Effect of V2O5 Additive on Micro-Arc Oxidation Coatings Fabricated on Magnesium Alloys with Different Loading Voltages
Abstract
:1. Introduction
2. Experimental
2.1. Material Preparation
2.2. Coating Fabrication
2.3. Coating Characterization
3. Results and Discussion
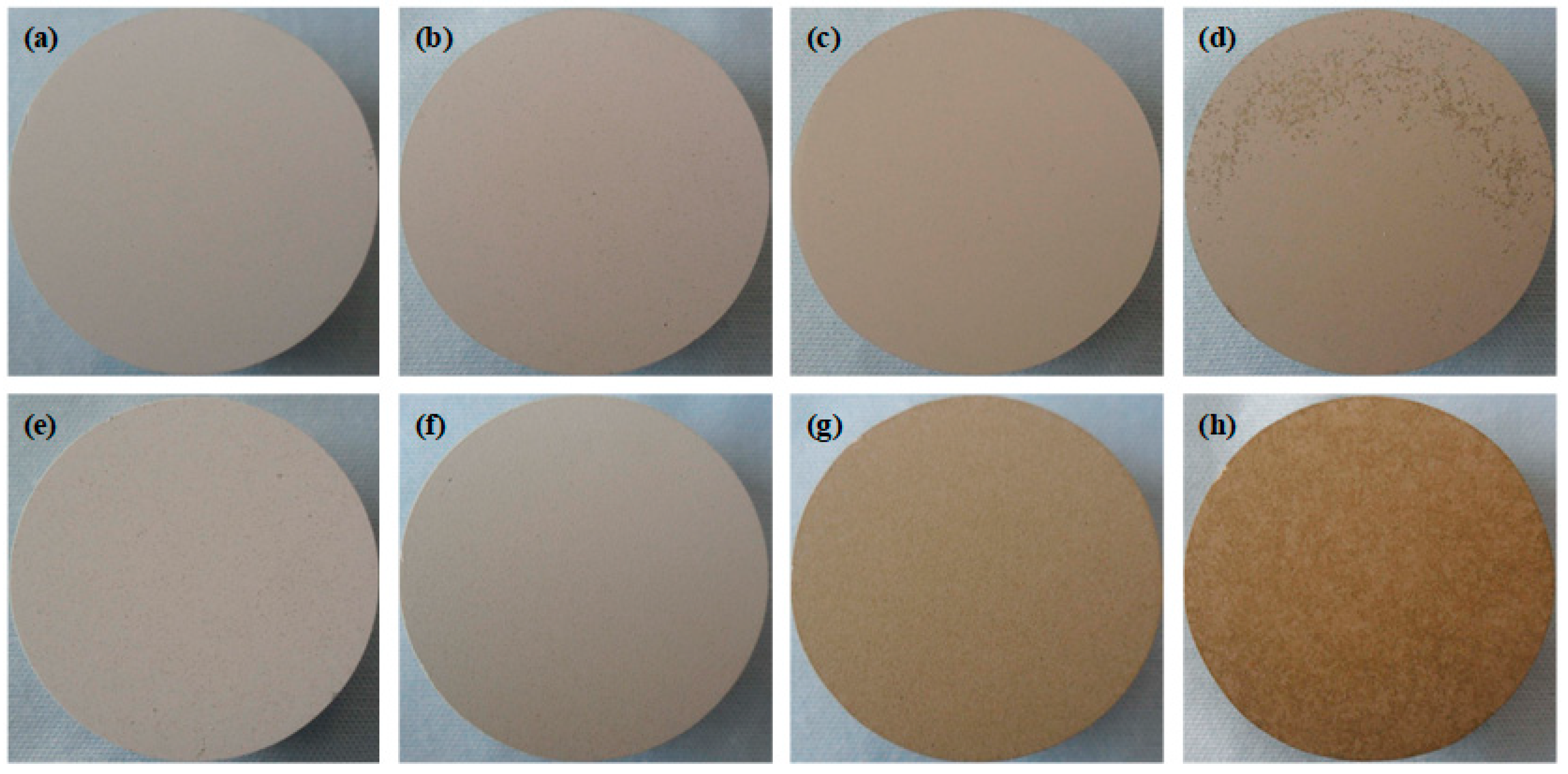
3.1. Macroscopic Morphologies
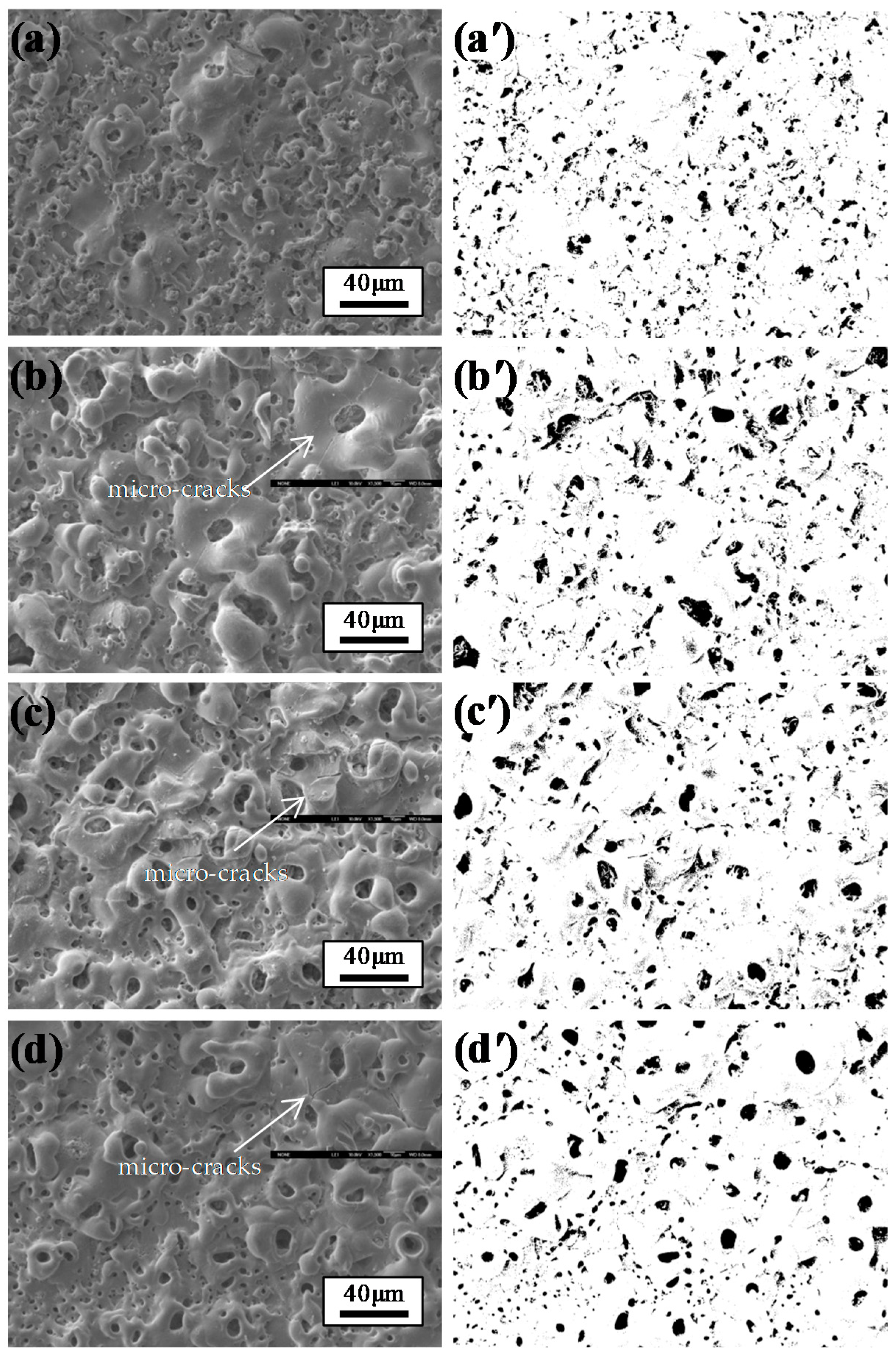
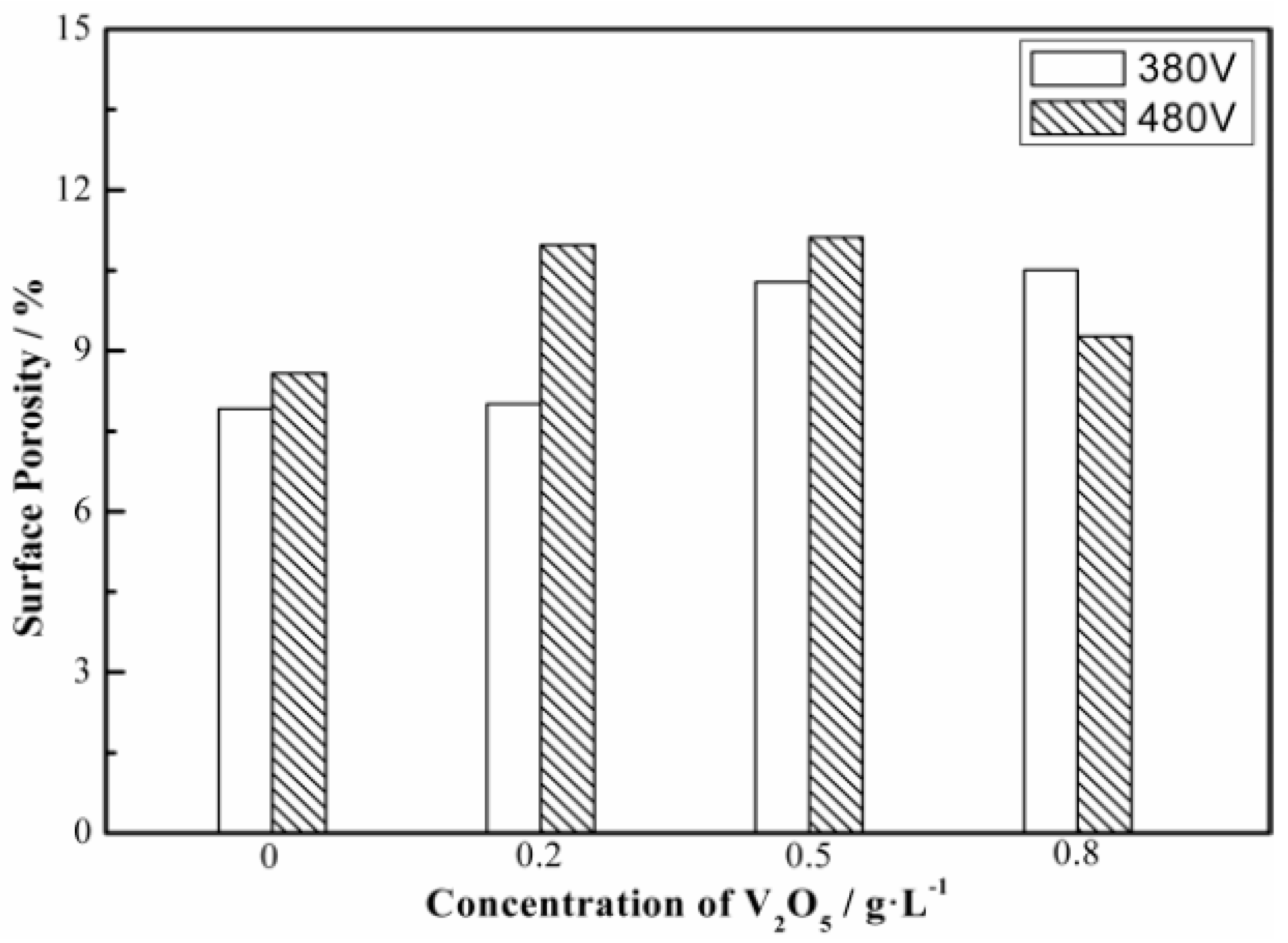
3.2. Microscopy Morphologies
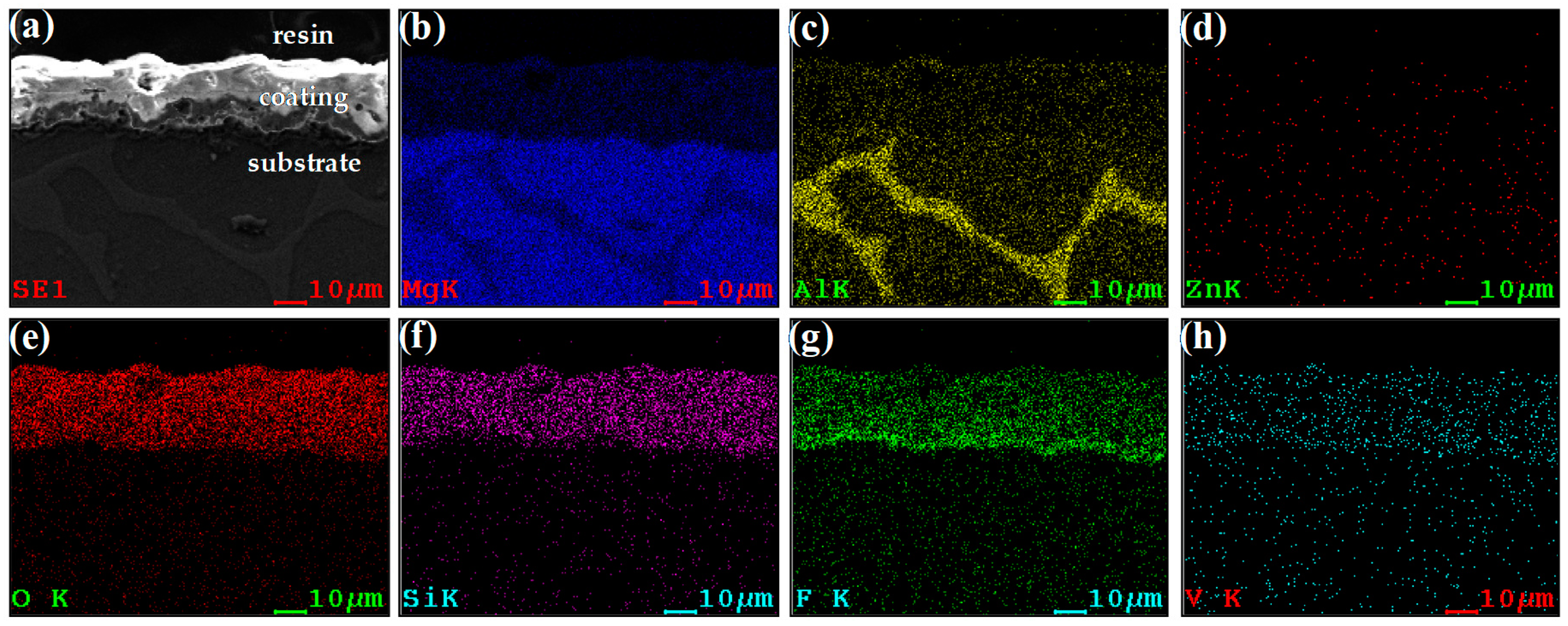
3.3. Chemical Composition of MAO Coating
3.4. Corrosion Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baghni, I.M.; Yinshun, W.; Jiuqing, L.; Du, C.; Zhang, W. Mechanical properties and potential applications of magnesium alloys. Trans. Nonferrous Met. Soc. China 2003, 13, 1253–1259. [Google Scholar]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Enhanced corrosion resistance and in-vitro biodegradation of plasma electrolytic oxidation coatings prepared on AZ91 Mg alloy using ZnO nanoparticles-incorporated electrolyte. Surf. Coat. Technol. 2019, 360, 153–171. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Liang, J.; Balajeee, R.G.; Blawert, C.; Störmer, M.; Dietzel, W. Effect of pulse frequency on the microstructure, phase composition and corrosion performance of a phosphate-based plasma electrolytic oxidation coated AM50 magnesium alloy. Appl. Surf. Sci. 2010, 256, 3928–3935. