Bulk and Surface Low Temperature Phase Transitions in the Mg-Alloy EZ33A
Abstract
1. Introduction
2. Background, Materials and Methods
2.1. Background
2.2. Material
2.3. Methods
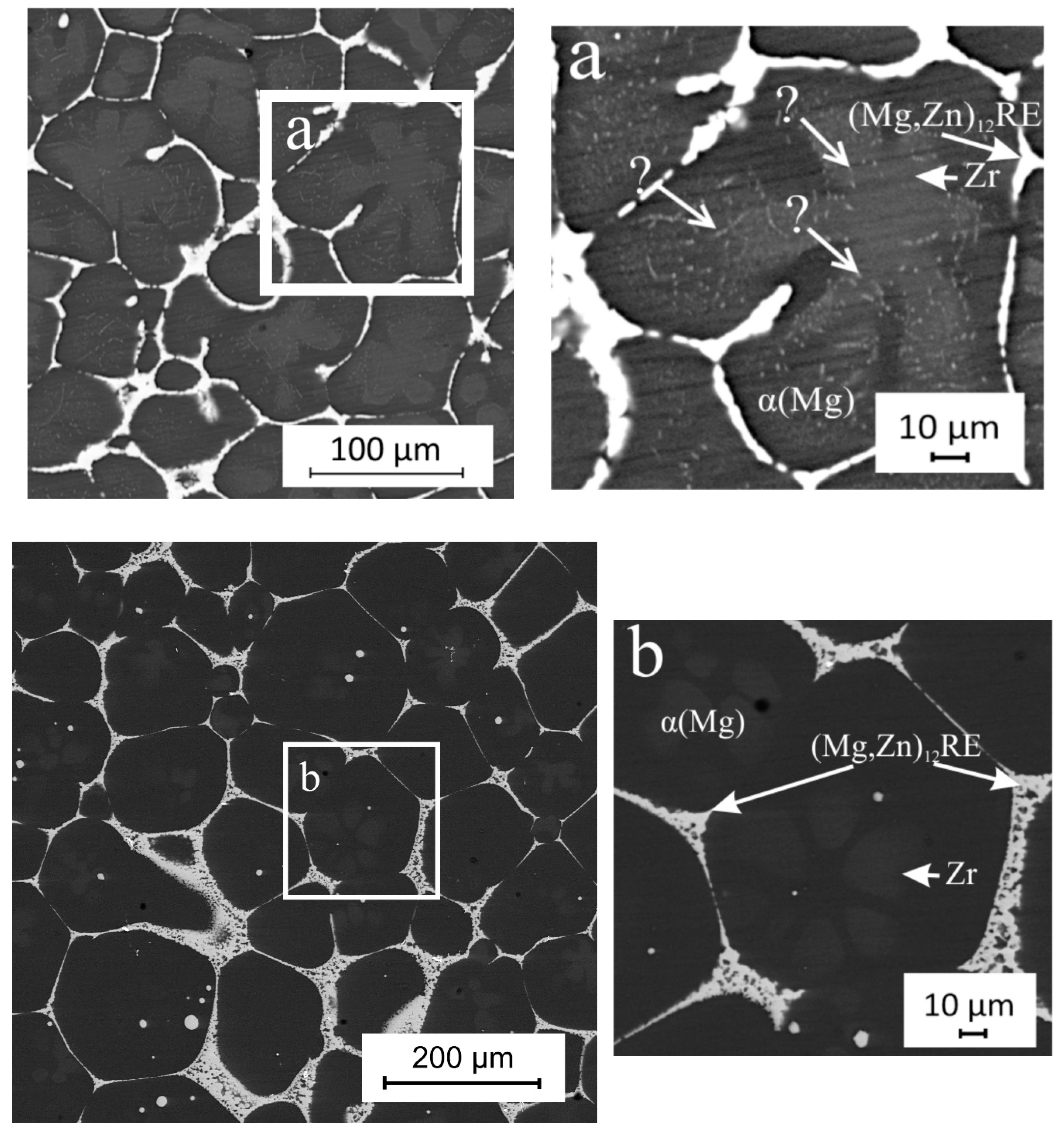
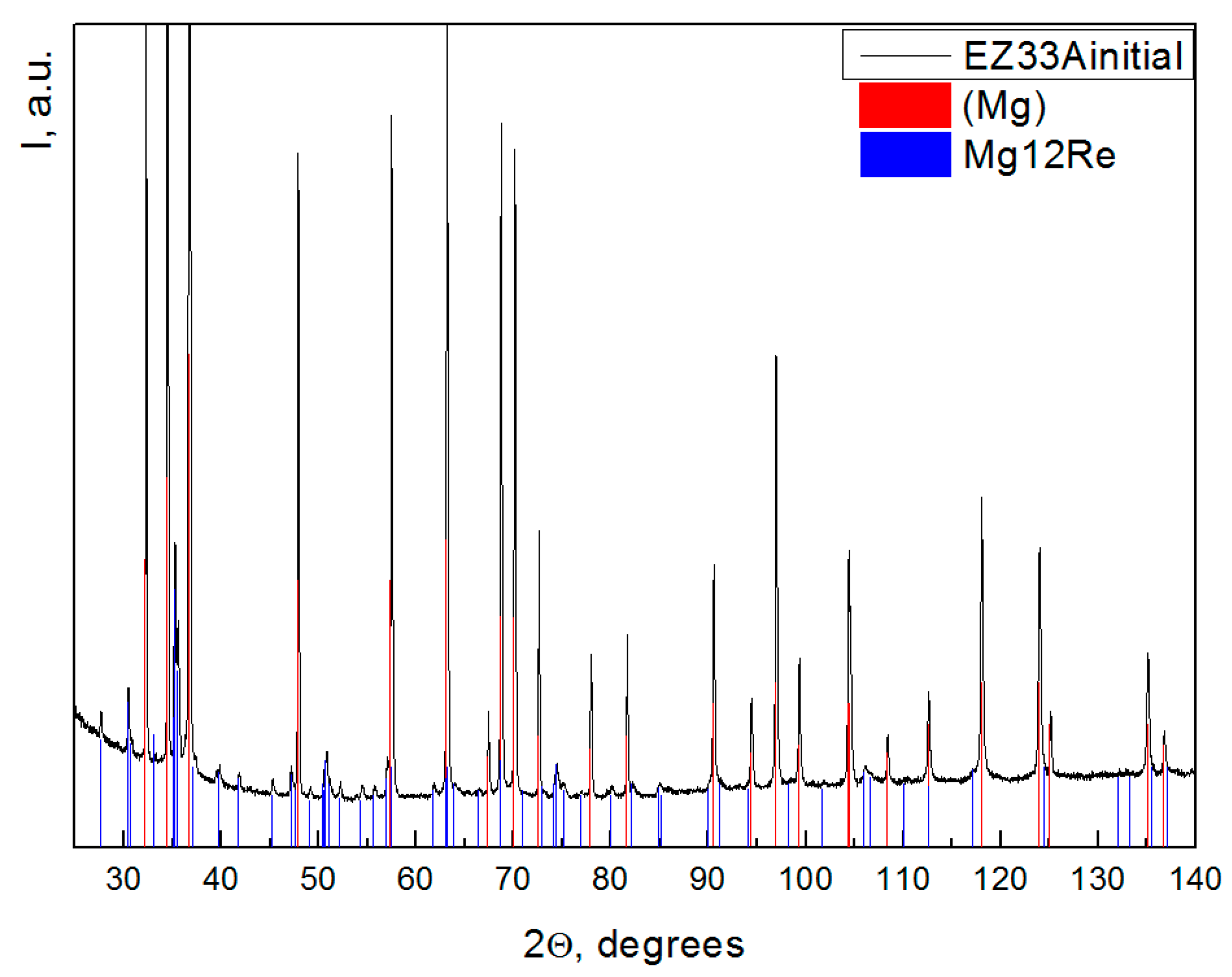
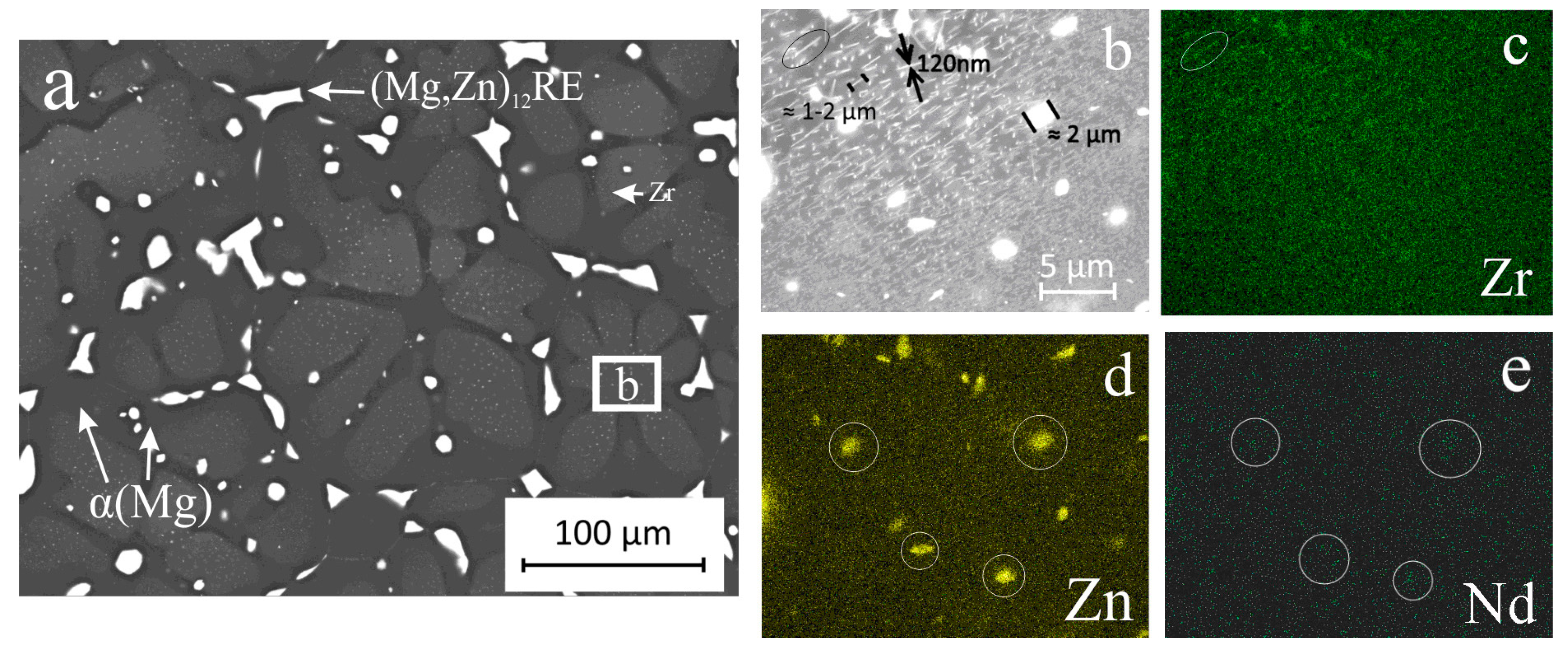
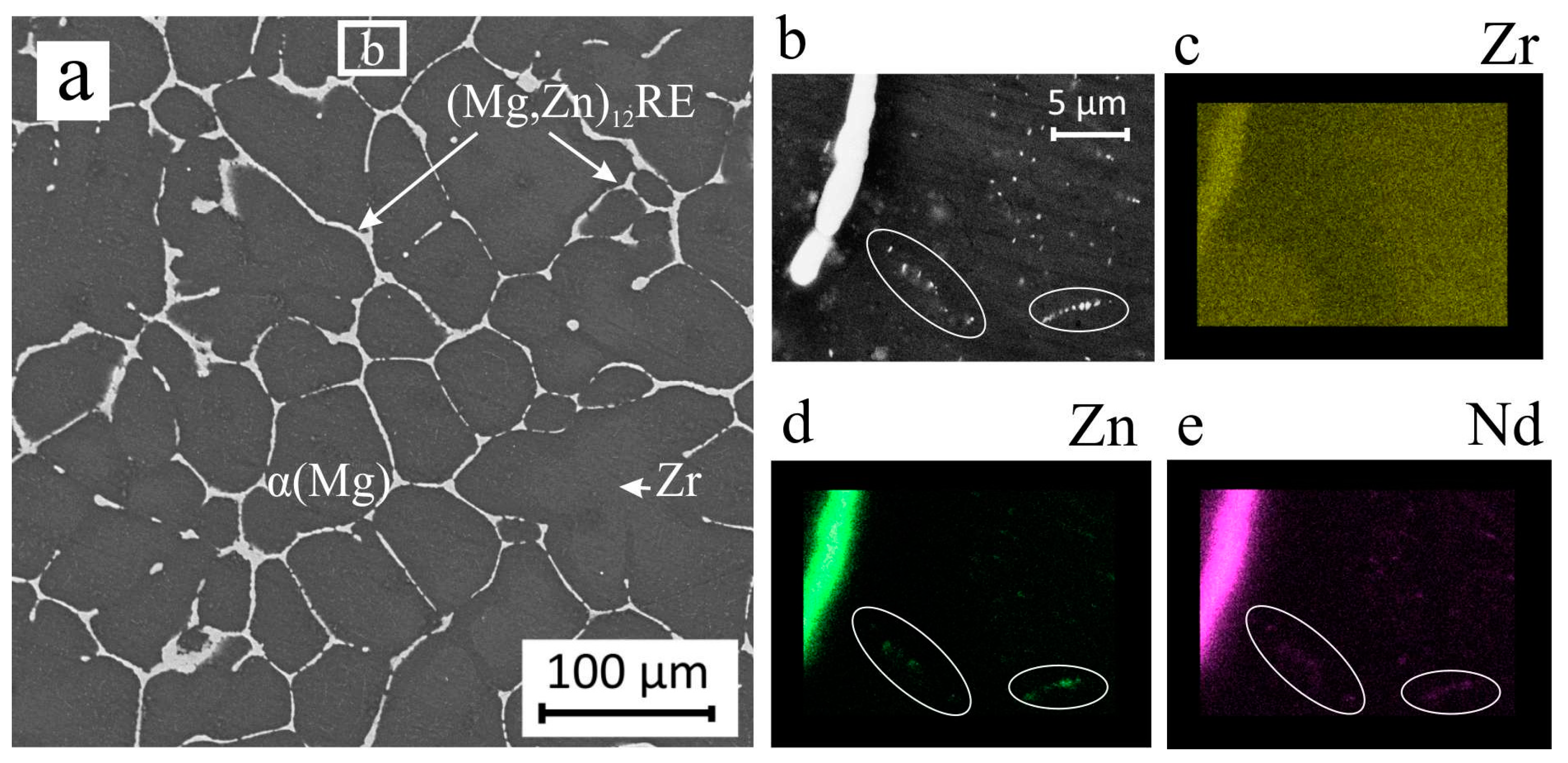
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bryła, K.; Morgiel, J.; Faryna, M.; Edalati, K.; Horita, Z. Effect of high-pressure torsion on grain refinement, strength enhancement and uniform ductility of EZ magnesium alloy. Mater. Lett. 2018, 212, 323–326. [Google Scholar] [CrossRef]
- Bryła, K.; Krystian, M.; Horky, J.; Mingler, B.; Mroczka, K.; Kurtyka, P.; Lityńska-Dobrzyńska, L. Improvement of strength and ductility of an EZ magnesium alloy by applying two different ECAP concepts to processable initial states. Mater. Sci. Eng. A 2018, 737, 318–327. [Google Scholar] [CrossRef]
- Rogal, L.; Kania, A.; Berent, K.; Janus, K.; Lityńska-Dobrzyńska, L. Microstructure and mechanical properties of Mg–Zn–RE–Zr alloy after thixoforming. J. Mater. Res. Technol. 2019, 8, 1121–1131. [Google Scholar] [CrossRef]
