Graphene Family Nanomaterial Reinforced Magnesium-Based Matrix Composites for Biomedical Application: A Comprehensive Review
Abstract
:1. Introduction
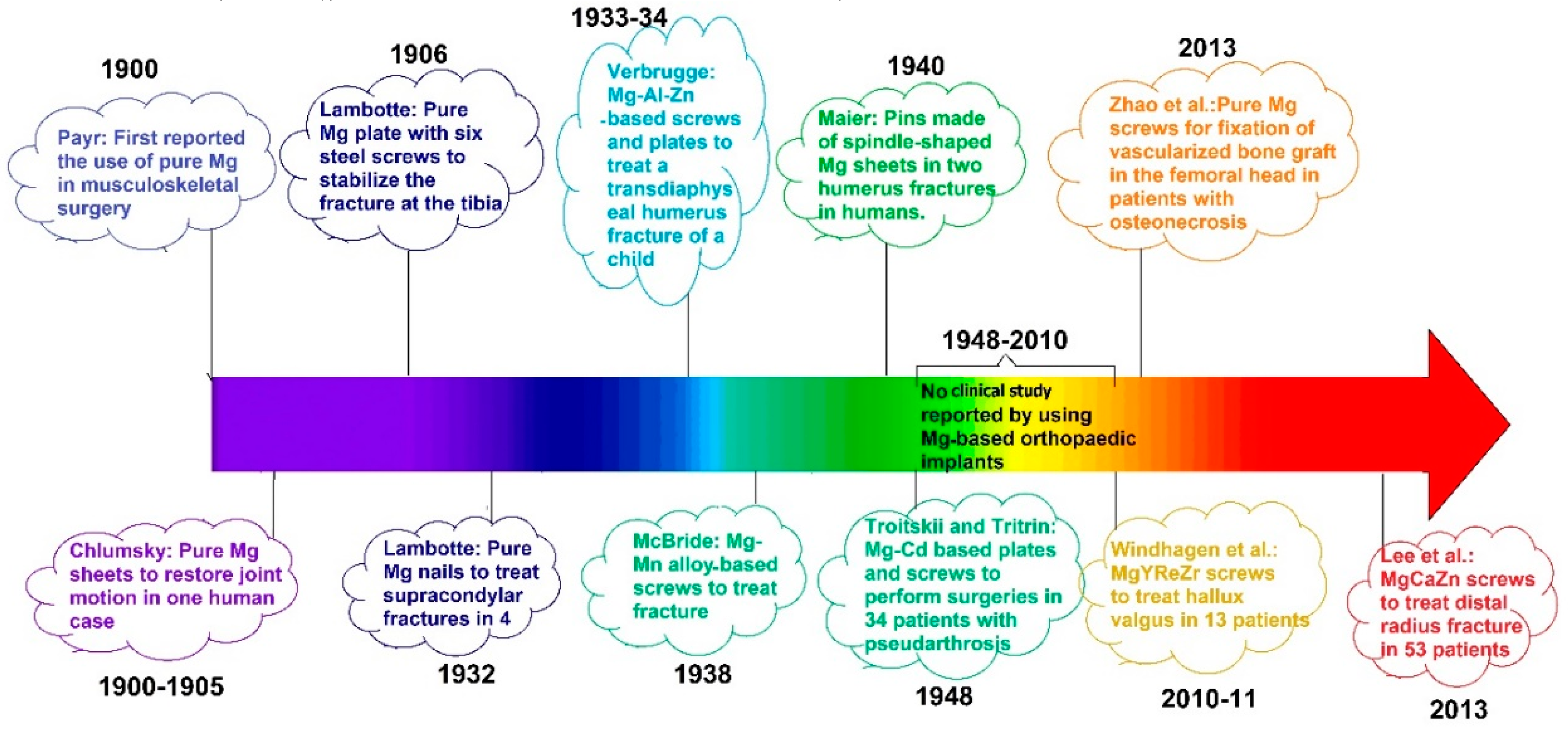
2. Development of Mg-Based Biodegradable Metals
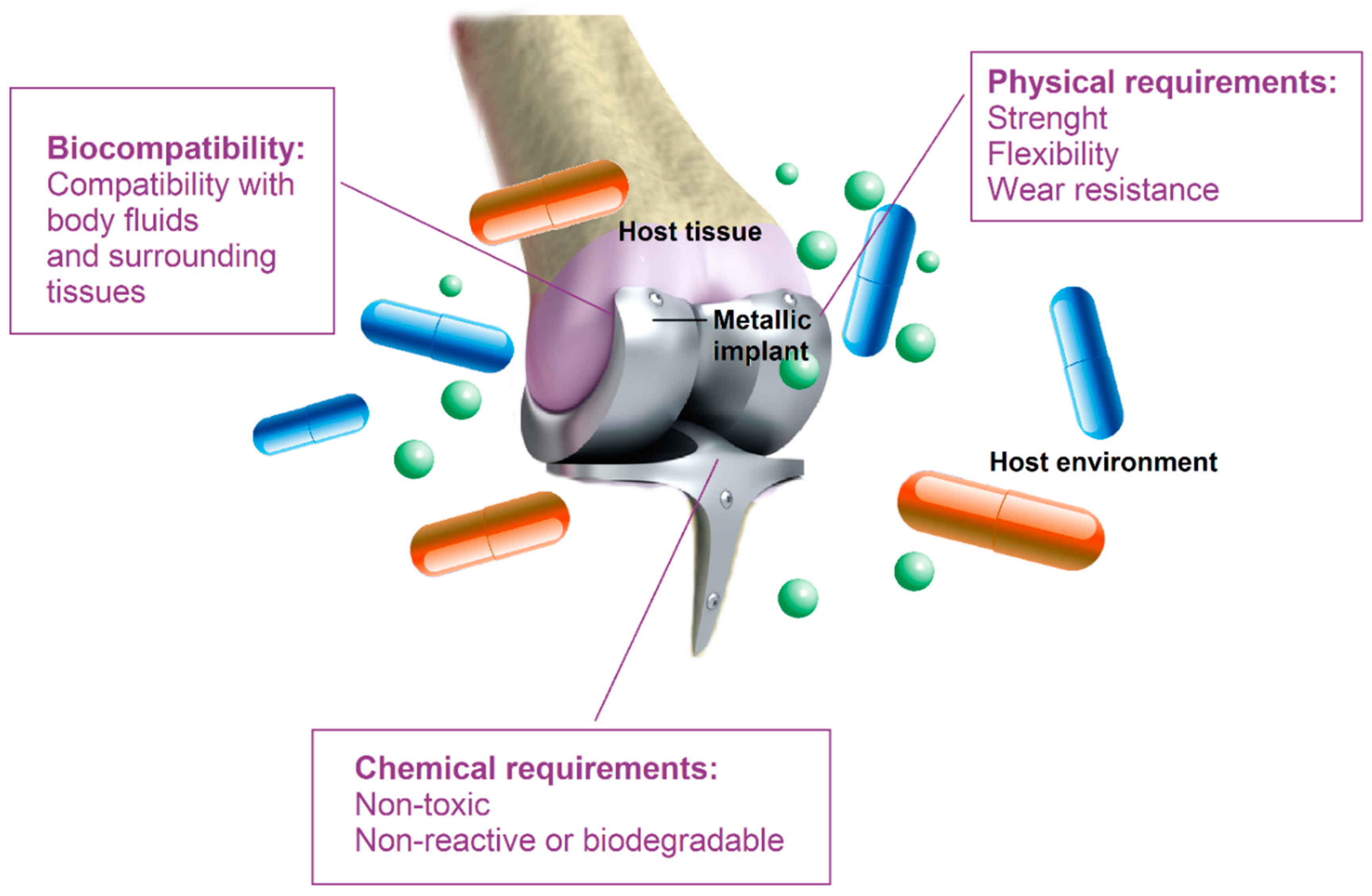
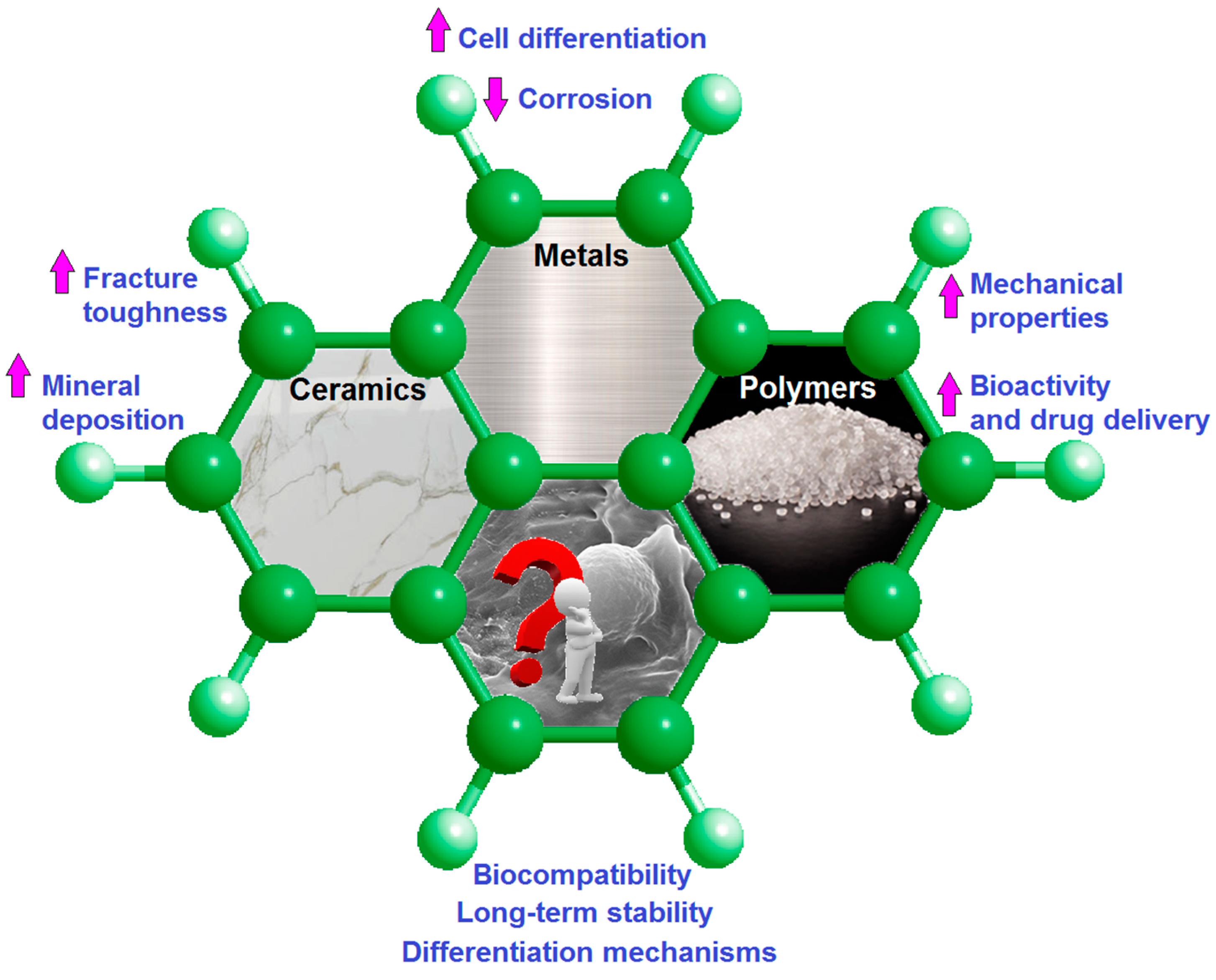
3. Mg-Based Biocomposites and Bio-Alloys
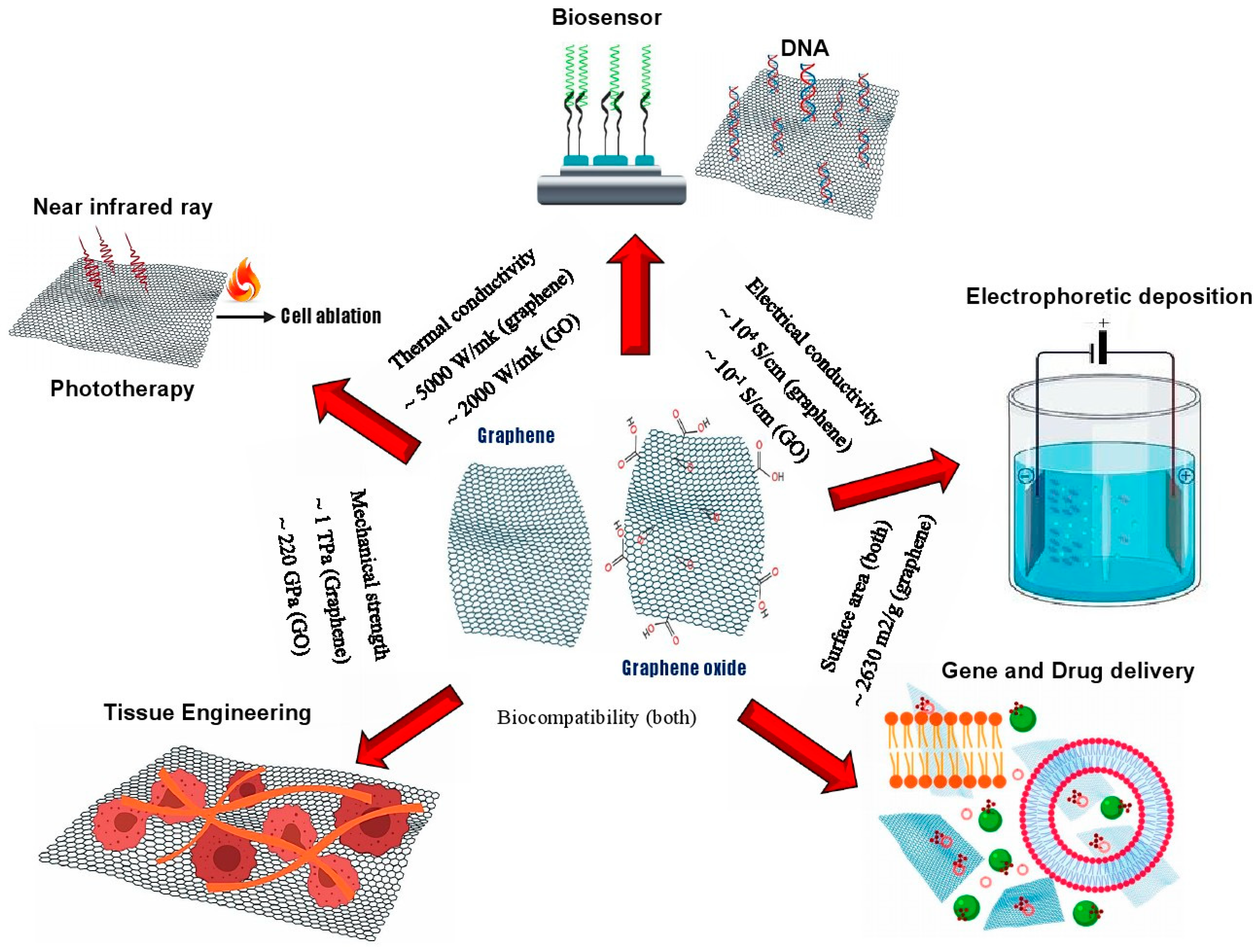
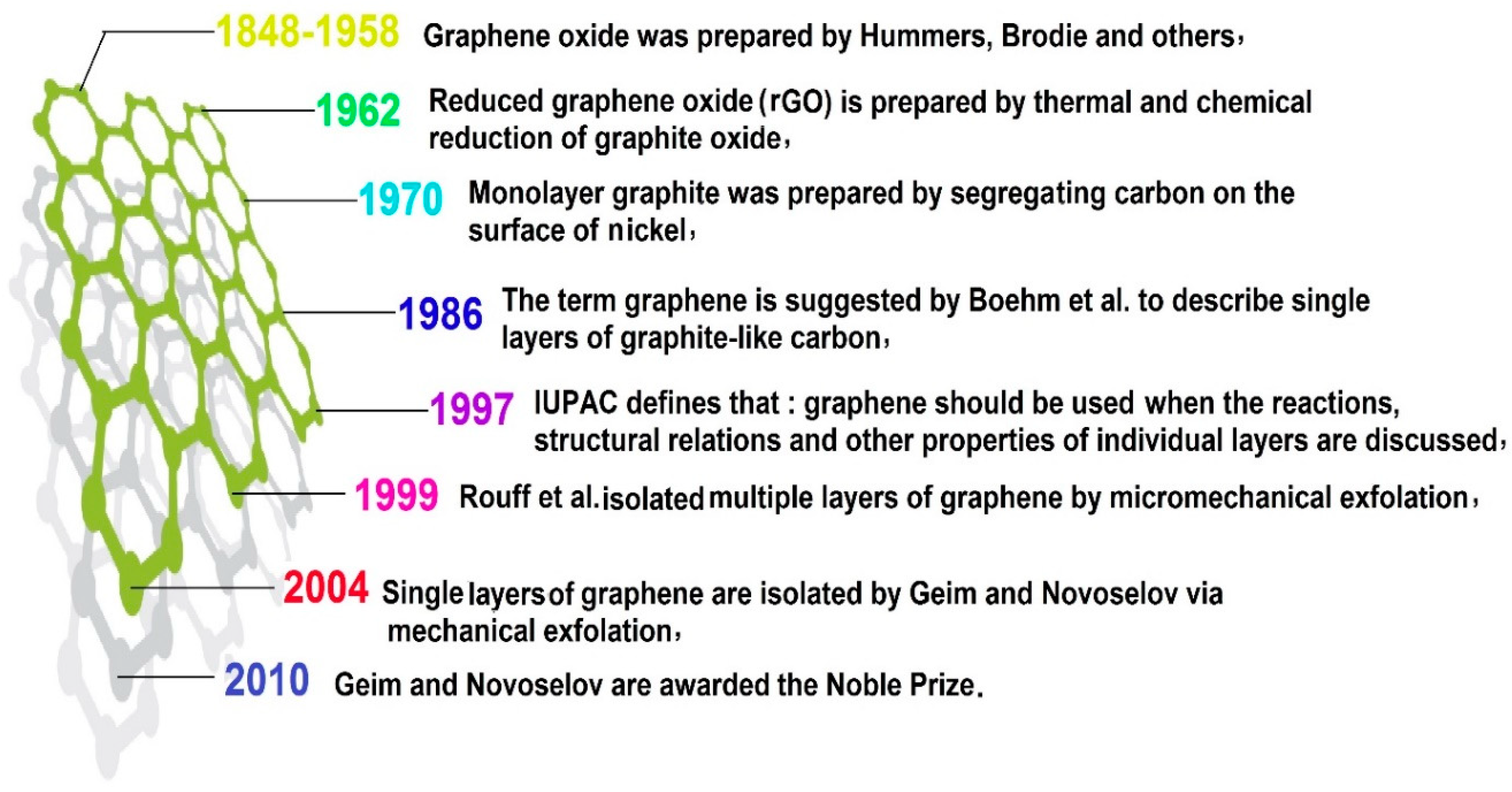
4. Graphene Family Nanomaterials
5. Fabrication of Graphene-Mg MMNCs
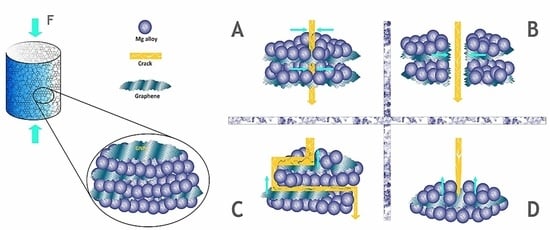
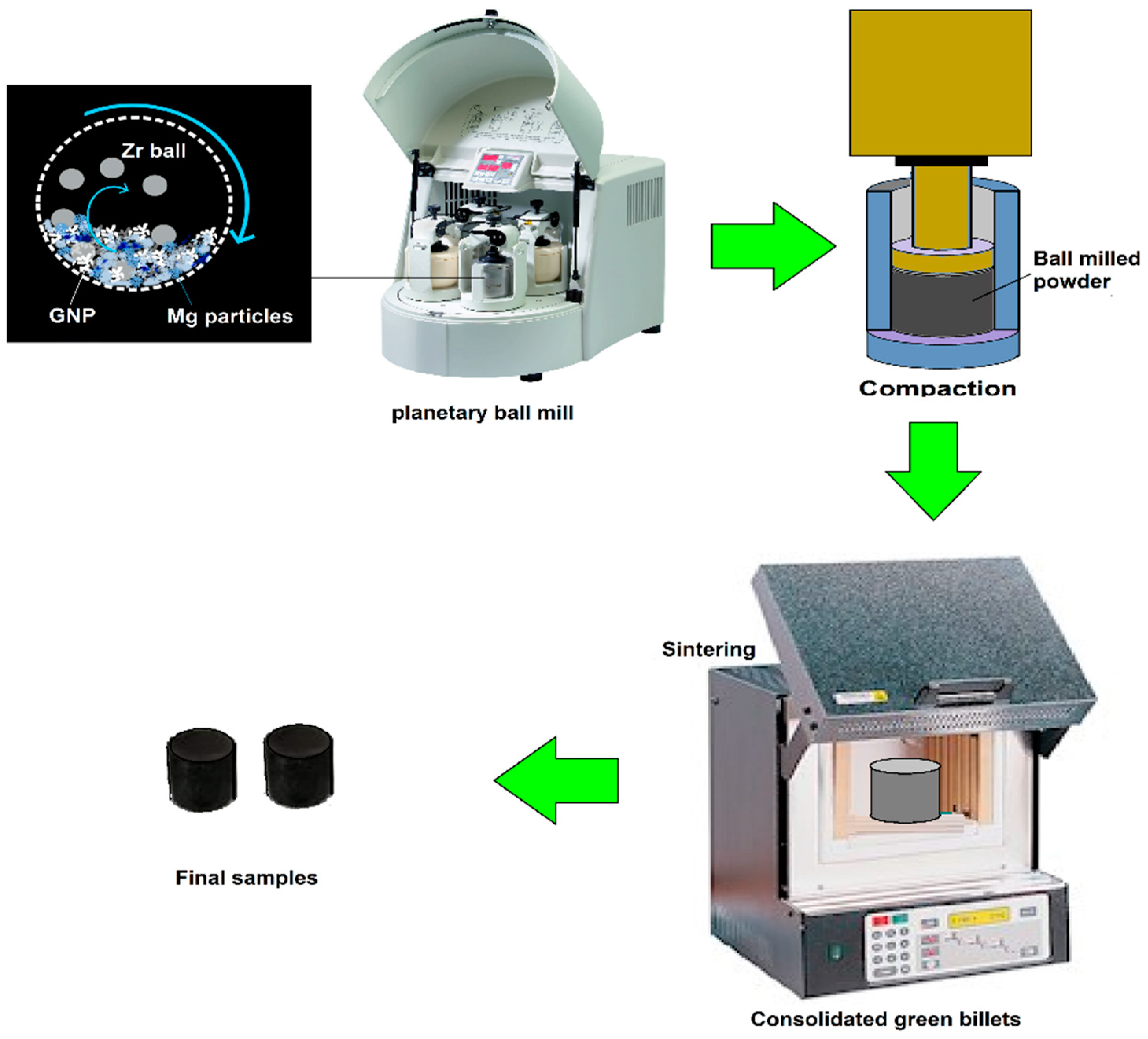
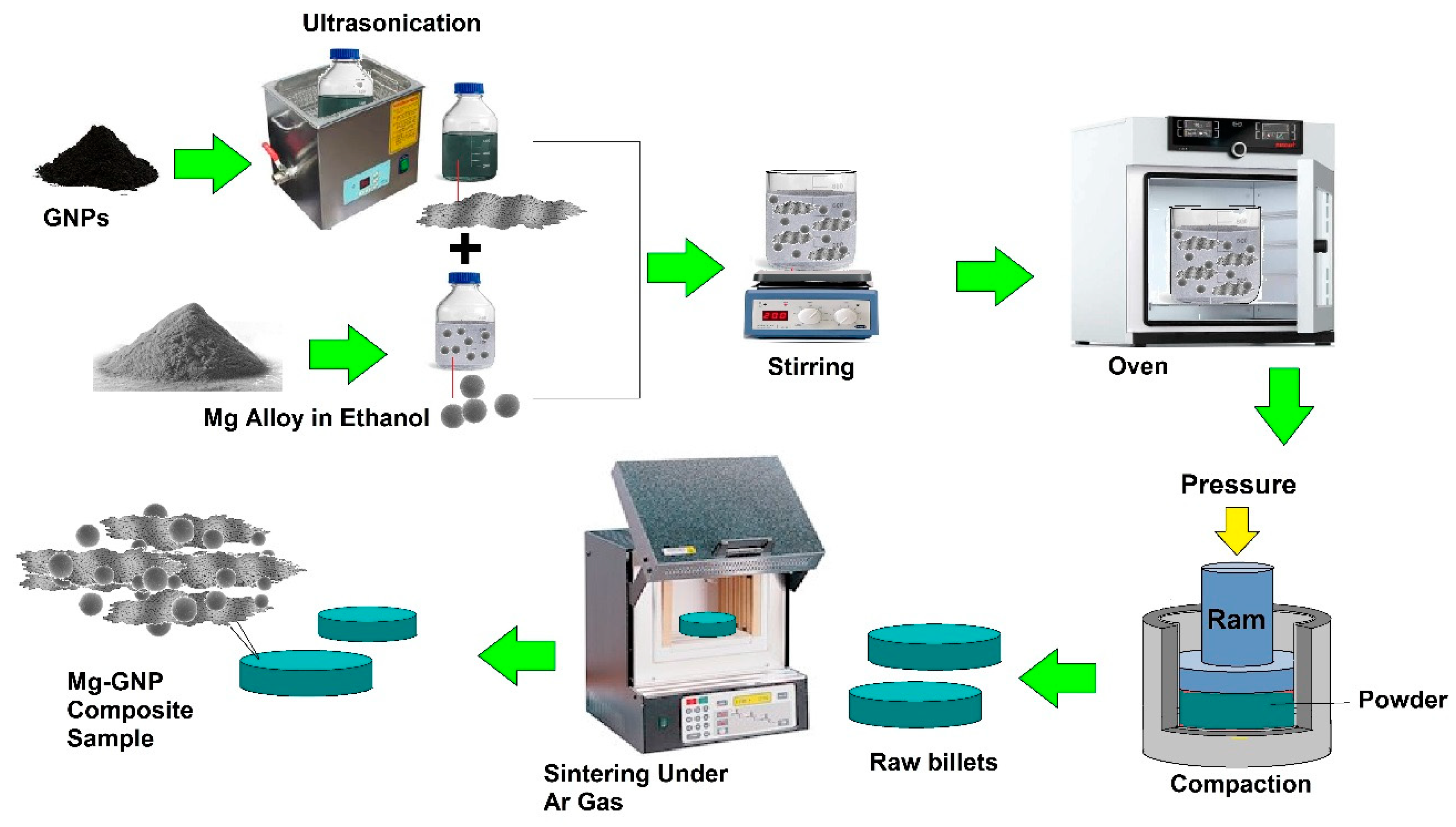
5.1. Powder Metallurgy
5.2. Stirring Casting
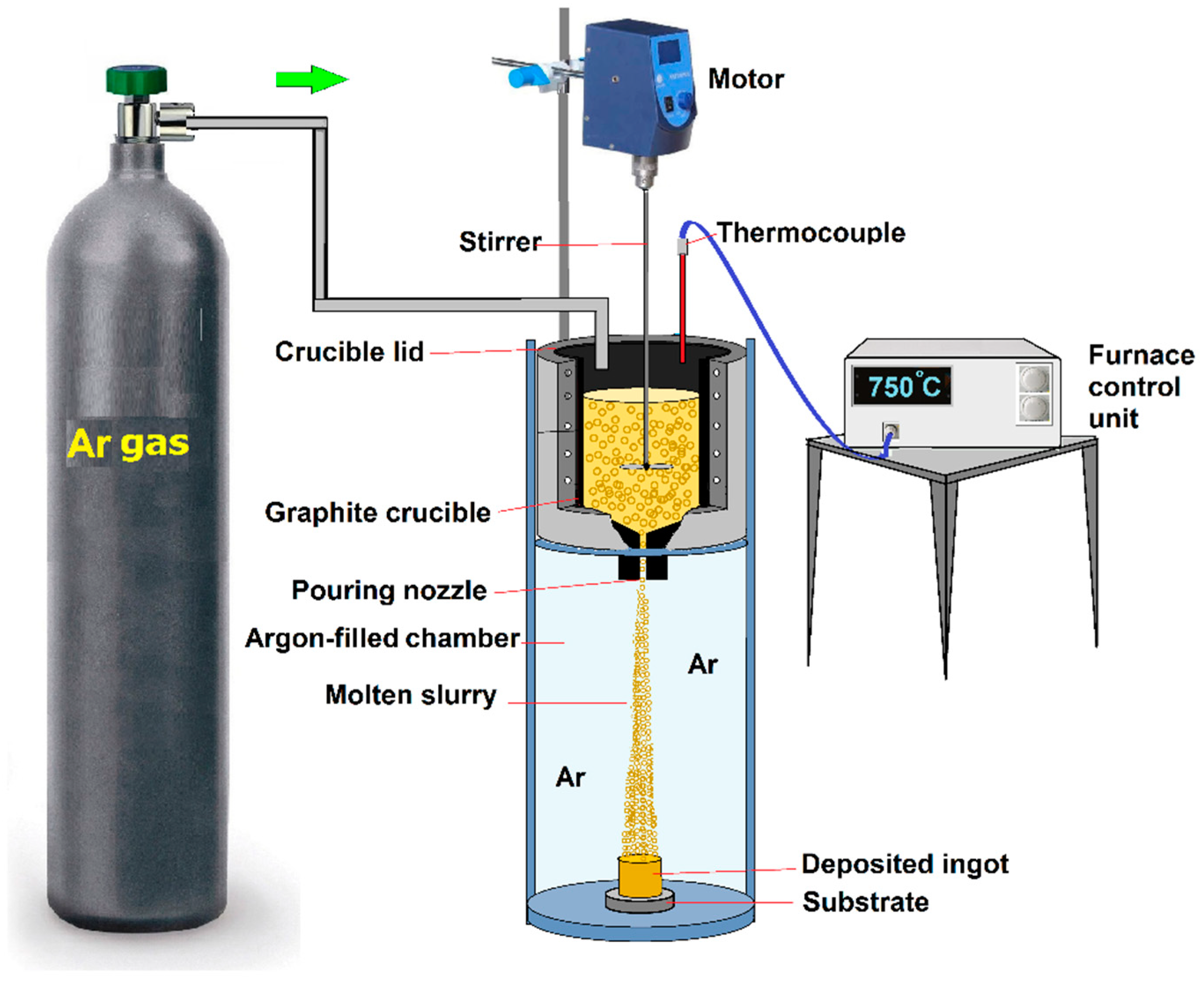
5.3. Disintegrated Melt Deposition
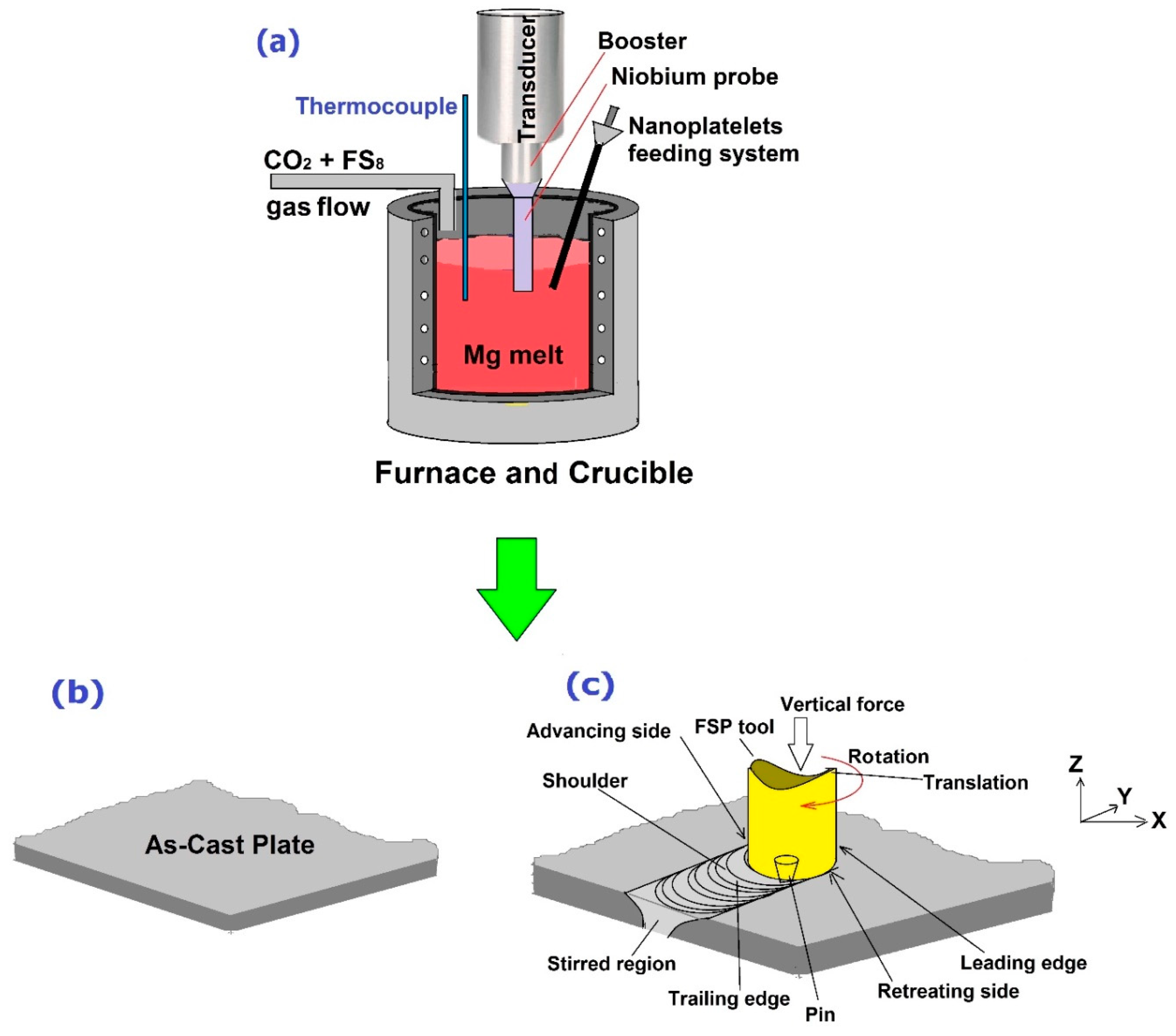
5.4. Friction Stir Processing
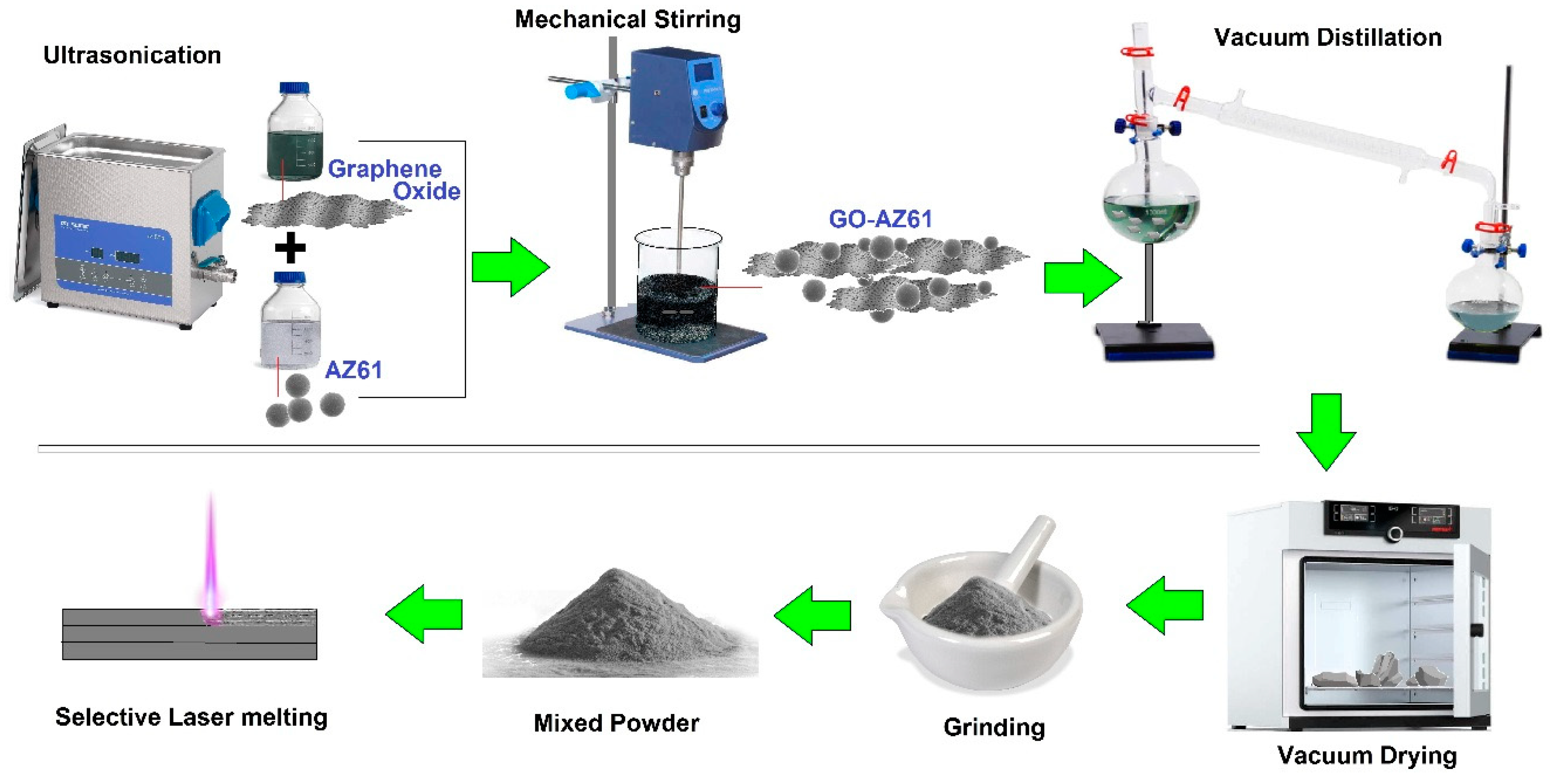
5.5. Selective Laser Melting
5.6. Multi-Step Dispersion Route
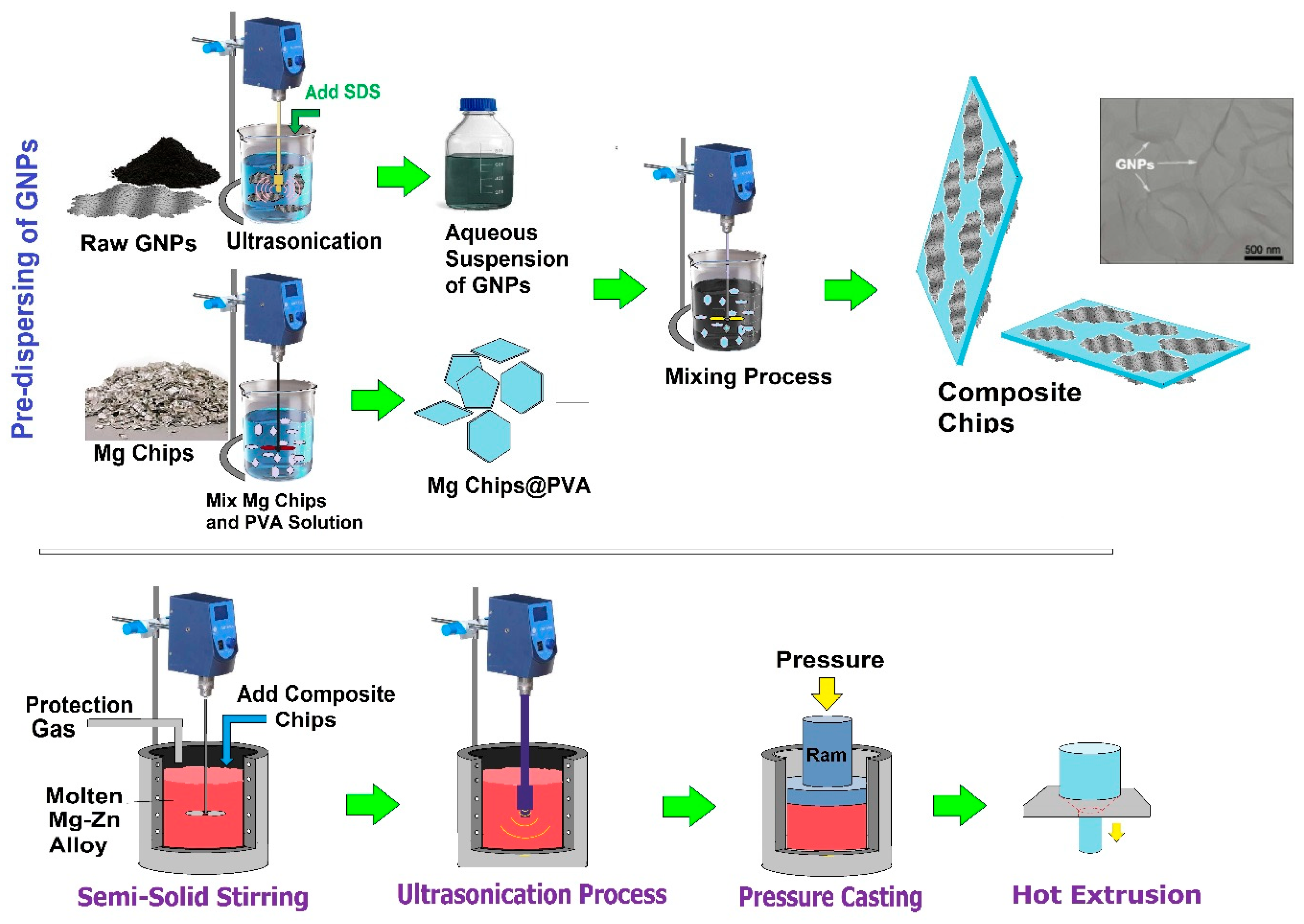
5.7. Semi-Powder Metallurgy
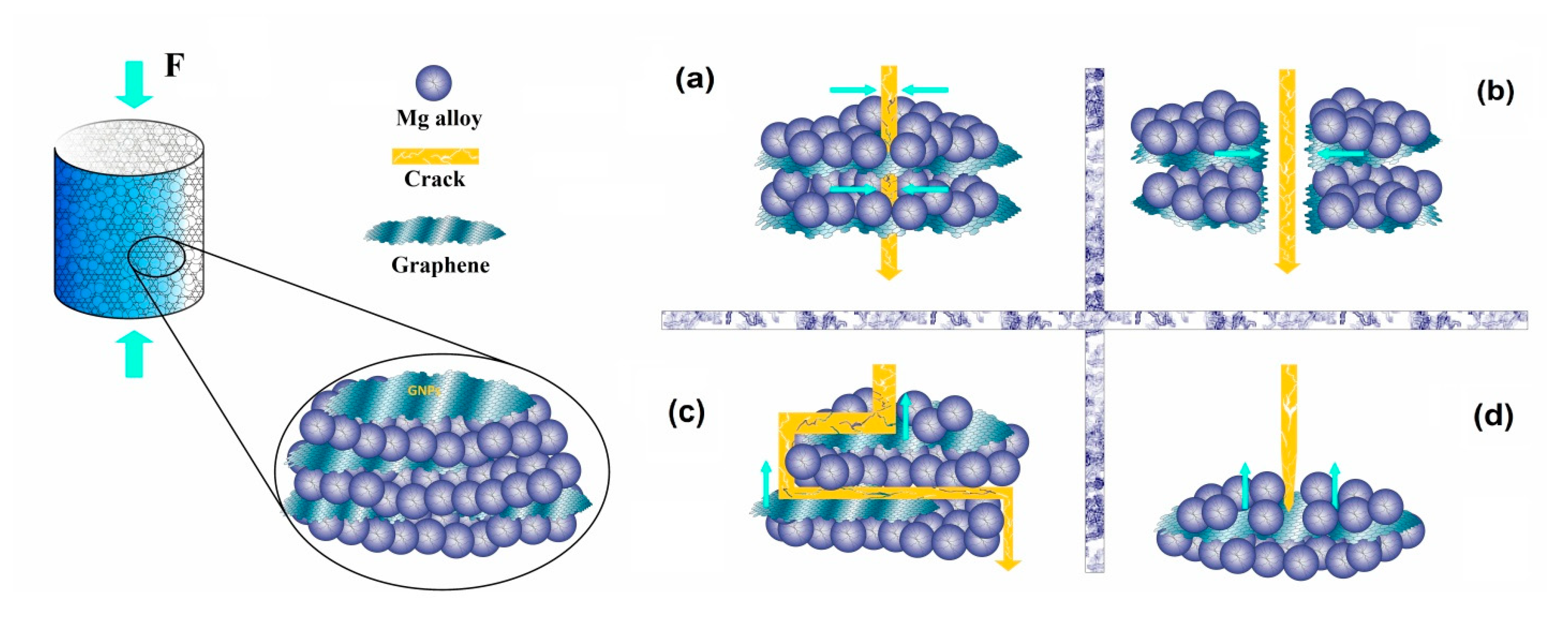
6. Mechanical Properties of Mg-GNP-Based Composites
7. Biocorrosion and Biodegradability of Mg-GFNs-Based Composites
8. Biodegradability of Mg-GFN-Based Composites
9. Cellular Response of Mg-GFN-Based Composites
10. Antibacterial Performance of Mg-GFN-Based Composites
11. Future research Directions in using GFNs for Bone Tissue Engineering
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MMCs | Metal matrix composites |
| MMNCs | Metal matrix nanocomposites |
| GFNs | Graphene family nanomaterials |
| Gr | Graphene |
| GNPs | Graphene nanoplatelets |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| GRO | Graphite oxide |
| CNTs | Carbon nanotubes |
| MWCNTs | Multi-walled carbon nanotubes |
| SBF | Simulated body fluid |
| GB | Grain boundary |
| CMG | Chemically modified graphene |
| BG | Bioglass |
| PM | Powder metallurgy |
| DMD | Disintegrated melt deposition |
| MSDR | Multi-step dispersion route |
| SPM | Semi-powder metallurgy |
| HTE | Hot extrusion |
| SC | Stir casting |
| HEBM | High energy ball milling |
| MBM | Mechanical ball milling |
| SLM | Selective laser melting |
| SSTS | Semi-solid treatment stirring |
| RC | Rheocasting |
| UTS | Ultimate tensile strength |
| TS | Tensile strength |
| TYS | Tensile yield strength |
| E | Elastic modulus |
| EL | Elongation |
| ET | Elastic modulus in tensile |
| CS | Compressive strength |
| UCS | Ultimate compressive strength |
| EC | Elastic modulus in compressive |
| CYS | Compressive yield strength |
| CTE | Coefficient of thermal expansion |
| FGs | Functional groups |
| CIP | Cold isostatic pressing |
| HP | Hot pressing |
| HIP | Hot isostatic pressing |
| SPS | Spark plasma sintering |
| HTE | Hot extrusion |
| HF | Hot forging |
| HR | Hot rolling |
| FSP | Friction stir processing |
| ECA | Equal channel angular processing |
| MA | Mechanical alloying |
| Icorr | Corrosion current density |
| Ecorr | Corrosion potentials |
| HE | Hydrogen evolution |
| WL | Weight loss |
| PDP | Potentio-dynamic polarization |
| Rp | Polarization resistance |
| PEO | Plasma electrolytic oxidation |
| FSW | Friction stir welding |
| TE | Tissue engineering |
| BTE | Bone tissue engineering |
| CVR | Cell viability ratio |
| ECM | Extracellular matrix |
| ROS | Reactive oxygen species |
| BMP | Bone morphogenetic protein |
References
- Mullan, B. Annual Report-Australia. Eur. Heal. Psychol. 2015, 17, 214–215. [Google Scholar]
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable Metals. Mater. Today 2019, 23, 57–71. [Google Scholar]
- Li, N.; Zheng, Y. Novel magnesium alloys developed for biomedical application: A review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Lee, J.-M.; Salvati, E.A.; Betts, F.; DiCarlo, E.F.; Doty, S.B.; Bullough, P.G. Size of metallic and polyethylene debris particles in failed cemented total hip replacements. J. Bone Joint. Surg. Br. 1992, 74, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Bakhsheshi-Rad, H.R.; Akbari, M.; Ismail, A.F.; Aziz, M.; Hadisi, Z.; Pagan, E.; Daroonparvar, M.; Chen, X. Coating biodegradable magnesium alloys with electrospun poly-L-lactic acid-åkermanite-doxycycline nanofibers for enhanced biocompatibility, antibacterial activity, and corrosion resistance. Surf. Coat. Technol. 2019, 37, 124898. [Google Scholar] [CrossRef]
- Hermawan, H. Biodegradable metals: State of the art. In Biodegradable Metals; Springer: Berlin, Germany 2012; pp. 13–22. [Google Scholar]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, H.B.; Heineman, W.; et al. Revolutionizing biodegradable metals. Mater. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- Moravej, M.; Mantovani, D. Biodegradable metals for cardiovascular stent application: Interests and new opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Agustin, J.; Wootton, D.; Zhou, J.G. Biomimetic design and fabrication of porous chitosan–gelatin liver scaffolds with hierarchical channel network. J. Mater. Sci. Mater. Med. 2014, 25, 113–120. [Google Scholar] [CrossRef]
- Onuma, Y.; Ormiston, J.; Serruys, P.W. Bioresorbable scaffold technologies. Circ. J. 2011, 75, 509. [Google Scholar] [CrossRef] [Green Version]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Daroonparvar, M.; Yajid, M.A.M.; Medraj, M. Preparation and characterization of NiCrAlY/nano-YSZ/PCL composite coatings obtained by combination of atmospheric plasma spraying and dip coating on Mg–Ca alloy. J. Alloys Compd. 2016, 658, 440–452. [Google Scholar] [CrossRef]
- Sanchez, A.H.M.; Luthringer, B.J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Dayaghi, E.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Akhavan-Farid, A.; Ismail, A.F.; Aziz, M.; Abdolahi, E. Magnesium-zinc scaffold loaded with tetracycline for tissue engineering application: In vitro cell biology and antibacterial activity assessment. Mater. Sci. Eng. C 2019, 102, 53–65. [Google Scholar] [CrossRef]
- Xiong, P.; Jia, Z.; Li, M.; Zhou, W.; Yan, J.; Wu, Y.; Cheng, Y.; Zheng, Y. Biomimetic Ca, Sr/P-doped silk fibroin films on Mg-1Ca alloy with dramatic corrosion resistance and osteogenic activities. ACS Biomater. Sci. Eng. 2018, 4, 3163–3176. [Google Scholar] [CrossRef]
- Nicolini, C.; Gupta, M. High Performance Lightweight Magnesium Nanocomposites for Engineering and Biomedical Applications. Nano World J. 2016, 2, 78–83. [Google Scholar]
- Miracle, D.B.; Donaldson, S.L.; Henry, S.D.; Moosbrugger, C.; Anton, G.J.; Sanders, B.R.; Hrivnak, N.; Terman, C.; Kinson, J.; Muldoon, K.; et al. ASM Handbook: Composite; ASM International: Geauga, OH, USA, 2001; Volume 21. [Google Scholar]
- Thakur, S.K.; Kwee, G.T.; Gupta, M. Development and characterization of magnesium composites containing nano-sized silicon carbide and carbon nanotubes as hybrid reinforcements. J. Mater. Sci. 2007, 42, 10040–10046. [Google Scholar] [CrossRef]