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.; Arrabal, R.; Matykina, E. 3-Dimensional structures of open-pores in PEO films on AZ31 Mg alloy. Mater. Lett. 2015, 161, 439–441. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Zhang, T.; Zhang, W.; Wang, F.; Lu, X.; Blawert, C.; Zheludkevich, M.L. Interaction effect between different constituents in silicate-containing electrolyte on PEO coatings on Mg alloy. Surf. Coat. Technol. 2016, 307, 825–836. [Google Scholar] [CrossRef]
- Peitao, G.; Mingyang, T.; Chaoyang, Z. Tribological and corrosion resistance properties of graphite composite coating on AZ31 Mg alloy surface produced by plasma electrolytic oxidation. Surf. Coat. Technol. 2019, 359, 197–205. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Kacalak, W.; Tandecka, K.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Malorny, W.; Matysek, D.; Dudek, L.; et al. Characterization of Porous Phosphate Coatings Enriched with Calcium, Magnesium, Zinc and Copper Created on CP Titanium Grade 2 by Plasma Electrolytic Oxidation. Metals 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- RehmanZ, U.; Koo, B.H. Effect of Na2SiO3·5H2O concentration on the microstructure and corrosion properties of two-step PEO coatings formed on AZ91alloy. Surf. Coat. Technol. 