- Straumal, A.B.; Tsoy, K.V.; Mazilkin, I.A.; Nekrasov, A.N.; Bryła, K. Grain boundary wetting and material performance in an industrial EZ33a Mg cast alloy. Acta Metall. Mater. 2019, 64, 869–873. [Google Scholar]
- Siebert-Timmer, A.; Fletcher, M.; Bichler, L.; Sediako, D. Creep performance of wrought AX30 and EZ33 magnesium alloys. Can. Met. Quart. 2013, 52, 430–438. [Google Scholar] [CrossRef]
- Fletcher, M.; Bichler, L.; Sediako, D.; Klassen, R. Compressive creep behaviour of extruded Mg alloys at 150 °C. In Magnesium Technology 2011; Sillekens, W.H., Agnew, S.R., Neelameggham, N.R., Mathaudhu, S.N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 79–83. [Google Scholar]
- Ryspaev, T.; Trojanová, Z.; Padalka, O.; Wesling, V. Microstructure of superplastic QE22 and EZ33 magnesium alloys. Mater. Lett. 2008, 62, 4041–4043. [Google Scholar] [CrossRef]
- Bettles, C.J.; Gibson, M.A. Microstructural design for enhanced elevated temperature properties in sand-castable magnesium alloys. Adv. Eng. Mater. 2003, 5, 859–865. [Google Scholar] [CrossRef]
- Wei, L.Y.; Dunlop, G.L.; Westengen, H. Precipitation hardening of Mg-Zn and Mg-Zn-RE alloys. MMTA 1995, 26, 1705–1716. [Google Scholar] [CrossRef]
- Bryła, K. Microstructure and mechanical characterisation of ECAP-ed ZE41A alloy. Mater. Sci. Eng. A 2020, 772, 138750. [Google Scholar] [CrossRef]