- Razzaghi, M.; Kasiri-Asgarani, M.; Bakhsheshi-Rad, H.R.; Ghayour, H. Microstructure, mechanical properties, and in-vitro biocompatibility of nano-NiTi reinforced Mg–3Zn-0.5Ag alloy: Prepared by mechanical alloying for implant applications. Compos. Part B Eng. 2020, 190, 107947. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Properties and deformation behaviour of Mg–Y2O3 nanocomposites. Acta Mater. 2007, 55, 5115–5121. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Development of high performance magnesium nano-composites using nano-Al2O3 as reinforcement. Mater Sci. Eng. A 2005, 392, 163–168. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Nayak, U.P.; Sabat, R.K.; Suwas, S.; Almajid, A.; Gupta, M. Nano-ZnO particle addition to monolithic magnesium for enhanced tensile and compressive response. J. Alloys Compd. 2014, 615, 211–219. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Seetharaman, S.; Gupta, M. Enhancing overall tensile and compressive response of pure Mg using nano-TiB2 particulates. Mater. Charact. 2014, 94, 178–188. [Google Scholar] [CrossRef]
- Tjong, S.C. Recent progress in the development and properties of novel metal matrix nanocomposites reinforced with carbon nanotubes and graphene nanosheets. Mater. Sci. Eng. R Rep. 2013, 74, 281–350. [Google Scholar] [CrossRef]
- Tjong, S.C. Novel nanoparticle-reinforced metal matrix composites with enhanced mechanical properties. Adv. Eng. Mater. 2007, 9, 639–652. [Google Scholar] [CrossRef]
- Haghshenas, M. Mechanical characteristics of biodegradable magnesium matrix composites: A review. J. Magnes Alloy 2017, 5, 189–201. [Google Scholar] [CrossRef]
- Munir, K.; Wen, C.; Li, Y. Graphene nanoplatelets-reinforced magnesium metal matrix nanocomposites with superior mechanical and corrosion performance for biomedical applications. J. Magnes Alloy 2020, 828, 154461. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Z.; Huang, L.; Essa, K.; Bilotti, E.; Zhang, H.; Peijs, T.; Hao, L. Additive manufacturing high performance graphene-based composites: A review. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105483. [Google Scholar] [CrossRef]
- Gao, C.; Feng, P.; Peng, S.; Shuai, C. Carbon nanotube, graphene and boron nitride nanotube reinforced bioactive ceramics for bone repair. Acta Biomater. 2017, 61, 1–20. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, T.; Gao, C.; Feng, P.; Xiao, T.; Yu, K.; Peng, S. Mechanical and structural characterization of diopside scaffolds reinforced with graphene. J. Alloys Compd. 2016, 655, 86–92. [Google Scholar] [CrossRef]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and antibacterial performance of novel polylactic acid-graphene oxide-silver nanoparticle hybrid nanocomposite mats prepared by electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Yu, Z.; Asif, M.; Lin, H.; Pan, R. Investigation on microstructural, mechanical and electrochemical properties of aluminum composites reinforced with graphene nanoplatelets. Prog. Nat. Sci. Mater. Int. 2015, 25, 460–470. [Google Scholar] [CrossRef] [Green Version]
- Saris, N.-E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion—a framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Rodrigues, L.R. Inhibition of bacterial adhesion on medical devices. In Bacterial Adhesion; Springer: Dordrecht, The Netherlands, 2011; Volume 715, pp. 351–367. [Google Scholar]
- Bellucci, D.; Cannillo, V.; Cattini, A.; Sola, A. A new generation of scaffolds for bone tissue engineering. Ind. Ceram. 2011, 31, 59–62. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef]
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [Green Version]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Hussein, M.A.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable materials and metallic implants—a review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munir, K.S.; Wen, C.; Li, Y. Carbon nanotubes and graphene as nanoreinforcements in metallic biomaterials: A review. Adv. Biosyst. 2019, 3, 1800212. [Google Scholar] [CrossRef] [PubMed]
- Yunus Basha, R.T.S.S.K.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater Sci. Eng., C 2015, 57, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Cao, T.; Rodríguez-Lozano, F.J.; Luong-Van, E.K.; Rosa, V. Graphene for the development of the next-generation of biocomposites for dental and medical applications. Dent. Mater. 2017, 33, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano. 2010, 4, 4317. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Saud, S.N.; Yaghoubidoust, F.; Akbari, E. Structure, corrosion behavior, and antibacterial properties of nano-silica/graphene oxide coating on biodegradable magnesium alloy for biomedical applications. Vacuum 2016, 131, 106–110. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.-H.; Yun, K.; Kim, S.J. Antibacterial efficiency of graphene nanosheets against pathogenic bacteria via lipid peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Park, M.R.; Kwon, D.N.; Kim, J.H. Antibacterial activity of dithiothreitol reduced graphene oxide. J. Ind. Eng. Chem. 2013, 19, 1280–1288. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano. 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Han, H. A new function of graphene oxide emerges: Inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. J. Nanopart. Res. 2013, 15, 1658. [Google Scholar] [CrossRef]
- Ahmed, F.; Rodrigues, D.F. Investigation of acute effects of graphene oxide on wastewater microbial community: A case study. J. Hazard Mater. 2013, 256, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Eizenberg, M.; Blakely, J.M. Carbon monolayer phase condensation on Ni (111). Surf. Sci. 1979, 82, 228–236. [Google Scholar] [CrossRef]
- Aizawa, T.; Souda, R.; Otani, S.; Ishizawa, Y.; Oshima, C. Anomalous bond of monolayer graphite on transition-metal carbide surfaces. Phys. Rev. Lett. 1990, 64, 768. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly (sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Berichte der Dtsch. Chem. Gesellschaft. 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Boehm, H.-P.; Clauss, A.; Fischer, G.O.; Hofmann, U. Das adsorptionsverhalten sehr dünner kohlenstoff-folien. Zeitschrift für Anorg. und Allg. Chemie 1962, 316, 119–127. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite Materials. Nature 2006, 442, 282–286. [Google Scholar]
- Schniepp, H.C.; Li, J.L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [Green Version]
- McAllister, M.J.; Li, J.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Kumar, H.G.P.; Xavior, M.A. Graphene reinforced metal matrix composite (GRMMC): A review. Procedia Eng. 2014, 97, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.L.C.; Kessler, F.; Dubey, N.; Rosa, V.; Fechine, G.J.M. CVD graphene transfer procedure to the surface of stainless steel for stem cell proliferation. Surf. Coat. Technol. 2017, 311, 10–18. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W. RS Ruoff The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Xie, H.; Dubey, N.; Madanagopal, T.T.; Rajan, S.S.; Morin, J.L.P.; Islam, I.; Neto, A.H.C. Graphene oxide-based substrate: Physical and surface characterization, cytocompatibility and differentiation potential of dental pulp stem cells. Dent Mater. 2016, 32, 1019–1025. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Jeon, C.H.; Jeong, Y.H.; Seo, J.J.; Tien, H.N.; Hong, S.T.; Yum, Y.J.; Hur, S.H.; Lee, K.J. Material properties of graphene/aluminum metal matrix composites fabricated by friction stir processing. Int. J. Precis Eng. Manuf. 2014, 15, 1235–1239. [Google Scholar] [CrossRef]
