Microstructural Evolution and Growth of Intermetallic Compounds at the Interface between Solid Cast Iron and Liquid Al–Si Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
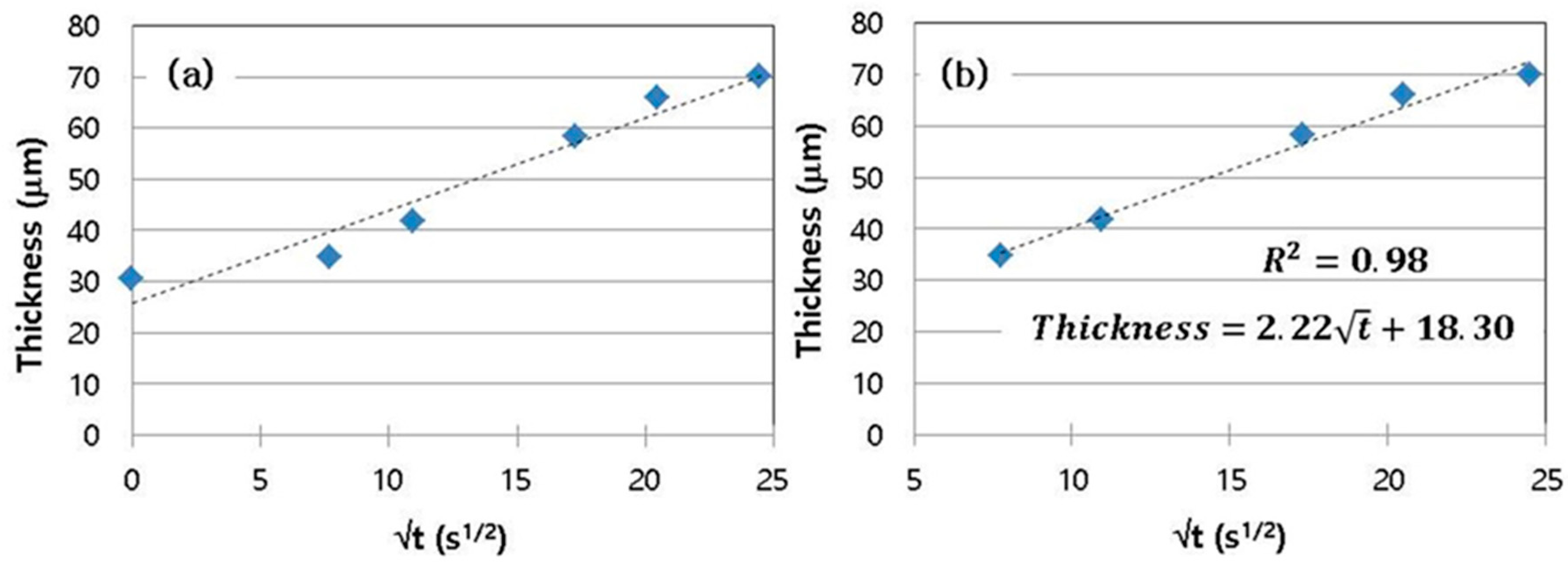
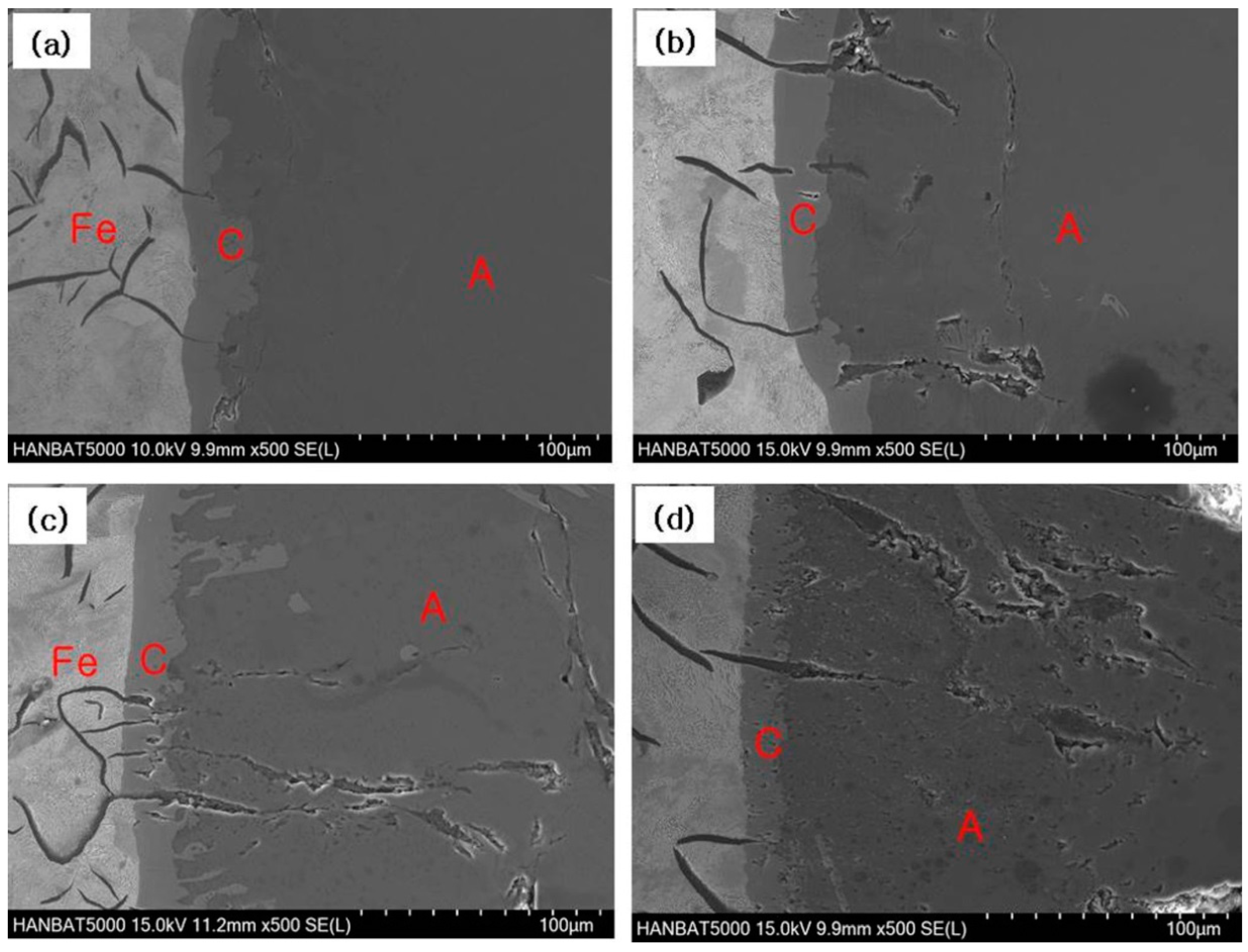
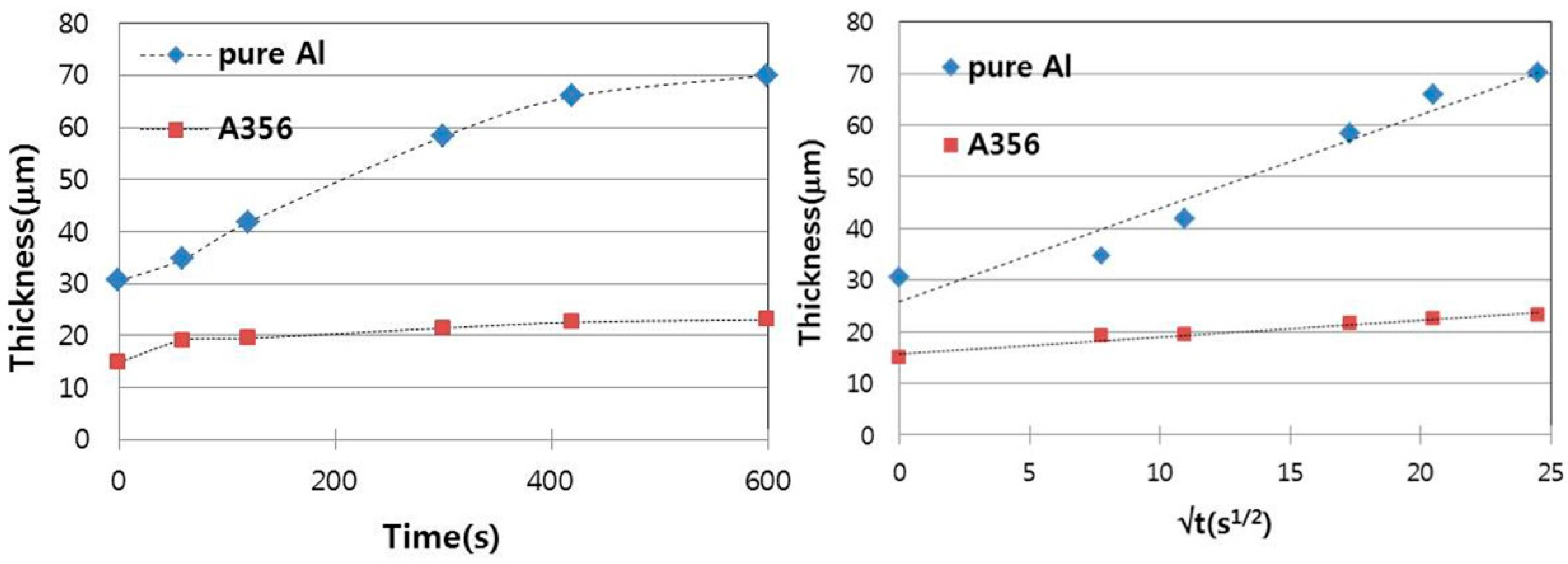
3.1. Cast Iron/Pure Aluminum Interface
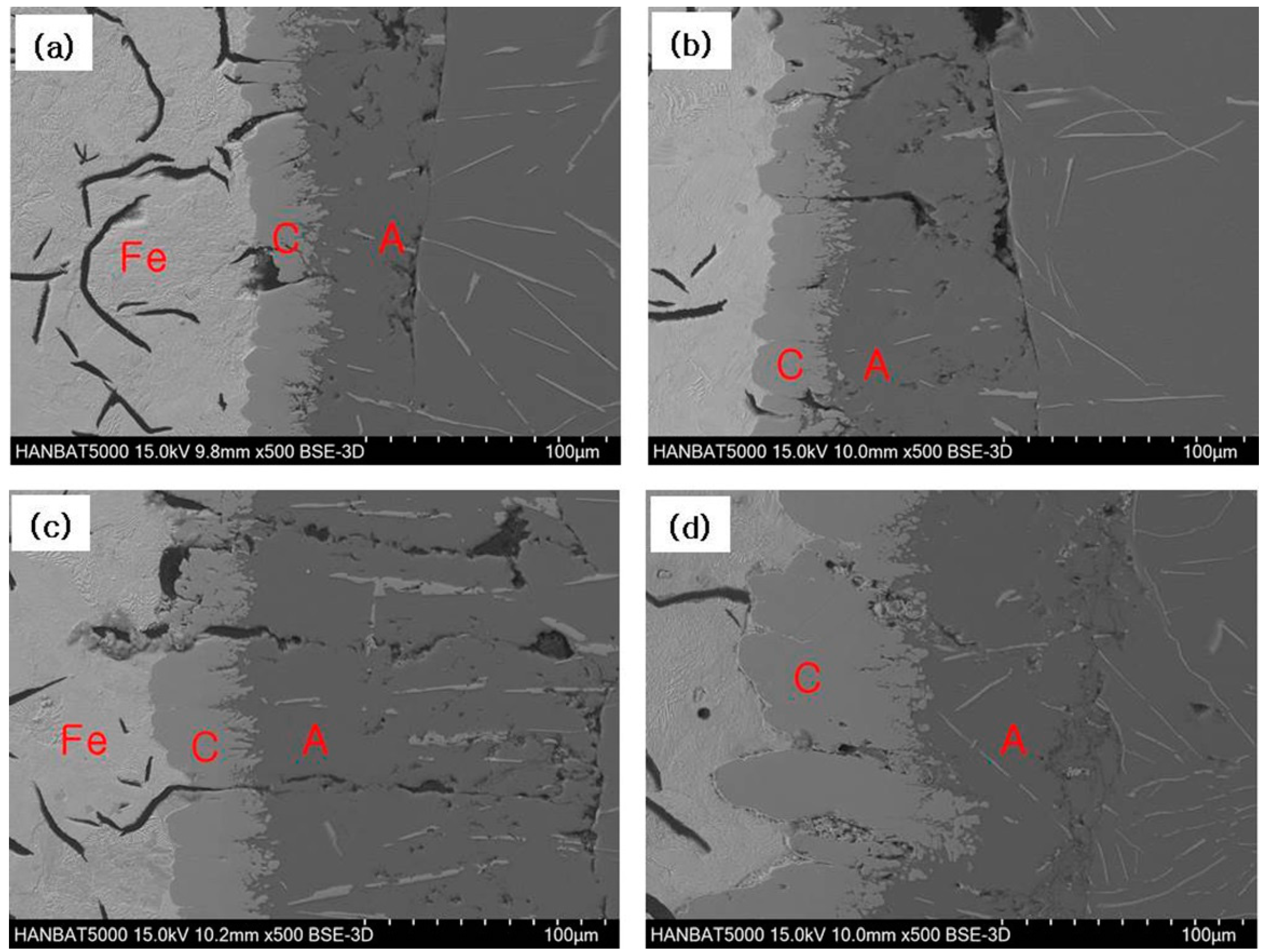
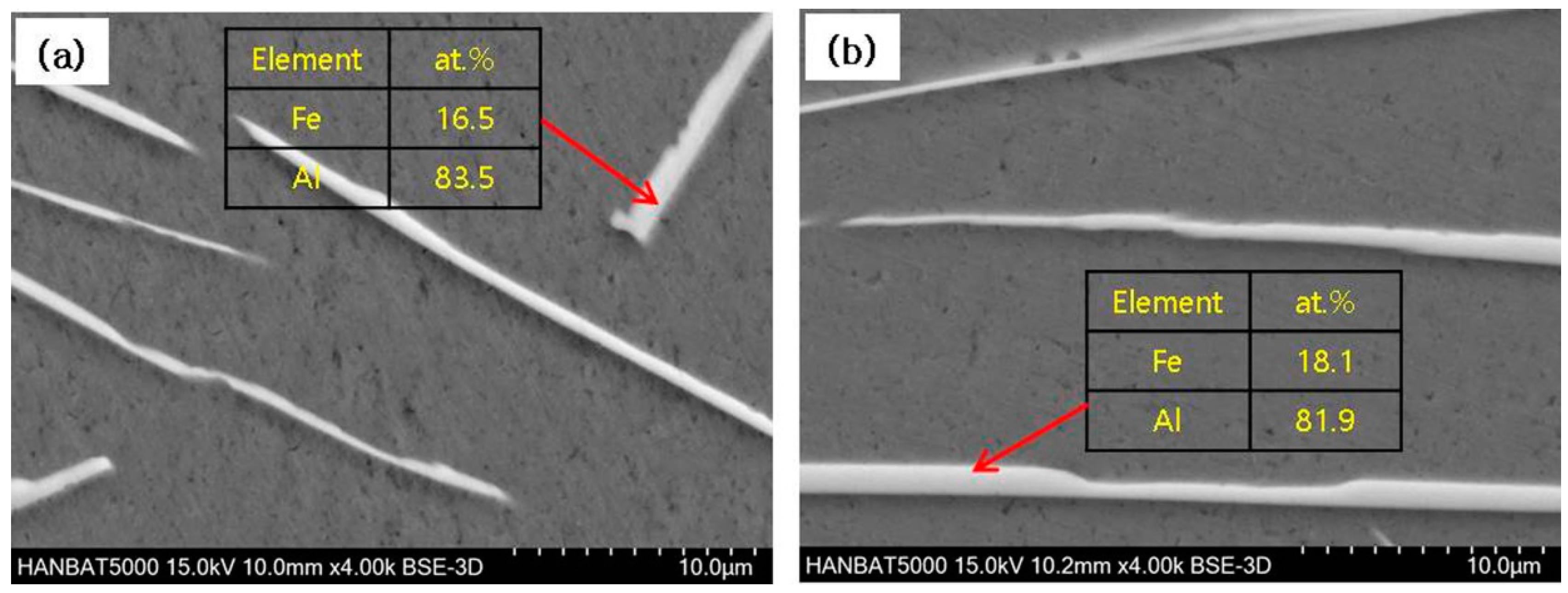
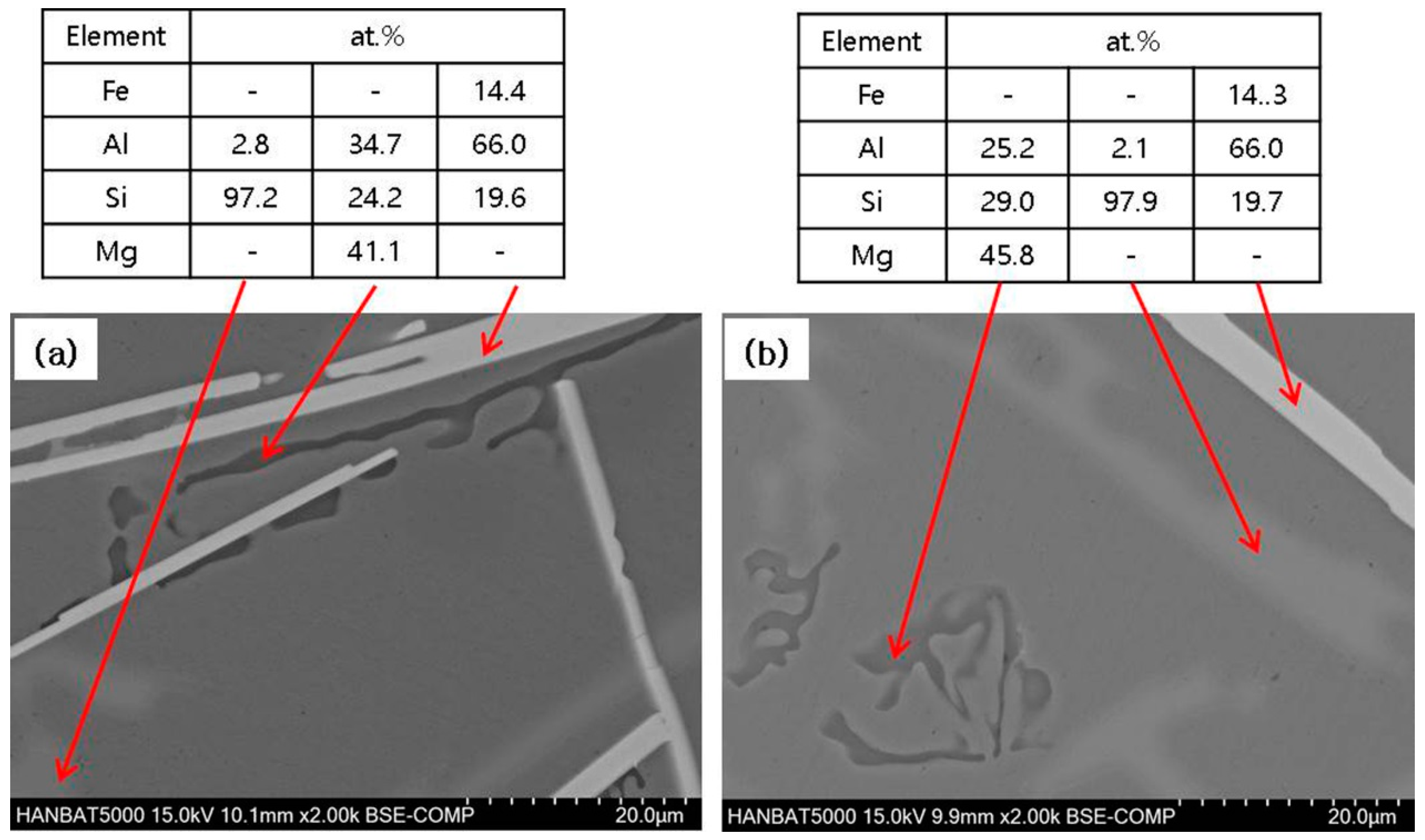
3.2. Cast Iron/Al–Si Alloy Interface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dangi, B.; Brown, T.W.; Kulkarni, K.N. Effect of silicon, manganese and nickel present in iron on the intermetallic growth at iron-aluminum alloy interface. J. Alloys Compd. 2018, 769, 777–787. [Google Scholar] [CrossRef]
- Jiang, W.; Li, G.; Jiang, Z.; Wu, Y.; Fan, Z. Effect of heat treatment on microstructures and mechanical properties of Al/Fe bimetal. Mater. Sci. Technol. 2018, 34, 1519–1528. [Google Scholar] [CrossRef]