- Straumal, B.B.; Baretzky, B.; Kogtenkova, O.A.; Straumal, A.B.; Sidorenko, A.S. Wetting of grain boundaries in Al by the solid Al3Mg2 phase. J. Mater. Sci. 2010, 45, 2057–2061. [Google Scholar] [CrossRef]
- Protasova, S.G.; Kogtenkova, O.A.; Straumal, B.B.; Zięba, P.; Baretzky, B. Inversed solid-phase grain boundary wetting in the Al–Zn system. J. Mater. Sci. 2011, 46, 4349–4353. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kogtenkova, O.A.; Kolesnikova, K.I.; Straumal, A.B.; Bulatov, M.F.; Nekrasov, A.N. Reversible “wetting” of grain boundaries by the second solid phase in the Cu–In system. Jetp Lett. 2014, 100, 535–539. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kogtenkova, O.A.; Straumal, A.B.; Baretzky, B. Grain boundary wetting-related phase transformations in Al and Cu-based alloys. Lett. Mater. 2018, 8, 364–371. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kogtenkova, O.A.; Straumal, A.B.; Kuchyeyev, Y.O.; Baretzky, B. Contact angles by the solid-phase grain boundary wetting in the Co–Cu system. J. Mater. Sci. 2010, 45, 4271–4275. [Google Scholar] [CrossRef]