- Xiang, S.; Wang, X.; Gupta, M.; Wu, K.; Hu, X.; Zheng, M. Graphene nanoplatelets induced heterogeneous bimodal structural magnesium matrix composites with enhanced mechanical properties. Sci. Rep. 2016, 6, 38824. [Google Scholar] [CrossRef]
- Tabandeh-Khorshid, M.; Kumar, A.; Omrani, E.; Kim, C.; Rohatgi, P. Synthesis, characterization, and properties of graphene reinforced metal-matrix nanocomposites. Compos. Part B Eng. 2020, 183, 107664. [Google Scholar] [CrossRef]
- Salleh, E.M.; Zuhailawati, H.; Ramakrishnan, S.; Dhindaw, B.K. Enhanced mechanical properties and corrosion behavior of biodegradable Mg-Zn/HA composite. Metall. Mater. Trans. A 2017, 48, 2519–2528. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Kadir, M.R.A.; Izman, S.; Bakhsheshi-Rad, H.R.; Farahany, S. Effect of mechanical alloying on the phase evolution, microstructure and bio-corrosion properties of a Mg/HA/TiO2/MgO nanocomposite. Ceram. Int. 2014, 40, 16743–16759. [Google Scholar] [CrossRef]
- Saheban, M.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Hamzah, E.; Najafinezhad, A. Effect of zeolite on the corrosion behavior, biocompatibility and antibacterial activity of porous magnesium/zeolite composite scaffolds. Mater. Technol. 2019, 34, 258–269. [Google Scholar] [CrossRef]
- Wan, Y.; Cui, T.; Li, W.; Li, C.; Xiao, J.; Zhu, Y.; Ji, D.; Xiong, G.; Luo, H. Mechanical and biological properties of bioglass/magnesium composites prepared via microwave sintering route. J. Compos. Mater. 2016, 99, 521–527. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Xi, Y.L.; Chai, D.L. In vitro degradation and cytotoxicity of Mg/Ca composites produced by powder metallurgy. Acta Biomater. 2010, 6, 1783–1791. [Google Scholar] [CrossRef]
- Chen, L.Y.; Konishi, H.; Fehrenbacher, A.; Ma, C.; Xu, J.Q.; Choi, H.; Xu, H.F.; Pfefferkorn, F.E.; Li, X.C. Novel nanoprocessing route for bulk graphene nanoplatelets reinforced metal matrix nanocomposites. Scr. Mater. 2012, 67, 29–32. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; She, J.; Gou, J.; Mao, J.; Hu, H. Development of magnesium-graphene nanoplatelets composite. J Compos. Mater. 2015, 49, 285–293. [Google Scholar] [CrossRef]
- Du, X.; Du, W.; Wang, Z.; Liu, K.; Li, S. Ultra-high strengthening efficiency of graphene nanoplatelets reinforced magnesium matrix composites. Mater. Sci. Eng. A 2018, 711, 633–642. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, H.; Wang, Y.; Hu, M.; Ji, Z. Investigation of the microstructure and mechanical properties of AZ31/graphene composite fabricated by semi-solid isothermal treatment and hot extrusion. JOM 2019, 71, 4162–4170. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium-based composites reinforced with graphene nanoplatelets as biodegradable implant materials. J. Alloys Compd. 2020, 828, 154461. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Lin, D.; Asif, M. High temperature mechanical behavior of AZ61 magnesium alloy reinforced with graphene nanoplatelets. J. Compos. Mater. 2016, 89, 1242–1250. [Google Scholar] [CrossRef]
- Ramezanzade, S.; Ebrahimi, G.R.; Parizi, M.T.; Ezatpour, H.R. Microstructure and mechanical characterizations of graphene nanoplatelets-reinforced Mg–Sr–Ca alloy as a novel composite in structural and biomedical applications. J. Compos. Mater. 2020, 54, 711–728. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Y.; Wang, L.D.; Zhu, Y.P.; Wang, X.J.; Sheng, J.; Yang, Z.Y.; Shi, H.L.; Shi, Z.D.; Fei, W.D. Achieving high strength and ductility in graphene/magnesium composite via an in-situ reaction wetting process. Carbon 2018, 139, 954–963. [Google Scholar] [CrossRef]
- Du, X.; Du, W.; Wang, Z.; Liu, K.; Li, S. Defects in graphene nanoplatelets and their interface behavior to reinforce magnesium alloys. Appl. Surf. Sci. 2019, 484, 414–423. [Google Scholar] [CrossRef]
- Xiang, S.L.; Gupta, M.; Wang, X.J.; Wang, L.D.; Hu, X.S.; Wu, K. Enhanced overall strength and ductility of magnesium matrix composites by low content of graphene nanoplatelets. Compos. Part A Appl. Sci. Manuf. 2017, 100, 183–193. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M. Exploring mechanical behavior of Mg–6Zn alloy reinforced with graphene nanoplatelets. Mater. Sci. Eng. A 2016, 649, 263–269. [Google Scholar] [CrossRef]
- Arab, M.; Marashi, S.P.H. Graphene nanoplatelet (GNP)-incorporated AZ31 magnesium nanocomposite: Microstructural, mechanical and tribological properties. Tribol. Lett. 2018, 66, 156. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, B.; Yang, Y.; Peng, S.; Gao, C. 3D honeycomb nanostructure-encapsulated magnesium alloys with superior corrosion resistance and mechanical properties. Compos. Part B Eng. 2019, 162, 611–620. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Peng, J.-Y.; Xu, J.-Q.; Choi, H.; Li, X.-C. Achieving uniform distribution and dispersion of a high percentage of nanoparticles in metal matrix nanocomposites by solidification processing. Scr. Mater. 2013, 69, 634–637. [Google Scholar] [CrossRef]
- Nie, K.B.; Wang, X.J.; Wu, K.; Xu, L.; Zheng, M.Y.; Hu, X.S. Processing, microstructure and mechanical properties of magnesium matrix nanocomposites fabricated by semisolid stirring assisted ultrasonic vibration. J. Alloys Compd. 2011, 509, 8664–8669. [Google Scholar] [CrossRef]
- Turan, M.E.; Sun, Y.; Akgul, Y. Mechanical, tribological and corrosion properties of fullerene reinforced magnesium matrix composites fabricated by semi powder metallurgy. J. Alloys Compd. 2018, 740, 1149–1158. [Google Scholar] [CrossRef]
- Turan, M.E.; Zengin, H.; Sun, Y. Dry sliding wear behavior of (MWCNT + GNPs) reinforced AZ91 magnesium matrix hybrid composites. Met. Mater. Int. 2020, 26, 540–550. [Google Scholar] [CrossRef]
- Turan, M.E.; Sun, Y.; Akgul, Y.; Turen, Y.; Ahlatci, H. The effect of GNPs on wear and corrosion behaviors of pure magnesium. J. Alloys Compd. 2017, 724, 14–23. [Google Scholar] [CrossRef]
- Turan, M.E.; Sun, Y.; Aydin, F.; Zengin, H.; Turen, Y.; Ahlatci, H. Effects of carbonaceous reinforcements on microstructure and corrosion properties of magnesium matrix composites. Mater. Chem. Phys. 2018, 218, 182–188. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M.; Tang, A. Powder metallurgy of Mg–1%Al–1%Sn alloy reinforced with low content of graphene nanoplatelets (GNPs). J. Ind. Eng. Chem. 2014, 20, 4250–4255. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H. Magnesium-graphene nano-platelet composites: Corrosion behavior, mechanical and biological properties. J. Alloys Compd. 2020, 821, 153379. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; Aamir, M. Synergetic effect of graphene nanoplatelets (GNPs) and multi-walled carbon nanotube (MW-CNTs) on mechanical properties of pure magnesium. J. Alloys Compd. 2014, 603, 111–118. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Srivastava, I.; Wang, Z.; Song, H.; Yu, Z.Z.; Koratkar, N. Fracture and fatigue in graphene nanocomposites. Small 2010, 6, 179–183. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Schultz, B.F.; Venugopalan, D.; Lopez, H.F.; Rohatgi, P.K.; Cho, K.; Kim, C.-S. On the superposition of strengthening mechanisms in dispersion strengthened alloys and metal-matrix nanocomposites: Considerations of stress and energy. Met. Mater. Int. 2014, 20, 375–388. [Google Scholar] [CrossRef]