- Moosavi-Khoonsari, E.; Jalilian, F.; Paray, F.; Emadi, D.; Drew, R.A.L. Cast joining of cast iron to aluminium casting matrix. Mater. Sci. Technol. 2011, 27, 1707–1717. [Google Scholar] [CrossRef]
- Yeremenko, V.N.; Natanzon, Y.V.; Dybkov, V.I. The effect of dissolution on the growth of the Fe2Al5 interlayer on the solid iron-liquid aluminium system. J. Mater. Sci. 1981, 16, 1748–1756. [Google Scholar] [CrossRef]
- Yan, M.; Fan, Z. Review: Durability of materials in molten aluminium alloys. J. Mater. Sci. 2001, 36, 1748–1756. [Google Scholar] [CrossRef]
- Denner, S.G.; Jones, R.D. Kinetic interactions between aluminium and iron/steel for conditions applicable to hot-dip aluminizing. Met. Technol. 1977, 4, 167–174. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, Q.; Lu, W.; Ge, X.; Cao, S.; Sun, S.; Yang, T.; Xia, M.; Li, J. Microstructural evolution and growth behavior of intermetallic compounds at the liquid Al/Solid Fe interface by synchrotron X-ray radiography. Mater. Charact. 2018, 136, 157–164. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Liu, X.; Liu, F. Effects of hot-dip galvanizing and aluminizing on interfacial microstructures and mechanical properties of aluminum/iron bimetallic composites. J. Alloys Compd. 2016, 688, 742–751. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Morphology and formation of Fe-Al intermetallic layers on iron hot-dipped in Al-Mg-Si alloy melt. Intermetallics 2014, 54, 136–142. [Google Scholar] [CrossRef]
- Shin, J.; Kim, T.; Lim, K.; Cho, H.; Yang, D.; Jeong, C.; Yi, S. Effects of steel type and sandblasting pretreatment on the solid-liquid compound casting characteristics of Zn-coated steel/aluminum bimetals. J. Alloys Compd. 2019, 778, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.J.; Wang, C.J. Observation of high-temperature phase transformation in the Si-modified aluminide coating on mild steel using EBSD. Mater. Charact. 2010, 61, 467–473. [Google Scholar] [CrossRef]