- Straumal, B.B.; Kucheev, Y.O.; Efron, L.I.; Petelin, A.L.; Dutta Majumdar, J.; Manna, I. Complete and incomplete wetting of ferrite grain boundaries by austenite in the low-alloyed ferritic steel. J. Mater. Eng. Perform. 2012, 21, 667–670. [Google Scholar] [CrossRef]
- Straumal, B.B.; Gornakova, A.S.; Kucheev, Y.O.; Baretzky, B.; Nekrasov, A.N. Grain boundary wetting by a second solid phase in the Zr–Nb alloys. J. Mater. Eng. Perform. 2012, 21, 721–724. [Google Scholar] [CrossRef]
- Gornakova, A.S.; Prokofiev, S.I.; Straumal, B.B.; Kolesnikova, K.I. Growth of (αTi) grain boundary layers in Ti–Co alloys. Russ. J. Non-Ferr. Met. 2016, 57, 703–709. [Google Scholar] [CrossRef]
- Gornakova, A.S.; Straumal, B.B.; Nekrasov, A.N.; Kilmametov, A.; Afonikova, N.S. Grain boundary wetting by a second solid phase in Ti–Fe alloys. J. Mater. Eng. Perform. 2018, 27, 4989–4992. [Google Scholar] [CrossRef]
- Rabkin, E.I.; Shvindlerman, L.S.; Straumal, B.B. Grain boundaries: Phase transitions and critical phenomena. Int. J. Mod. Phys. B 1991, 5, 2989–3028. [Google Scholar] [CrossRef]
- Noskovich, O.I.; Rabkin, E.I.; Semenov, V.N.; Straumal, B.B.; Shvindlerman, L.S. Wetting and premelting phase transitions in 38°[100] tilt grain boundaries in (Fe–12at.%Si) Zn alloy in the vicinity of the A2–B2 bulk ordering in Fe–12at.%Si alloy. Acta Metall. Mater. 1991, 39, 3091–3098. [Google Scholar] [CrossRef]