- Ramezanzade, S.; Ebrahimi, G.R.; Parizi, M.T.; Ezatpour, H.R. Synergetic effect of GNPs and MgOs on the mechanical properties of Mg–Sr–Ca alloy. Mater. Sci. Eng. A 2019, 761, 138025. [Google Scholar] [CrossRef]
- Meng, L.; Hu, X.; Wang, X.; Zhang, C.; Shi, H.; Xiang, Y.; Liu, N.; Wu, K. Graphene nanoplatelets reinforced Mg matrix composite with enhanced mechanical properties by structure construction. Mater. Sci. Eng. A 2018, 733, 414–418. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhou, G.; Liao, L.; Liu, Y.; Luo, L. Interfacial structure in AZ91 alloy composites reinforced by graphene nanosheets. Carbon 2018, 127, 177–186. [Google Scholar] [CrossRef]
- Kandemir, S. Development of Graphene Nanoplatelet-Reinforced AZ91 Magnesium Alloy by Solidification Processing. J Mater. Eng. Perform. 2018, 27, 3014–3023. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; Hussain, S.; Gou, J.; Mao, J. Improved strength and ductility of magnesium with addition of aluminum and graphene nanoplatelets (Al + GNPs) using semi powder metallurgy method. J. Ind. Eng. Chem. 2015, 23, 243–250. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.S.; Asif, M.; Ullah, A. Improved mechanical properties of magnesium–graphene composites with copper–graphene hybrids. Mater. Sci. Technol. 2015, 31, 1452–1461. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M. Magnesium matrix composites reinforced with graphene nanoplatelets. Graphene Mater. Sci. Eng. A 2015, 630, 36–44. [Google Scholar] [CrossRef]
- Clyne, T.W.; Withers, P.J. An Introduction to Metal Matrix Composites; Cambridge university press: Cambridge, UK, 1995. [Google Scholar]
- Courtney, T.H. Mechanical Behavior of Materials; Waveland Press: Long Grove, IL, USA, 2005. [Google Scholar]
- Şenel, M.C.; Gürbüz, M.; Koc, E. Fabrication and characterization of synergistic Al-SiC-GNPs hybrid composites. Compos. Part B Eng. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Tiwari, A.; Syväjärvi, M. Graphene Materials: Fundamentals and Emerging Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Rashad, M.; Pan, F.; Zhang, J.; Asif, M. Use of high energy ball milling to study the role of graphene nanoplatelets and carbon nanotubes reinforced magnesium alloy. J. Alloys Compd. 2015, 646, 223–232. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Liu, Y.; Chen, X.; Lin, H.; Pan, R.; Asif, M.; She, J. High temperature formability of graphene nanoplatelets-AZ31 composites fabricated by stir-casting method. J. Magnes Alloy 2016, 4, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Torabi Parizi, M.; Ebrahimi, G.R.; Ezatpour, H.R. Effect of graphene nanoplatelets content on the microstructural and mechanical properties of AZ80 magnesium alloy. Mater. Sci. Eng. A 2019, 742, 373–389. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Lu, Y.; Asif, M.; Hussain, S.; She, J.; Gou, J.; Mao, J. Effect of graphene nanoplatelets (GNPs) addition on strength and ductility of magnesium-titanium alloys. J. Magnes Alloy 2013, 1, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Manivasagam, G.; Suwas, S. Biodegradable Mg and Mg based alloys for biomedical implants. Mater. Sci. Technol. 2014, 30, 515–520. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, W.; Cheng, Y.; Zheng, Y.F. A study on alkaline heat treated Mg–Ca alloy for the control of the biocorrosion rate. Acta Biomater. 2009, 5, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.W. Interfacial bioengineering to enhance surface biocompatibility. Med. Device Technol. 2002, 13, 18–21. [Google Scholar]
- Ruedi, T.P. AO principles of fracture management; Thieme: New York, NY, USA, 2000. [Google Scholar]
- Erinc, M.; Sillekens, W.H.; Mannens, R.; Werkhoven, R.J. Applicability of existing magnesium alloys as biomedical implant Materials. In Proceedings of the Magnesium Technology 2009, San Francisco, CA, USA, 15–19 February 2009; pp. 209–214, Conference code: 76923. [Google Scholar]
- Atrens, A.D.; Gentle, I.; Atrens, A. Possible dissolution pathways participating in the Mg corrosion reaction. Corros. Sci. 2015, 92, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Huo, K.; Tao, H.; Tang, G.; Chu, P.K. Influence of aggressive ions on the degradation behavior of biomedical magnesium alloy in physiological environment. Acta Biomater. 2008, 4, 2008–2015. [Google Scholar] [CrossRef]
- Raucci, M.G.; Giugliano, D.; Longo, A.; Zeppetelli, S.; Carotenuto, G.; Ambrosio, L. Comparative facile methods for preparing graphene oxide–hydroxyapatite for bone tissue engineering. J. Tissue Eng. Regen Med. 2017, 11, 2204–2216. [Google Scholar] [CrossRef]
- Kurapati, R.; Russier, J.; Squillaci, M.A.; Treossi, E.; Ménard-Moyon, C.; Del Rio-Castillo, A.E.; Vazquez, E.; Samorì, P.; Palermo, V.; Bianco, A. Dispersibility-dependent biodegradation of graphene oxide by myeloperoxidase. Small 2015, 11, 3985–3994. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Schiller, T.; Medhekar, N.; Birbilis, N. Exploring graphene as a corrosion protection barrier. Corros. Sci. 2012, 56, 1–4. [Google Scholar] [CrossRef]
- Raman, R.S.; Banerjee, P.C.; Lobo, D.E.; Gullapalli, H.; Sumandasa, M.; Kumar, A.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Protecting copper from electrochemical degradation by graphene coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Neupane, M.P.; Lee, S.J.; Kang, J.Y.; Park, I.S.; Bae, T.S.; Lee, M.H. Surface characterization and corrosion behavior of silanized magnesium coated with graphene for biomedical application. Mater. Chem. Phys. 2015, 163, 229–235. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M.; Chen, X. Corrosion behavior of magnesium-graphene composites in sodium chloride solutions. J. Magnes Alloy 2017, 5, 271–276. [Google Scholar] [CrossRef]
- Kavimani, V.; Soorya Prakash, K.; Arun Pandian, M. Influence of r-GO addition on enhancement of corrosion and wear behavior of AZ31 MMC. Appl. Phys. A 2017, 123, 514. [Google Scholar] [CrossRef]
- Kavimani, V.; Soorya Prakash, K.; Thankachan, T. Investigation of graphene-reinforced magnesium metal matrix composites processed through a solvent-based powder metallurgy route. Bull. Mater. Sci. 2019, 42, 39. [Google Scholar] [CrossRef] [Green Version]
- Aydin, F.; Ayday, A.; Turan, M.E.; Zengin, H. Role of graphene additive on wear and electrochemical corrosion behaviour of plasma electrolytic oxidation (PEO) coatings on Mg–MWCNT nanocomposite. Surf. Eng. 2019, 1–9. [Google Scholar] [CrossRef]