- Takata, N.; Nishimoto, M.; Kobayashi, S.; Takeyama, M. Crystallography of Fe2Al5 phase at the interface between solid Fe and liquid Al. Intermetallics 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Bouayad, A.; Gerometta, C.; Belkebir, A.; Ambari, A. Kinetic interactions between solid iron and molten aluminium. Mater. Sci. Eng. A 2003, A363, 53–61. [Google Scholar] [CrossRef]
- Deqing, W.; Ziyuan, S.; Longjiang, Z. A liquid aluminum corrosion resistance surface on steel substrate. Appl. Surf. Sci. 2003, 214, 304–311. [Google Scholar] [CrossRef]
- Cheng, W.J.; Wang, C.J. Effect of silicon on the formation of intermetallic phases in aluminide coating on mild steel. Intermetallics 2011, 19, 1455–1460. [Google Scholar] [CrossRef]
- Soderhjelm, C. Multi-Material Metal Casting: Metallurgical Bonding Aluminum to Ferrous Inserts. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 25 April 2017. [Google Scholar]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Fedian, D.; Josse, C.; Nquyen, P.; Gey, N.; Ratel-Ramond, N.; Parseval, P.; Thebault, Y.; Malard, B.; Lacaze, J.; Salvo, L. Chinese script vs plate-like precipitation of beta-Al9Fe2Si2 phase in an Al-6.5Si-1Fe alloy. Metal. Mater. Trans. A 2015, 46, 2814–2818. [Google Scholar] [CrossRef] [Green Version]
- Sacinti, M.; Cubuklusu, E.; Birol, Y. Effect of iron on microstructure and mechanical properties of primary AlSi7Mg0.3 alloy. Int. J. Cast Met. Res. 2016, 59, 1586–1600. [Google Scholar] [CrossRef]
- Sakow, S.; Tokunaga, T.; Ohno, M.; Matsuura, K. Microstructure refinement and mechanical properties improvement of Al-Si-Fe alloys by hot extrusion using a specially designed high-strain die. J. Mater. Process. Tech. 2020, 277, 1116447. [Google Scholar] [CrossRef]




| Alloy | C | Si | Mg | Mn | Fe | Al |
|---|---|---|---|---|---|---|