- Morgan, J.E.; Mordike, B.L. An investigation into creep-resistant, as-cast magnesium alloys containing yttrium, zinc, neodymium and zirconium. MTA 1981, 12, 1581–1585. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straumal, A.; Mazilkin, I.; Tzoy, K.; Straumal, B.; Bryła, K.; Baranchikov, A.; Eggeler, G. Bulk and Surface Low Temperature Phase Transitions in the Mg-Alloy EZ33A. Metals 2020, 10, 1127. https://doi.org/10.3390/met10091127
Straumal A, Mazilkin I, Tzoy K, Straumal B, Bryła K, Baranchikov A, Eggeler G. Bulk and Surface Low Temperature Phase Transitions in the Mg-Alloy EZ33A. Metals. 2020; 10(9):1127. https://doi.org/10.3390/met10091127
Chicago/Turabian StyleStraumal, Alexander, Ivan Mazilkin, Kristina Tzoy, Boris Straumal, Krzysztof Bryła, Alexander Baranchikov, and Gunther Eggeler. 2020. "Bulk and Surface Low Temperature Phase Transitions in the Mg-Alloy EZ33A" Metals 10, no. 9: 1127. https://doi.org/10.3390/met10091127
APA StyleStraumal, A., Mazilkin, I., Tzoy, K., Straumal, B., Bryła, K., Baranchikov, A., & Eggeler, G. (2020). Bulk and Surface Low Temperature Phase Transitions in the Mg-Alloy EZ33A. Metals, 10(9), 1127. https://doi.org/10.3390/met10091127