- Tsetseris, L.; Pantelides, S.T. Graphene: An impermeable or selectively permeable membrane for atomic species? Carbon 2014, 67, 58–63. [Google Scholar] [CrossRef]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The Enzymatic Oxidation of Graphene Oxide. ACS Nano. 2011, 5, 2098–2108. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Newman, L.; Lozano, N.; Zhang, M.; Iijima, S.; Yudasaka, M.; Bussy, C.; Kostarelos, K. Hypochlorite degrades 2D graphene oxide sheets faster than 1D oxidised carbon nanotubes and nanohorns. NPJ 2D Mater. Appl. 2017, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bal, B.S.; Rahaman, M.N. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium matrix nanocomposites for orthopedic applications: A review from mechanical, corrosion, and biological perspectives. Acta Biomater. 2019, 96, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shi, M.; Yan, B.; Ma, H.; Li, N.; Hu, Y.; Ye, M. Covalent attaching protein to graphene oxide via diimide-activated amidation. Colloids Surf., B 2010, 81, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Girase, B.; Shah, J.S.; Misra, R.D.K. Cellular mechanics of modulated osteoblasts functions in graphene oxide reinforced elastomers. Adv. Eng. Mater. 2012, 14, 101–111. [Google Scholar] [CrossRef]
- Neagu, M.; Piperigkou, Z.; Karamanou, K.; Engin, A.B.; Docea, A.O.; Constantin, C.; Negrei, C.; Nikitovic, D.; Tsatsakis, A. Protein bio-corona: Critical issue in immune nanotoxicology. Arch. Toxicol. 2017, 91, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- La, W.G.; Jin, M.; Park, S.; Yoon, H.H.; Jeong, G.J.; Bhang, S.H.; Park, H.; Char, K.; Kim, B.S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9, 107–116. [Google Scholar]
- Ku, S.H.; Park, C.B. Myoblast differentiation on graphene oxide. Biomaterials 2013, 34, 2017–2023. [Google Scholar] [CrossRef]
- Hronik-Tupaj, M.; Rice, W.L.; Cronin-Golomb, M.; Kaplan, D.L.; Georgakoudi, I. Osteoblastic differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed. Eng. Online 2011, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Aaron, R.K.; Boyan, B.D.; Ciombor, D.M.; Schwartz, Z.; Simon, B.J. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin. Orthop. Relat. Res. 2004, 419, 30–37. [Google Scholar] [CrossRef]
- Gong, H.; Anasori, B.; Dennison, C.R.; Wang, K.; Kumbur, E.C.; Strich, R.; Zhou, J.G. Fabrication, biodegradation behavior and cytotoxicity of Mg-nanodiamond composites for implant application. J. Mater. Sci. Mater. Med. 2015, 26, 110. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based Materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide–silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Kong, W.; Sun, M.; Yang, Y.; Liu, W.; Lv, M.; Song, S.; Wang, L.; Song, H.; Hao, R. Highly stable graphene-based nanocomposite (GO–PEI–Ag) with broad-spectrum, long-term antimicrobial activity and antibiofilm effects. ACS Appl. Mater. Interfaces 2018, 10, 17617–17629. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the antimicrobial activities of graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Guo, W.; Wu, P.; Yang, W.; Hu, S.; Xia, Y.; Feng, P. A graphene oxide-Ag co-dispersing nanosystem: Dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem. Eng. J. 2018, 347, 322–333. [Google Scholar] [CrossRef]
- Saito, N.; Haniu, H.; Usui, Y.; Aoki, K.; Hara, K.; Takanashi, S.; Shimizu, M.; Narita, N.; Okamoto, M.; Kobayashi, S.; et al. Safe clinical use of carbon nanotubes as innovative Biomaterials. Chem. Rev. 2014, 114, 6040–6079. [Google Scholar] [CrossRef]
- Rahim, M.I.; Ullah, S.; Mueller, P.P. Advances and challenges of biodegradable implant materials with a focus on magnesium-alloys and bacterial infections. Metals 2018, 8, 532. [Google Scholar] [CrossRef] [Green Version]
- Peron, M.; Torgersen, J.; Berto, F. Mg and its alloys for biomedical applications: Exploring corrosion and its interplay with mechanical failure. Metals 2017, 7, 252. [Google Scholar] [CrossRef] [Green Version]
- Azarniya, A.; Safavi, M.S.; Sovizi, S.; Azarniya, A.; Chen, B.; Madaah Hosseini, H.R.; Ramakrishna, S. Metallurgical challenges in carbon nanotube-reinforced metal matrix nanocomposites. Metals 2017, 7, 384. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, J.; Russell, T.; Sankar, J.; Yun, Y. The biological responses to magnesium-based biodegradable medical devices. Metals 2017, 7, 514. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Qu, X.; Ren, L.; Fan, L.; Zhang, Y.; Guo, Y.; Quan, G.; Tang, Q.; Liu, B.; Sun, H. The effects of carbon nanotubes on the mechanical and wear properties of AZ31 alloy. Materials 2017, 10, 1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saboori, A.; Dadkhah, M.; Fino, P.; Pavese, M. An overview of metal matrix nanocomposites reinforced with graphene nanoplatelets; mechanical, electrical and thermophysical properties. Metals 2018, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Saboori, A.; Moheimani, S.K.; Dadkhah, M.; Pavese, M.; Badini, C.; Fino, P. An overview of key challenges in the fabrication of metal matrix nanocomposites reinforced by graphene nanoplatelets. Metals 2018, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.-N.; Kim, J.-H.; Kim, B.-S.; Jeong, H.-M.; Huh, S.-C. Study on the thermal conductivity characteristics of graphene prepared by the planetary ball mill. Metals 2016, 6, 234. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Fan, L.; Zhou, M.; Zhou, Y.; Jiang, K.; Chen, Y. Hot compression deformation and activation energy of nanohybrid-reinforced AZ80 magnesium matrix composite. Metals 2020, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Liu, H.; Tang, Y.; Xu, X.; Long, H.; Yan, Y.; Luo, Z.; Zhang, Y.; Yu, K.; Zhu, Y. A potential biodegradable Mg-Y-Ag implant with strengthened antimicrobial properties in orthopedic applications. Metals 2018, 8, 948. [Google Scholar] [CrossRef] [Green Version]
- Kafri, A.; Ovadia, S.; Goldman, J.; Drelich, J.; Aghion, E. The suitability of Zn–1.3% Fe alloy as a biodegradable implant material. Metals 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.Q.; Pham, D.N.; Kai, N.; Dinh, H.V.; Hiromoto, S.; Kobayashi, E. In vitro corrosion properties of Mg matrix in situ composites fabricated by spark plasma sintering. Metals 2017, 7, 358. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.-J.; Hung, F.-Y.; Lee, H.-P.; Yeh, M.-L. Development of a novel degradation-controlled magnesium-based regeneration membrane for future guided bone regeneration (GBR) therapy. Metals 2017, 7, 481. [Google Scholar] [CrossRef] [Green Version]
- Vinogradov, A.; Vasilev, E.; Kopylov, V.I.; Linderov, M.; Brilevesky, A.; Merson, D. High performance fine-grained biodegradable Mg-Zn-Ca alloys processed by severe plastic deformation. Metals 2019, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Tun, K.S.; Zhang, Y.; Parande, G.; Manakari, V.; Gupta, M. Enhancing the hardness and compressive response of magnesium using complex composition alloy reinforcement. Metals 2018, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Bakhsheshi-Rad, H.R.; Dayaghi, E.; Ismail, A.F.; Madzlan, A.Z.I.Z.; Akhavan-Farid, A.; Xiongbiao, C. Synthesis and in-vitro characterization of biodegradable porous magnesium-based scaffolds containing silver for bone tissue engineering. Trans. Nonferrous Met. Soc. China 2019, 29, 984–996. [Google Scholar] [CrossRef]