2017, 317, 117–124. [Google Scholar] [CrossRef]
- Kazek-Kesik, A.; Dercz, G.; Kalemba, I.; Suchanek, K.; Kukharenko, A.I.; Korotin, D.M.; Michalska, J.; Krząkała, A.; Piotrowski, J.; Kurmaeve, E.Z.; et al. Surface characterisation of Ti–15Mo alloy modified by a PEO process in various suspensions. Mater. Sci. Eng. C 2014, 39, 259–272. [Google Scholar] [CrossRef]
- Weiyi, M.; Yong, H. Study on micro-arc oxidized coatings on magnesium in three different electrolytes. Rare Met. Mater. Eng. 2010, 39, 1129–1134. [Google Scholar] [CrossRef]
- Ko, Y.G.; Namgung, S.; Shin, D.H. Correlation between KOH concentration and surface properties of AZ91 magnesium alloy coated by plasma electrolytic oxidation. Surf. Coat. Technol. 2010, 205, 2525–2531. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Shin, S.H.; Hussain, I. Structure and corrosion properties of the two-step PEO coatings formed on AZ91DMg alloy in K2ZrF6-based electrolyte solution. Surf. Coat. Technol. 2016, 307, 484–490. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Wang, H.; Peng, J. The influence of additives on the stability behavior of electrolyte, discharges and PEO films characteristics. J. Alloys Compd. 2010, 493, 445–452. [Google Scholar] [CrossRef]
