | - | (hexagonal phase) ⚫ |
| H-Zn-Mg2Zn11 (eutectic constituent) | Point (c), Figure 2b,c | ⚫ + (cubic phase) ▲ | ||
| ST | η-Zn solid solution | - | (hexagonal phase) ⚫ | |
| η-Zn-Mg2Zn11 (eutectic constituent) | Point (b), Figure 4a,b | ⚫ + (cubic phase) ▲ | ||
| Extruded | η-Zn solid solution | - | (hexagonal phase) ⚫ | |
| Mg4Zn7 precipitates | - | (monoclinic phase) ⬟ | ||
| Mg0.971Zn0.029 intermetallic compound | - | (hexagonal phase) 🟄 | ||
| Zn-12.5Ag1Mg | As-cast | η-Zn solid solution | - | (hexagonal phase) ⚫ |
| ε-AgZn3 phase | Point (f), Figure 2e,f | (hexagonal phase) ★ | ||
| η-Zn solid solution + AgZn solid solution with Mg enrichment (modified eutectic) | Point (g), Figure 2e,g | ⚫ + (cubic phase) ◆ | ||
| Ag0.15MgZn1.85 Mg-rich intermetallic compound | Point (h), Figure 2e,h | (hexagonal phase) ▄ | ||
| ST | η-Zn solid solution | - | (hexagonal phase) ⚫ | |
| η-Zn solid solution + AgZn solid solution | Point (d), Figure 4c,d | ⚫ + (cubic phase) ◆ | ||
| ε-AgZn3 phase | Point (e), Figure 4c,e | (hexagonal phase) ★ | ||
| Ag0.15MgZn1.85 Mg-rich intermetallic compound | Point (f), Figure 4c,f | (hexagonal phase) ▄ | ||
| Extruded | η-Zn solid solution | - | (hexagonal phase) ⚫ | |
| ε-AgZn3 phase | Point (e), Figure 5d,e | (hexagonal phase) ★ | ||
| η-Zn solid solution + AgZn solid solution | Point (f), Figure 5d,f | ⚫ + (cubic phase) ◆ | ||
| Ag0.15MgZn1.85 Mg-rich intermetallic compound | Point (g), Figure 5d,g | (hexagonal phase) ▄ |
| Specimen | As-cast | 3 h at 370 °C | 5 h at 370 °C | Extruded at 280 °C |
|---|---|---|---|---|
| Zn | 30.0 ± 2.8 | 29.5 ± 1.4 | 29.2 ± 1.2 | 50.7 ± 2.8 |
| Zn–1Mg | 55.4 ± 1.9 | 66. 6 ± 5.6 | 61.6 ± 4.0 | 33.4 ± 1.9 |