- Razzaghi, M.; Asgarani, M.K.; Bakhsheshi-Rad, H.R.; Ghayour, H. In vitro degradation, antibacterial activity and cytotoxicity of Mg-3Zn-xAg nanocomposites synthesized by mechanical alloying for implant applications. J. Mater. Eng. Perform. 2019, 28, 1441–1455. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Staiger, M.P.; Dias, G.J.; Hadisi, Z.; Saheban, M.; Kashefian, M. Drug release, cytocompatibility, bioactivity, and antibacterial activity of doxycycline loaded Mg-Ca-TiO2 composite scaffold. Mater. Des. 2018, 139, 212–221. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdellahi, M.; Hamzah, E.; Daroonparvar, M.; Rafiei, M. Introducing a composite coating containing CNTs with good corrosion properties: Characterization and simulation. RSC Adv. 2016, 6, 108498–108512. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Tok, H.Y.; Kasiri-Asgarani, M.; Jabbarzare, S.; Medraj, M. Microstructure, In Vitro corrosion behavior and cytotoxicity of biodegradable Mg-Ca-Zn and Mg-Ca-Zn-Bi alloys. J. Mater. Eng. Perform. 2017, 26, 653–666. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Farahany, S.; Staiger, M.P. The mechanical properties and corrosion behavior of quaternary Mg-6Zn-0.8Mn-xCa alloys. J. Mater. Eng. Perform. 2015, 24, 598–608. [Google Scholar] [CrossRef]
- Tok, H.Y.; Hamzah, E.; Bakhsheshi-Rad, H.R. The role of bismuth on the microstructure and corrosion behavior of ternary Mg-1.2Ca-xBi alloys for biomedical application. J. Alloys Compd. 2015, 640, 335–346. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Fereidouni-Lotfabadi, A.; Daroonparvar, M.; Yajid, M.A.M.; Mezbahul-Islam, M.; Kasiri-Asgarani, M.; Medraj, M. Microstructure and bio-corrosion behavior of Mg–Zn and Mg–Zn–Ca alloys for biomedical applications. Mater Corros. 2014, 65, 1178–1187. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg–Ca–Mn–Zn alloys compared with binary Mg–Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdul-Kadir, M.R.; Idris, M.H.; Farahany, S. Relationship between the corrosion behavior and the thermal characteristics and microstructure of Mg–0.5Ca–xZn alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar] [CrossRef] [Green Version]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul Kadir, M.R.; Farahany, S. Microstructure analysis and corrosion behavior of biodegradable Mg–Ca implant alloys. Mater. Des. 2012, 33, 88–97. [Google Scholar]
- Farahany, S.; Bakhsheshi-Rad, H.R.; Idris, M.H.; Kadir, M.R.A.; Lotfabadi, A.F.; Ourdjini, A. In-situ thermal analysis and macroscopical characterization of Mg–xCa and Mg–0.5Ca–xZn alloy systems. Thermochim. Acta 2012, 527, 180–189. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R. Preparation and corrosion resistance of a nanocomposite plasma electrolytic oxidation coating on Mg-1%Ca alloy formed in aluminate electrolyte containing titania nano-additives. J. Alloys Compd. 2016, 688, 841–857. [Google Scholar] [CrossRef]
- Safari, N.; Toroghinejad, M.R.; Kharaziha, M. Influence of copper on the structural, mechanical, and biological characteristics of Mg–1Al–Cu alloy. Mater. Chem. Phys. 2019, 237, 121838. [Google Scholar] [CrossRef]
- Golshirazi, A.; Kharaziha, M.; Golozar, M.A. Polyethylenimine/kappa carrageenan: Micro-arc oxidation coating for passivation of magnesium alloy. Carbohydr. Polym. 2017, 167, 185–195. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Pillado, B.; Mohedano, M.; Arrabal, R.; Matykina, E. Calcium doped flash-PEO coatings for corrosion protection of Mg alloy. Metals 2020, 10, 916. [Google Scholar] [CrossRef]
- Feliu, S., Jr. Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: Brief review and challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Mitchell, J.; Crow, N.; Nieto, A. Effect of surface roughness on pitting corrosion of AZ31 Mg alloy. Metals 2020, 10, 651. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Pei, Z.; Yan, M. Mg-based materials with quasiamorphous phase produced by vertical twin-roll casting process. Metals 2020, 10, 452. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Wang, H.; Chen, L.; Zhang, H.; Chen, G.; Chen, L.; Du, Z. Preparation and mechanical properties of ZK61-Y magnesium alloy wheel hub via liquid forging—isothermal forging process. Metals 2020, 10, 385. [Google Scholar] [CrossRef] [Green Version]
- Kunčická, L.; Král, P.; Dvořák, J.; Kocich, R. Texture evolution in biocompatible Mg-Y-Re alloy after friction stir processing. Metals 2019, 9, 1181. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-T.; Hung, F.-Y.; Syu, J.-C. Biodegradable implantation material: Mechanical properties and surface corrosion mechanism of Mg-1Ca-0.5Zr alloy. Metals 2019, 9, 857. [Google Scholar] [CrossRef] [Green Version]


| Material | Fabrication Method | Bacteria Strains | Killing Rate | Incubation time (h) | Material Concentration (mg/L) | cfu/mL | Ref. |
|---|---|---|---|---|---|---|---|
| Gr | Chemical exfoliation | Escherichia coli (E. coli) | 59% | 1 | - | 108 | [51] |
| Gr | Chemical exfoliation | Staphylococcus aureus (S. aureus) | 74% | 1 | - | 108 | [51] |
| rGO | Hummers and Offeman | E. coli | 88% | 4 | 100 | 106 | [51] |
| rGO | Hummers and Offeman | E. coli | 81% | 2 | 150 | 106–107 | [51] |
| GO | Chemical exfoliation | E. coli | 84% | 1 | - | 108 | [54] |
| GO | Chemical exfoliation | S. aureus | 95% | 1 | - | 108 | [54] |
| GO | Modified Hummers | Fusarium graminearum (F. graminearum) | 90% | 7 | 500 | 3 × 107 | [56] |
| GO | Modified Hummers | Fusarium oxysporum (F. oxysporum) | 80% | 7 | 500 | 3 × 107 | [56] |
| GO | Modified Hummers | E. coli | 69% | 2 | 80 | 106–107 | [57] |
| GO | Hummers and Offeman | Xanthomonas oryzae pv. oryzae (X. o. pv. oryzae) | 100% | 4 | 250 | 107–108 | [58] |
| GO | Modified Hummers | Activated sludge | 35% | 5 | 100 | 5 × 105 | [59] |
| GO | Hummers and Offeman | Pseudomonas aeruginosa (P. aeruginosa) | 100% | 2 | 175 | 106 | [60] |
| Samples | Processing route | E (GPa) | Tensile Performances | Compressive Performances | Hardness (HV) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.2%TYS (MPa) | UTS (MPa) | Ductility or Elongation (%) | CYS (MPa) | UCS (MPa) | Failure Sstrain δ (%) | |||||
| Pure Mg | SPM | 13.2 ± 0.3 | 187 ± 4 | 219 ± 5 | 3.45 ± 0.5 | - | - | - | 57.5 ± 2 | [91] |
| Mg-0.3GNPs | SPM | 14.6 ± 0.2 | 197 ± 3.1 | 238 ± 6 | 3.11 ± 0.4 | - | - | - | 68.5 ± 2 | [91] |
| Mg-6Zn | DMD + Sintering + the | - | 159 ± 5 | 276 ± 7 | 17 ± 1.5 | 109 ± 4.5 | 426 ± 6.1 | 21 ± 1.7 | 62.5 | [91] |
| Mg-6Zn-0.5GNPs | DMD + Sintering + HTE | - | 171 ± 4 | 295 ± 3.5 | 18 ± 1.9 | 226 ± 4.7 | 480 ± 5.6 | 20 ± 2 | 75 | [100] |
| Mg-6Zn-1.5GNPs | DMD + Sintering + HTE | - | 214 ± 2 | 313 ± 5.2 | 21 ± 1.1 | 226 ± 4.7 | 480 ± 5.6 | 16 ± 2.9 | 70 | [100] |
| pure Mg | DMD + Sintering + HTE | - | 96 ± 4 | 163 ± 3 | 7.5 ± 1.5 | - | - | - | - | [99] |
| Mg-0.10 GNPs | DMD + Sintering + HTE | - | 105 ± 1 | 176 ± 2 | 10.3 ± 0.6 | - | - | - | - | [99] |
| Mg-0.25 GNPs | DMD + Sintering + HTE | - | 122 ± 2 | 202 ± 3 | 14.5 ± 1.2 | - | - | - | - | [99] |