| Cast Iron | 2.65 | 0.31 | - | 0.15 | Balanced | - |
| A356 | - | 6.96 | 0.31 | - | - | Balanced |
| Time | 0 min | 1 min | 5 min | 10 min | ||||
|---|---|---|---|---|---|---|---|---|
| Location | C | A | C | A | C | A | C | A |
| Fe | 23.0 (38.2) | 0.2 (0.4) | 23.5 (38.9) | 0.7 (1.4) | 26.5 (42.8) | 0.2 (0.4) | 26.4 (42.6) | 0.2 (0.3) |
| Al | 77.0 (61.8) | 99.8 (99.6) | 76.5 (61.1) | 99.3 (98.6) | 73.5 (57.2) | 99.8 (99.6) | 73.6 (57.4) | 99.8 (99.7) |
| Time | 0 min | 1 min | 5 min | 10 min | ||||
|---|---|---|---|---|---|---|---|---|
| Location | C | A | C | A | C | A | C | A |
| Fe | 15.6 (27.4) | - | 16.1 (28.3) | - | 17.5 (30.3) | - | 15.2 (26.8) | - |
| Al | 67.1 (57.2) | 97.8 (97.7) | 67.7 (57.3) | 92.3 (92.1) | 68.6 (57.4) | 98.1 (98.1) | 66.8 (57.1) | 98.6 (98.6) |
| Si | 17.1 (15.1) | 1.8 (1.9) | 16.0 (14.1) | 7.2 (7.5) | 13.7 (12.0) | 1.5 (1.6) | 17.8 (15.8) | 0.9 (1.0) |
| Mn | 0.2 (0.3) | - | 0.2 (0.3) | - | 0.2 (0.3) | - | 0.2 (0.3) | - |
| Mg | - | 0.4 (0.4) | - | 0.5 (0.4) | - | 0.4 (0.3) | - | 0.5 (0.4) |
| Phase | Al4.5FeSi | Al8Fe2Si | Al13Fe4 | Al5Fe2 | Al | Ferrite |
|---|---|---|---|---|---|---|
| Fraction | 0.19 | 0.19 | 0.14 | 0.21 | 0.13 | 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-M.; Shin, K.; Shin, J.-S. Microstructural Evolution and Growth of Intermetallic Compounds at the Interface between Solid Cast Iron and Liquid Al–Si Alloy. Metals 2020, 10, 759. https://doi.org/10.3390/met10060759
Kim J-M, Shin K, Shin J-S. Microstructural Evolution and Growth of Intermetallic Compounds at the Interface between Solid Cast Iron and Liquid Al–Si Alloy. Metals. 2020; 10(6):759. https://doi.org/10.3390/met10060759
Chicago/Turabian StyleKim, Jeong-Min, Keesam Shin, and Je-Sik Shin. 2020. "Microstructural Evolution and Growth of Intermetallic Compounds at the Interface between Solid Cast Iron and Liquid Al–Si Alloy" Metals 10, no. 6: 759. https://doi.org/10.3390/met10060759