- Li, K.; Li, W.; Zhang, G.; Guo, Z. Preparation of black PEO layers on Al-Si alloy and the colorizing analysis. Vacuum 2015, 111, 131–136. [Google Scholar] [CrossRef]
- Bahramian, A.; Raeissi, K.; Hakimizad, A. An investigation of the characteristics of Al2O3/TiO2 PEO nanocomposite coating. Appl. Surf. Sci. 2015, 351, 13–26. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, D.; Yu, J.; Di, S. Effects of Al2O3Nano-additive on Performance of Micro-arc Oxidation Coatings Formed on AZ91D Mg Alloy. J. Mater. Sci. Technol. 2014, 30, 984–990. [Google Scholar] [CrossRef]
- Fatimah, S.; Kamil, M.P.; Kwon, J.H.; Kaseem, M.; Ko, Y.G.; Kwon, J.H. Dual incorporation of SiO2 and ZrO2 nanoparticles into the oxide layer on 6061 Al alloy via plasma electrolytic oxidation: Coating structure and corrosion properties. J. Alloys Compd. 2017, 707, 358–364. [Google Scholar] [CrossRef]
- Mengesha, G.A.; Chu, J.P.; Lou, B.S.; Lee, J.-W. Effects of Processing Parameters on the Corrosion Performance of Plasma Electrolytic Oxidation Grown Oxide on Commercially Pure Aluminum. Metals 2020, 10, 394. [Google Scholar] [CrossRef] [Green Version]