| AZ31 alloy | PM + Sintering + Extrusion | - | 195 ± 5 | 285 ± 2.9 | 14.5 ± 1.5 | 160 ± 6 | 363 ± 3.5 | 16.3 ± 1.5 | 58 ± 3 | [125] |
| AZ31-0.3GNPs | PM + Sintering + Extrusion | - | 173 ± 6.2 | 275 ± 5.7 | 21.7 ± 2.8 | 161 ± 4.5 | 397 ± 5.3 | 16.3 ± 1.5 | 71 ± 2.1 | [125] |
| Pure Mg | SPM + Vacuum sintering + HTE | ET: 7.0 ± 0.3 EC: 6.4 ± 0.4 | 104 ± 4 | 164 ± 5 | 6.2±0.2 | 136 ± 3 | 286 ± 6 | 12 ± 0.2 | 46 ± 2 | [111] |
| AZ31 Alloy | SC | - | 183 ± 4.3 | 267 ± 6.5 | 9.74 ±1.5 | 100 ± 3.3 | 398 ± 5.1 | 21.0 ± 1.4 | 61 | [126] |
| AZ31-1.5GNPs | SC | - | 187 ± 3.5 | 284 ± 5.4 | 12.34 ± 3.4 | 121 ± 4.7 | 415 ± 3.4 | 21.2 ±2.1 | 65 | [126] |
| AZ31-3GNPs | SC | - | 195 ± 4.5 | 299 ± 6.2 | 12.56 ± 4.3 | 120 ± 2.8 | 406 ± 4.1 | 19.3±1.8 | 68.9 | [126] |
| Mg-1Al | SPM + Vacuum sintering + HTE | ET: 12.8 ± 0.4 EC: 5.0 ± 0.3 | 155 ± 3 | 202 ± 3 | 6.9 ± 0.5 | 100 ± 2 | 377 ± 8 | 18 ± 0.5 | 50 ± 4 | [111] |
| Mg-1Al-0.60 GNPs | SPM + Vacuum sintering + HTE | ET: 17.2 ± 0.1 EC: 7.6 ± 0.5 | 204 ± 9 | 265 ± 8 | 230 ± 5 | 407 ± 3 | - | 63 ± 2 | [111] | |
| Pure Mg | SPM + Vacuum sintering + HTE | ET: 7.4 ± 0.3 EC: 6.3 ± 0.4 | 104 ± 4 | 164 ± 5 | 6.2 ± 1.8 | 123 ± 5 | 264 ± 6 | 9 ± 2.5 | 40 ± 3 | [119] |
| Mg-1Cu-0.18 Gr | SPM + Vacuum sintering + HTE | ET: 10.6 ± 0.4 EC: 5.9 ± 0.3 | 160 ± 6 | 240 ± 2 | 10.4 ± 2.1 | 140 ± 4 | 335 ± 8 | - | 44 ± 2 | [119] |
| Mg-1Cu-0.36 Gr | SPM + Vacuum sintering + HTE | ET: 12.4 ± 0.25 EC: 8.4 ± 0.35 | 184 ± 3 | 252 ± 3 | 12.2 ± 1.3 | 143 ± 6 | 338 ± 5 | - | 46 ± 3 | [119] |
| Mg-1Cu-0.54 Gr | SPM + Vacuum sintering + HTE | ET: 14 ± 0.16 EC: 7.7 ± 0.21 | 226 ± 5 | 260 ± 5 | 4.8 ± 2.5 | 166 ± 3 | 420 ± 6 | - | 56.7 ± 1 | [119] |
| ZK60 | SPM+ HTE + SC + HTE | - | 158 ± 2.0 | 282 ± 3.0 | 11 ± 0.8 | 126 ± 3.0 | 364 ± 2.8 | 9 ± 0.3 | 68 ± 2.8 | [92] |
| ZK60-0.05GNPs | SPM+ HTE + SC + HTE | - | 256 ± 4.0 | 336 ± 4.0 | 13 ± 1.2 | 249 ± 4.0 | 473 ± 6.2 | 10 ± 1.0 | 78 ± 2.0 | [92] |
| ZK60-0.1GNPs | SPM+ HTE + SC + HTE | - | 283 ± 3.5 | 343 ± 3.8 | 17 ± 2.0 | 279 ± 3.4 | 463 ± 5.0 | 12 ± 1.1 | 75 ± 2.5 | [92] |
| AZ61 Alloy | DMD + HTE | - | 184 ± 5.5 | 300 ± 7.1 | 11.5 ± 1.9 | 170 ± 5.1 | 461 ± 6.8 | 16.7 ± 2.1 | 75.7 ± 2.5 | [95] |
| AZ61-3GNPs | DMD + HTE | - | 232 ± 4.9 | 335 ± 6.6 | 10.7 ± 2.1 | 226 ± 4.7 | 480 ± 5.6 | 15.1 ± 3.5 | 87.5 ± 1.8 | [95] |
| AZ80-0.5Ca | RC + Extrusion | - | 104 ± 5.2 | 271 ± 13.5 | 4.4 ± 0.22 | 78 ± 3.9 | 340 ± 17 | 9.5 ± 0.33 | 71.3 ± 2 | [127] |
| AZ80-0.5Ca-0.1GNP | RC + Extrusion | - | 146 ± 7.3 | 310 ± 15.5 | 6.6 ± 0.33 | 90 ± 4.5 | 419 ± 21 | 13 ± 0.6 | 77.6 ± 2.6 | [127] |
| AZ80-0.5Ca-0.6GNP | RC + Extrusion | - | 160 ± 16 | 325 ± 32 | 9.3 ± 0.85 | 102 ± 10 | 361 ± 36 | 11.5 ± 0.7 | 88.9 ± 5 | [127] |
| AZ31 | - | - | 215 | 256 | 13.3 | - | - | - | 56 | [101] |
| AZ31-GNPs | FSP | - | 217 | 278 | 15.8 | - | - | - | 79 | [101] |
| AZ31-0.3Gr | MBM + HTE | - | 214.82 | 310.79 | 5.99 | - | - | - | - | [93] |
| Mg-0.3Sr-0.3Ca | SC + HTE | - | 174 | 233 | 7.4 | 68 | 300 | 15.2 | [114] | |
| Mg-0.3Sr-0.3Ca-GNP | SC + HTE | - | 213 | 235 | 10.2 | 90 | 303 | 16.9 | 55 | [114] |
| Mg-0.3Sr-0.3Ca-GNPs + MgO | SC + HTE | - | 224 | 239 | 13.8 | 96 | 330 | 18.3 | 68 | [114] |
| Pure Mg | SPM + Vacuum sintering + HTE | - | 162 ± 5 | 195 ± 4 | 3.7 ± 2.5 | - | - | - | 41 ± 4 | [118] |
| Mg-0.5Al-0.18GNPs | SPM + Vacuum sintering + HTE | - | 173 ± 4 | 230 ± 5.1 | 10.7 ±3 | - | - | - | 55 ± 2 | [118] |
| Mg-1.0Al-0.18GNPs | SPM + Vacuum sintering + HTE | - | 190 ± 5.3 | 254 ± 3 | 15.5 ± 3.4 | - | - | - | 58 ± 3.5 | [118] |
| Mg-1.5Al-0.18GNPs | SPM + Vacuum sintering + HTE | - | 209 ± 3.9 | 268 ± 4.5 | 12.7 ± 2 | - | - | - | 60 ± 3 | [118] |
| Mg-1Al-1Sn | SPM + Extrusion | - | 161 ± 04 | 236 ± 5.1 | 16.7 ± 03 | - | - | - | - | [109] |
| Mg-1Al-1Sn-0.18GNPs | SPM + Extrusion | - | 208 ± 5.3 | 269 ± 03 | 10.9 ± 3.4 | - | - | - | - | [109] |
| Mg-0.3Sr-0.3Ca | SC + HTE | - | 171 ± 8.5 | 228 ± 11.4 | 6 ± 0.3 | 65 ± 3.2 | 339 ± 17 | 14.3 ± 0.7 | - | [96] |
| Mg-0.3Sr-0.3Ca-0.1GNP | SC + HTE | - | 184 ± 9.2 | 232 ± 11 | 8.1 ± 0.4 | 67 ± 3.4 | 335 ± 16 | 15.5 ± 0.8 | - | [96] |
| Mg-0.3Sr-0.3Ca-0.2GNPs | SC+ HTE | - | 210 ± 10.5 | 231 ± 11.5 | 10 ± 0.5 | 93 ± 4.7 | 339 ± 18 | 18.3 ± 0.95 | - | [96] |
| Mg-0.3Sr-0.3Ca-0.4GNPs | SC + HTE | - | 223 ± 13.5 | 245 ± 15 | 8.8 ± 0.55 | 82 ± 5 | 309 ± 17 | 14.3 ± 0.9 | - | [96] |
| AZ91-0.25GNPs | SPM | - | 116 ± 9 | 172 ± 10 | 3.4 ± 0.7 | - | - | - | 65 ± 1.5 | [117] |
| AZ91-0.50GNPs | SPM | - | 128 ± 13 | 190 ± 14 | 2.8 ± 0.9 | - | - | - | 69 ± 2.5 | [117] |
| Mg | SPM + Sintering + HTE | 5.98 | 119 ± 5 | 186 ± 6 | 9.7 ± 3 | - | - | - | 41 ± 3.5 | [120] |
| Mg-1Al-0.09GNPs | SPM + Sintering + HTE | 13.40 | 148 ± 3 | 206 ± 4 | 10.5 ± 3.4 | - | - | - | 48 ± 2.9 | [120] |
| Mg-1Al-0.18GNPs | SPM + Sintering + HTE | 12.18 | 162 ± 4.1 | 223 ± 5 | 15.2 ± 2 | - | - | - | 51 ± 3 | [120] |
| Mg-1Al-0.30GNPs | SPM + Sintering + HTE | 13.84 | 178 ± 2.9 | 246 ± 3.5 | 16.9 ± 3 | - | - | - | 55 ± 4 | [120] |
| Mg-10Ti | SPM + Extrusion | - | 141 ± 04 | 212 ± 5.1 | 11 ± 03 | - | - | - | [128] | |
| Mg-10Ti-0.18 GNPs | SPM + Extrusion | - | 160 ± 5.3 | 230 ± 03 | 14 ± 3.4 | - | - | - | [128] | |
| AZ91 | SPM + HTE | - | 168 ± 5.0 | 215 ± 6.0 | 7.0 ± 0.2 | - | - | - | 72.4 ± 2.0 | [116] |
| AZ91-0.1GNPs | SPM + HTE | - | 223 ± 3.6 | 276 ± 4.2 | 7.0 ± 0.2 | - | - | - | 78.2 ± 1.5 | [116] |
| AZ91-0.3GNPs | SPM + HTE | - | 268 ± 4.6 | 318 ± 5.0 | 8.2 ± 0.1 | - | - | - | 84.4 ± 1.2 | [116] |
| AZ91-0.5GNPs | SPM + HTE | - | 296 ± 3.7 | 335 ± 4.8 | 8.2 ± 0.1 | - | - | - | 88.5 ± 1.0 | [116] |
| AZ91-0.8GNPs | SPM + HTE | - | 252 ± 5.5 | 307 ± 5.0 | 6.8 ± 0.1 | - | - | - | 81.6 ± 1.4 | [116] |
| AZ91-1.2GNPs | SPM + HTE | - | TYS: 234 ± 3.0 | 287 ± 5.0 | 6.5 ± 0.2 | - | - | - | 74.7 ± 1.2 | [116] |
| Mg-0.25GNPs | Sprayed GNPs on Mg foils (laminated composite) + HTE + Rolling | - | - | 160 | 4.9 | - | - | - | - | [115] |
| Mg-0.75GNPs | Sprayed GNPs on Mg foils (laminated composite) + HTE + Rolling | - | - | 179 | 2.7 | - | - | - | - | [115] |
| ZK60 | Mechanical agitation + SC + HTE | 161 | 281 | 15.6 | - | - | - | - | [98] | |
| ZK60-1GNPs | Mechanical agitation + SC + HTE | - | 261 | 336 | 16.6 | - | - | - | - | [98] |
| Mg-6Zn | In situ reaction wetting process + SC + HTE procedure | 46.9 ± 0.7 | 136 ± 5 | 269 ± 6 | 19.5 ± 2.0 | - | - | - | - | [97] |
| Mg-6Zn-0.1(GO-ZnO) | In situ reaction wetting process + SC + HTE | 47.4 ± 0.3 | 206 ± 2 | 306 ± 5 | 15.1 | - | - | - | 71 ± 2.9 | [97] |
| Mg-6Zn-0.3(GO-ZnO) | In situ reaction wetting process + SC + THE | 47.8 ± 0.5 | 221 ± 4 | 316 ± 3 | 14.8 | - | - | - | 86 ± 3.6 | [97] |
| Mg-3Zn-1Ca | SPM | - | - | - | - | - | 8559 N | - | 48 | [110] |
| Mg-3Zn-1Ca-0.5GNP | SPM | - | - | - | - | - | 14,900 N | - | 57 | [110] |
| Mg-3Zn-1Ca-1GNPs | SPM | - | - | - | - | - | 20,586 N | 60 | [110] | |
| Mg-3Zn-1Ca-2GNPs | SPM | - | - | - | - | - | 2002 N | 62 | [110] | |
| Pure Mg | HEBM + Compaction + Sintering | - | - | - | - | 59 ± 2 | 85 ± 10 | 6 ± 0.5 | - | [30] |
| Mg-0.1GNP Particle size (15 μm) | HEBM + Compaction + Sintering | - | - | - | - | 99 ± 1 | 146 ± 5 | 12 ± 2.4 | - | [30] |