| Zn–12.5Ag–1Mg | 127.6 ± 5.0 | 177.6 ± 9.8 | 164.5 ± 8.1 | 151.5 ± 4.8 |
| Specimen | n | YS (MPa) | UTS (MPa) | Elongation (%) |
|---|---|---|---|---|
| Zn | 0.23 | 42 ± 4 | 75 ± 4 | 71 ± 6 |
| Zn-1Mg | 0.33 | 80 ± 4 | 152 ± 6 | 11 ± 3 |
| Zn-12.5Ag-1Mg | 0.37(1), 0.76(2) | 64 ± 4 | 305 ± 17 | 21 ± 3 |
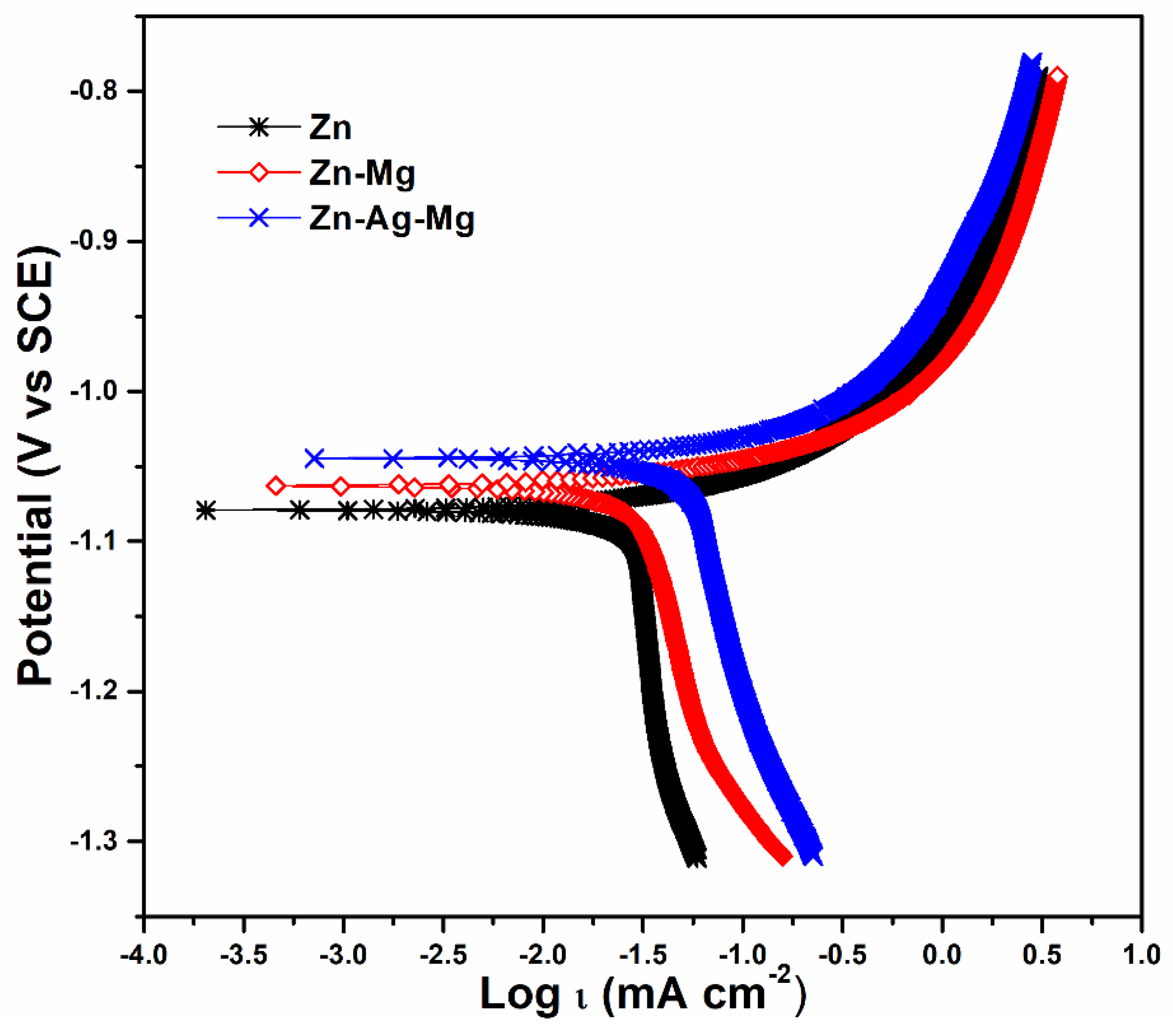
| Specimen | OCP (V) vs. SCE | ba (V) | Icorr (µA cm−2) | Corrosion Rate (mm year−1) | X2 |
|---|---|---|---|---|---|
| Zn | −1.09 | 0.167 | 35.1 | 0.53 | 8.90 × 10−4 |
| Zn-1Mg | −1.06 | 0.129 | 44.2 | 0.68 | 2.53 × 10−4 |
| Zn-12.5Ag-1Mg | −1.04 | 0.107 | 78.5 | 1.25 | 1.70 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez–Ledesma, A.L.; Roncagliolo–Barrera, P.; Paternoster, C.; Casati, R.; Lopez, H.; Vedani, M.; Mantovani, D. Microstructural Precipitation Evolution and In Vitro Degradation Behavior of a Novel Chill-Cast Zn-Based Absorbable Alloy for Medical Applications. Metals 2020, 10, 586. https://doi.org/10.3390/met10050586
Ramirez–Ledesma AL, Roncagliolo–Barrera P, Paternoster C, Casati R, Lopez H, Vedani M, Mantovani D. Microstructural Precipitation Evolution and In Vitro Degradation Behavior of a Novel Chill-Cast Zn-Based Absorbable Alloy for Medical Applications. Metals. 2020; 10(5):586. https://doi.org/10.3390/met10050586
Chicago/Turabian StyleRamirez–Ledesma, Ana Laura, Paola Roncagliolo–Barrera, Carlo Paternoster, Riccardo Casati, Hugo Lopez, Maurizio Vedani, and Diego Mantovani. 2020. "Microstructural Precipitation Evolution and In Vitro Degradation Behavior of a Novel Chill-Cast Zn-Based Absorbable Alloy for Medical Applications" Metals 10, no. 5: 586. https://doi.org/10.3390/met10050586