- Demirbaş, Ç.; Ayday, A. The influence of Nano-TiO2 and Nano-Al2O3 Particles in Silicate Based Electrolytes on Microstructure and Mechanical Properties of Micro Arc Coated Ti6Al4V Alloy. Mater. Res. 2018, 21, e20180092. [Google Scholar] [CrossRef]
- Fu, J.; Li, X.; Johansson, B.; Zhao, J. Improved Finnis-Sinclair potential for vanadium-rich V-Ti-Cr ternary alloys. J. Alloys Compd. 2017, 705, 369–375. [Google Scholar] [CrossRef]
- Schuth, S.; Horn, I.; Brüske, A.; Wolff, P.E.; Weyer, S. First vanadium isotope analyses of V-rich minerals by femtosecond laserablation and solution-nebulization MC-ICP-MS. Ore Geol. Rev. 2017, 81, 1271–1286. [Google Scholar] [CrossRef]
- Perfilyev, V.; Moshkovich, A.; Lapsker, I.; Laikhtman, A.; Rapoport, L. The effect of vanadium content and temperature on stick–slip phenomena under friction of CrV (x) N coatings. Wear 2013, 307, 44–51. [Google Scholar] [CrossRef]
- He, H.; Zan, L.; Zhang, Y. Effects of amorphous V2O5 coating on the electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion batteries. J. Alloys Compd. 2016, 680, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Vernardou, D.; Louloudakis, D.; Katsarakis, N.; Koudoumas, E.; Kazadojev, I.I.; O’Brien, S.; Pemble, M.E.; Povey, I.M. Electrochemical evaluation of vanadium pentoxide coatings grown by AACVD. Sol. Energy Mater. Sol. Cells 2015, 143, 601–605. [Google Scholar] [CrossRef]