| Mg-0.2GNP Particle size (15 μm) | HEBM + Compaction + Sintering | - | - | - | - | 130 ± 4 | 182 ± 14 | 6 ± 3.9 | - | [30] |
| Mg-0.3GNP Particle size (15 μm) | HEBM + Compaction + Sintering | - | - | - | - | 126 ± 6 | 246 ± 1 | 14 ± 1.7 | - | [30] |
| Mg-0.1GNP Particle size (5 μm) | HEBM + Compaction + Sintering | - | - | - | - | 76 ± 5 | 143 ± 14 | 9 ± 0.3 | - | [30] |
| Mg-0.2GNP Particle size (5 μm) | HEBM + Compaction + Sintering | - | - | - | - | 97 ± 6 | 183 ± 4 | 13 ± 0.2 | - | [30] |
| Mg-0.3GNP Particle size (5 μm) | HEBM + Compaction + Sintering | - | - | - | - | 110 ± 8 | 169 ± 18 | 9 ± 0.8 | - | [30] |
| AZ61-0.2GO | SPM + SLM | - | - | - | - | ∼177.5 | - | - | 93 | [102] |
| AZ61-0.4GO | SPM + SLM | - | - | - | - | ∼188.5 | - | - | 97 | [102] |
| AZ61-0.6GO | SPM + SLM | - | - | - | - | ∼202.5 | - | - | 100 | [102] |
| AZ61-0.8GO | SPM + SLM | - | - | - | - | ∼208.75 | - | - | 102 | [102] |
| AZ61-1GO | SPM + SLM | - | - | - | - | ∼221.05 | - | - | 104.5 | [102] |
| AZ61-1.2GO | SPM + SLM | - | - | - | - | ∼192.5 | - | - | 108.52 | [102] |
| Samples | Reinforcement | Processing Route | Reinforcement Particle Size | Corrosion Medium | Icorr (μA.cm−2) | Ecorr vs. SCE | Corrosion rate (mm/year) | Rp (Ω.cm2) | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non Polarized | Polarized | ||||||||||
| Immersion Time (h) | HE or WL | PDP | |||||||||
| Pure Mg | - | HEBM + Compaction+ Sintering | - | Hank’s | 0.81 ± 0.02 (mA.cm−2) | - | 24 | 32.52 ± 1.58 mL.cm−2.day−1 74.11 ± 3.61 mm/year | 25.02 ± 1.79 | [30] | |
| Mg | 0.1GNPs | Particle size (15 μm); thickness (5 nm) | 0.42 ± 0.02 (mA.cm−2) | - | 15.16 ± 0.41 mL.cm−2.day−1 34.57 ± 0.94 mm/year | 13.28 ± 0.27 | - | ||||
| 0.2GNPs | 0.58 ± 0.03 (mA.cm−2) | - | 26.93 ±1.08 mL.cm−2.day−1 61.38 ± 2.48 mm/year | 18.50 ± 0.83 | - | ||||||
| 0.3GNPs | 0.35 ± 0.04 (mA.cm−2) | - | 30.41 ± 1.39 mL.cm−2.day−1 69.32 ± 3.16 mm/year | 11.00 ± 1.08 | - | ||||||
| Mg | 0.1GNPs | Particle size (5 μm); thickness (9 nm) | 0.50 ± 0.05 (mA.cm−2) | - | 29.55 ± 2.61 mL.cm−2.day−1 67.35 ± 5.95 mm/year | 15.13 ± 0.91 | - | ||||
| 0.2GNPs | 0.69 ± 0.03 (mA.cm−2) | - | 31.54 ± 2.97 mL.cm−2.day−1 71.88 ± 6.77 mm/year | 21.45 ± 1.91 | - | ||||||
| 0.3GNPs | 0.91 ± 0.03 (mA.cm−2) | - | 34.93 ± 1.82 mL.cm−2.day−1 79.62 ± 4.17 mm/year | 28.74 ± 3.06 | - | ||||||
| Mg-Sr-Ca | - | SC + HTE | - | SBF | 7.373 | 1.832 | - | - | 0.241 | [114] | |
| Mg-Sr-Ca | GNPs | SC + HTE | - | 6.980 | 1.776 | - | - | 0.231 | - | ||
| Mg-Sr-Ca | GNPs + MgO | SC + HTE | - | 9.279 | 1.800 | - | - | 0.269 | - | ||
| AZ61 | - | SPM + SLM | - | SBF | 50 ± 4 | 1.54 ± 0.02 | 360 | - | 1.21 ± 0.09 | - | [102] |
| AZ61 | 0.2GO | Diameter (8–15 μm) | 89 ± 12 | 1.54 ± 0.02 | 2.03 ± 0.27 | - | |||||
| 0.4GO | 212 ± 16 | 1.52 ± 0.03 | 205.23 mL.cm−2 | 4.84 ± 0.36 | - | ||||||
| 0.6GO | 118 ± 13 | 1.57 ± 0.02 | 2.67 ± 0.30 | - | |||||||
| 0.8GO | 85 ± 6 | 1.51 ± 0.02 | 1.94 ± 0.14 | - | |||||||
| 1.0GO | 35 ± 3 | 1.56 ± 0.03 | 15.3 mL.cm−2 | 0.76 ± 0.07 | - | ||||||
| 1.2GO | 135 ± 15 | 1.53 ± 0.02 | 65.25 mL.cm−2 | 3.08 ± 0.34 | - | ||||||
| AZ31 | - | SPM | - | 0.1 M Na2SO4 | 371.54 | 1.416 | - | - | ∼4 | 108.6 (Ω) | [143] |
| AZ31 | 0.2r-GO | Thickness (up to 5.8 nm) | 992.08 | 1.456 | - | - | ∼11.5 | 465.7 (Ω) | |||
| 0.3r-GO | 207.25 | 1.464 | - | - | ∼2.4 | 755 (Ω) | |||||
| 0.4r-GO | 61.21 | 1.328 | - | - | ∼0.5 | 221.4 (Ω) | |||||
| 0.5r-GO | 207.41 | 1.464 | - | - | ∼1.8 | 754 (Ω) | |||||
| Mg-3Zn-Ca | 0.5GNPs | SPM | - | SBF | 186.54 | 1.46 | - | - | - | 134.57 | [110] |
| 1GNPs | - | 112.89 | 1.45 | - | - | - | 166.55 | ||||
| 2GNPs | - | 420.76 | 1.49 | - | - | - | 66. 21 | ||||
| AZ91 | GNPs | SPM | Diameter (5, 8 nm); surface area (about 750 m2/g) | 3.5 wt.% NaCl | 388.43 μA | 1.491 | - | - | 4.92 | - | [108] |
| AZ31 | - | SC | Diameter (5, 8 nm); surface area ( about 750 m2/g) | 3.5 wt.% NaCl | 15.47 μA | 1.453 | - | - | - | - | [141] |
| AZ31 | 1.5GNPs | 18.13 μA | 1.465 | - | - | - | - | ||||
| AZ31 | 3.0GNPs | 19.31 μA | 1.479 | - | - | - | - | ||||
| AZ61 | - | 11.54 μA | 1.457 | - | - | - | - | ||||
| AZ61 | 3.0GNPs | 14.21 μA | 1.476 | - | - | - | - | ||||
| Pure Mg | - | SPM | - | - | 0.12 (mA.cm−2) | 1.63 | - | - | 249.9 (mpy) | - | [107] |
| Mg | 0.1 GNPs | Thickness (5-8 nm); surface area (750 m2/g) | 3.5 wt.% NaCl | 0.51 (mA.cm−2) | 1.59 | - | - | 1048 (mpy) | - | ||
| 0.25 GNPs | 0.89 (mA.cm−2) | 1.58 | - | - | 1813 (mpy) | - | |||||
| 0.50 GNPs | 1.02 (mA.cm−2) | 1.59 | - | - | 2090 (mpy) | - | |||||
| Mg-0.5 MWCNT | GNPs | PEO Coating | - | 3.5 wt.% NaCl | 101 μA | 1.424 | - | - | 14.46 (mpy) | - | [144] |
| Materials | Processing Route | Reinforcement Particle Size | Cell Type | Cell Viability (%) | ALP Activity | Cell Attachment | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mg-0.1GNPs | HEBM + PM | Particle size (15 μm); thickness (5 nm) | SaOS-2 cells | CVR: 1.13 | - | Excellent | In vitro | [30] |
| Mg-0.2GNPs | HEBM + PM | Particle size (15 μm); thickness (5 nm) | SaOS-2 cells | CVR: 0.92 | - | Excellent | In vitro | [30] |
| Mg-0.3GNPs | HEBM + PM | Particle size (15 μm); thickness (5 nm) | SaOS-2 cells | CVR: 0.9 | - | Excellent | In vitro | [30] |
| AZ61-1GO | SPM + SLM | Diameter (8–15 μm) | MG-63 cells | Optic density = 1.7 after 5 days | - | Good | In vitro | [30] |
| Mg-3Zn-Ca-0.5GNPs | SPM | - | MG-63 cells | 83 % for 24 h 87 % for 48 h | 3.4 for 24 h 4.7 for 48 h | Adequate | In vitro | [102] |
| Mg-3Zn-Ca-1GNPs | SPM | - | MG-63 cells | 65 % for 24 h 60 % for 48 h | 3.7 for 24 h 5.5 for 48 h | Adequate | In vitro | [110] |
| Mg-3Zn-Ca-2GNPs | SPM | - | MG-63 cells | 100 % for 24 h 100 % for 48 h | 2.5for 24 h 3.4 for 48 h | Adequate | In vitro | [110] |
| Mg-1ND | PM | Particle size < 10 nm | L-929 cells | 93.2 % for 24 h 105.8 % for 72 h | - | Good | In vitro | [110] |
| Mg-3ND | PM | Particle size < 10 nm | L-929 cells | 94.1 % for 24 h 102.2 % for 72 h | - | Good | In vitro | [158] |
| Mg-5ND | PM | Particle size < 10 nm | L-929 cells | 95.4 % for 24 h 113.1 % for 72 h | - | Good | In vitro | [158] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. Graphene Family Nanomaterial Reinforced Magnesium-Based Matrix Composites for Biomedical Application: A Comprehensive Review. Metals 2020, 10, 1002. https://doi.org/10.3390/met10081002
Abazari S, Shamsipur A, Bakhsheshi-Rad HR, Ramakrishna S, Berto F. Graphene Family Nanomaterial Reinforced Magnesium-Based Matrix Composites for Biomedical Application: A Comprehensive Review. Metals. 2020; 10(8):1002. https://doi.org/10.3390/met10081002
Chicago/Turabian StyleAbazari, Somayeh, Ali Shamsipur, Hamid Reza Bakhsheshi-Rad, Seeram Ramakrishna, and Filippo Berto. 2020. "Graphene Family Nanomaterial Reinforced Magnesium-Based Matrix Composites for Biomedical Application: A Comprehensive Review" Metals 10, no. 8: 1002. https://doi.org/10.3390/met10081002
APA StyleAbazari, S., Shamsipur, A., Bakhsheshi-Rad, H. R., Ramakrishna, S., & Berto, F. (2020). Graphene Family Nanomaterial Reinforced Magnesium-Based Matrix Composites for Biomedical Application: A Comprehensive Review. Metals, 10(8), 1002. https://doi.org/10.3390/met10081002