- Lingyun, A.; Ying, M.; Yunpo, L.; Sun, L.; Wang, S.; Wang, Z. Effects of additives, voltage and their interactions on PEO coatings formed on magnesium alloys. Surf. Coat. Technol. 2018, 354, 226–235. [Google Scholar]
- Dou, J.; Zhao, Y.; Lu, L.; Gu, G.; Yu, H.; Chen, C. Effect of the second-step voltages on the structural and corrosion properties of silicon-calcium-phosphate (Si-CaP) coatings on Mg-Zn-Ca alloy. R. Soc. Open Sci. 2018, 5, 172410. [Google Scholar] [CrossRef] [Green Version]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Song, G. Recent Progress in Corrosion and Protection of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Altun, H.; Sen, S. Studies on the influence of chloride ion concentration and pH on the corrosion and electrochemical behaviour of AZ63 magnesium alloy. Mater. Des. 2004, 25, 637–643. [Google Scholar] [CrossRef]
- Zhao, M.C.; Liu, M.; Song, G.L.; Atrens, A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41. Corros. Sci. 2008, 50, 3168–3178. [Google Scholar] [CrossRef]
- Liang, J.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Influence of pH on the deterioration of plasma electrolytic oxidation coated AM50 magnesium alloy in NaCl solutions. Corros. Sci. 2010, 52, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Ying, M.; Junyan, F.; Yuezhou, M.; Hua, Z.; Wei, G. Comparative study on characterization of corrosion resistance of micro-arc oxidation coatings on magnesium alloys. J. Chin. Soc. Corros. Prot. 2010, 30, 442–447. [Google Scholar]
- Sowa, M.; Simka, W. Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation. Materials 2018, 11, 545. [Google Scholar] [CrossRef] [Green Version]
- Etim, U.J.; Bai, P.; Ullah, R.; Subhan, F.; Yan, Z. Vanadium Contamination of FCC catalyst: Understanding the Destruction and Passivation Mechanisms. Appl. Catal. A Gen. 2018, 555, 108–117. [Google Scholar] [CrossRef]
- Akah, A. Application of rare earths in fluid catalytic cracking: A review. J. Rare Earths 2017, 35, 941–956. [Google Scholar] [CrossRef]
- Zhang, R.F. Film formation in the second step of micro-arc oxidation on magnesium alloys. Corros. Sci. 2010, 52, 1285–1290. [Google Scholar] [CrossRef]
- Kaseem, M.; Choi, K.; Ko, Y.G. A highly compact coating responsible for enhancing corrosion properties of Al-Mg-Si alloy. Mater. Lett. 2017, 196, 316–319. [Google Scholar] [CrossRef]
- Hairong, D.; Ying, M.; Sheng, W.; Xiaoxin, Z.; Huixia, G.; Yuan, H. Effect of Growth Rate on Microstructure and Corrosion Resistance of Micro-arc Oxidation Coatings on Magnesium Alloy. Rare Met. Mater. Eng. 2017, 46, 2399–2404. [Google Scholar] [CrossRef]
- Mamiya, H.; Onoda, M. Electronic states of vanadium spinels MgV2O4 and ZnV2O4. Solid State Commun. 1995, 95, 217–221. [Google Scholar] [CrossRef]
- Kaur, R.; Maitra, T.; Nautiyal, T. Effect of spin–orbit coupling on magnetic and orbital order in MgV2O4. J. Phys. Condens. Matter 2013, 25, 065503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.J.; Yue, R.; Rateick, R.G.; Birss, V.I. Electrochemical Studies of AC/DC Anodized Mg Alloy in NaCl Solution. J. Electrochem. Soc. 2004, 151, B179–B187. [Google Scholar] [CrossRef]
- Murphy, O.J.; Bockris, J.O.; Pou, T.E. Chloride ion penetration of passive films on iron. J. Electrochem. Soc. 1983, 130, 1792–1794. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.W.; Wang, Y.J.; Chen, D.; Zhang, Y.C.; Du, K.; Oguzie, E.E.; Ma, X.L. Unmasking chloride attack on the passive filmof metals. Nat. Commun. 2018, 9, 2559. [Google Scholar] [CrossRef]


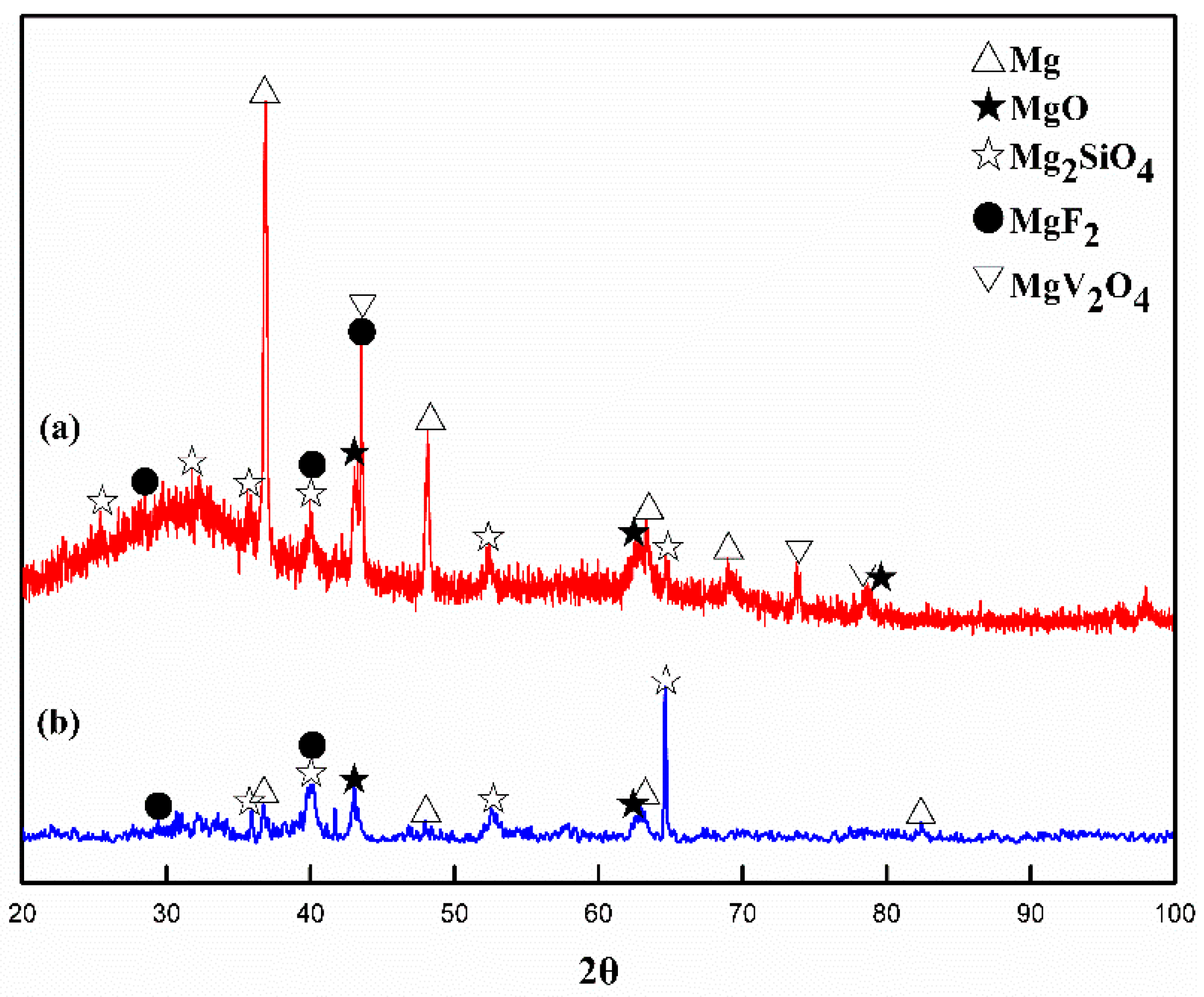
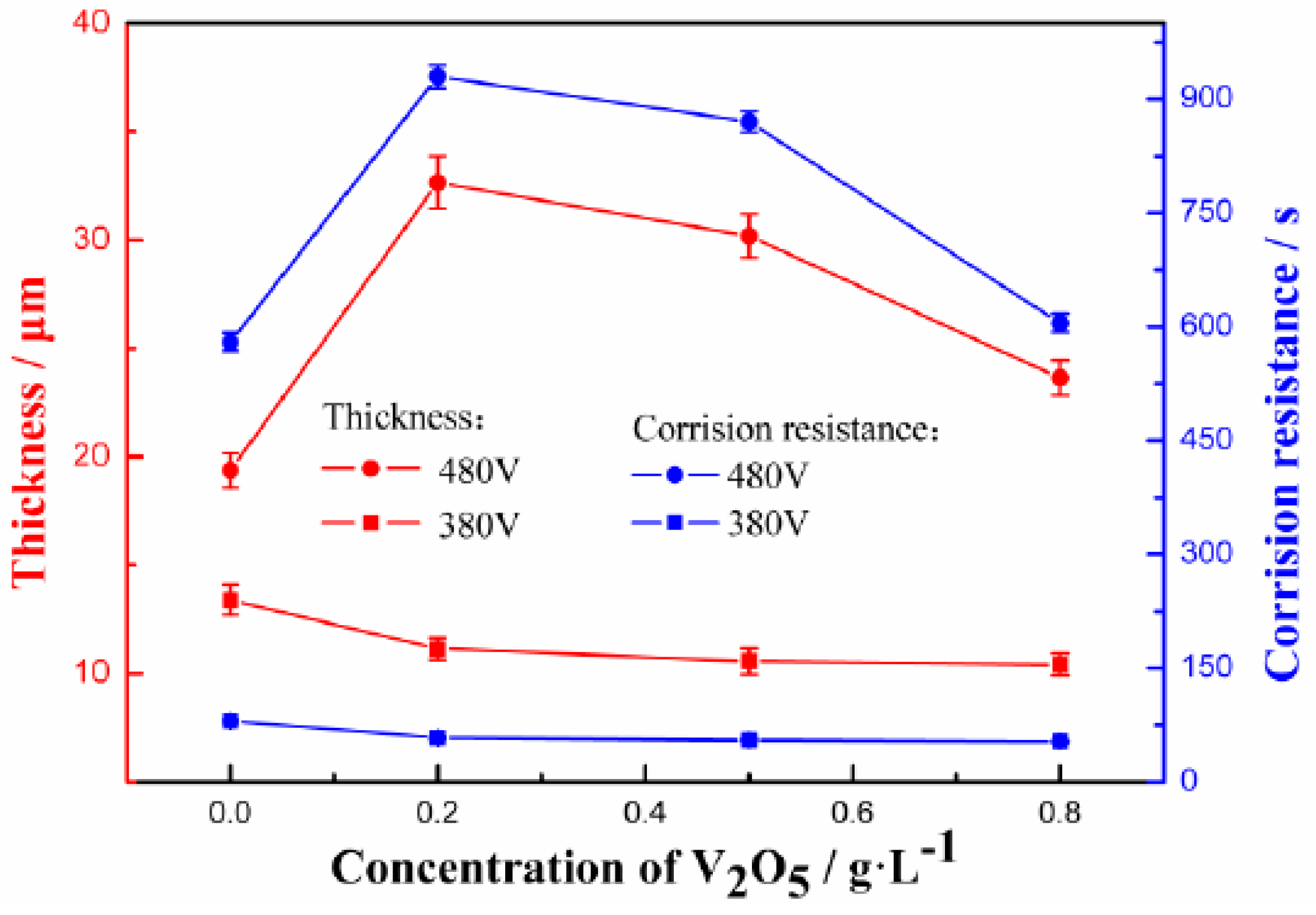
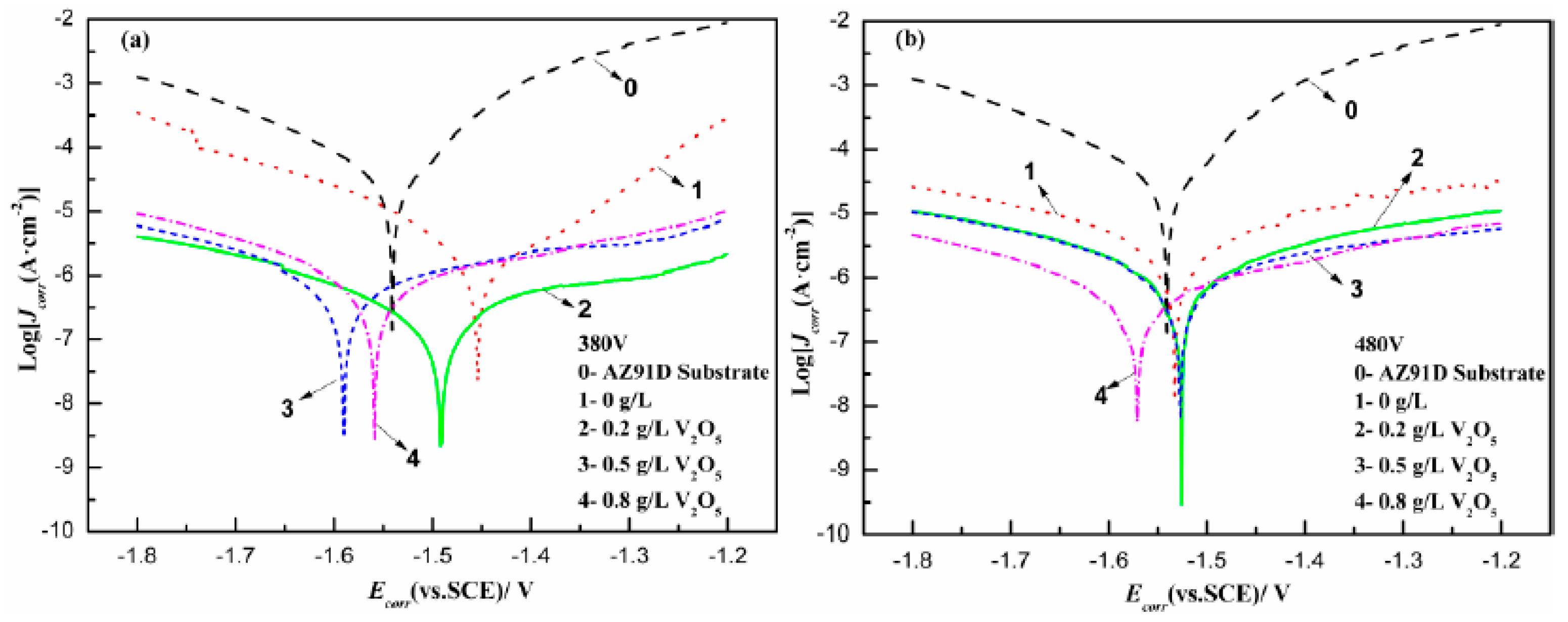
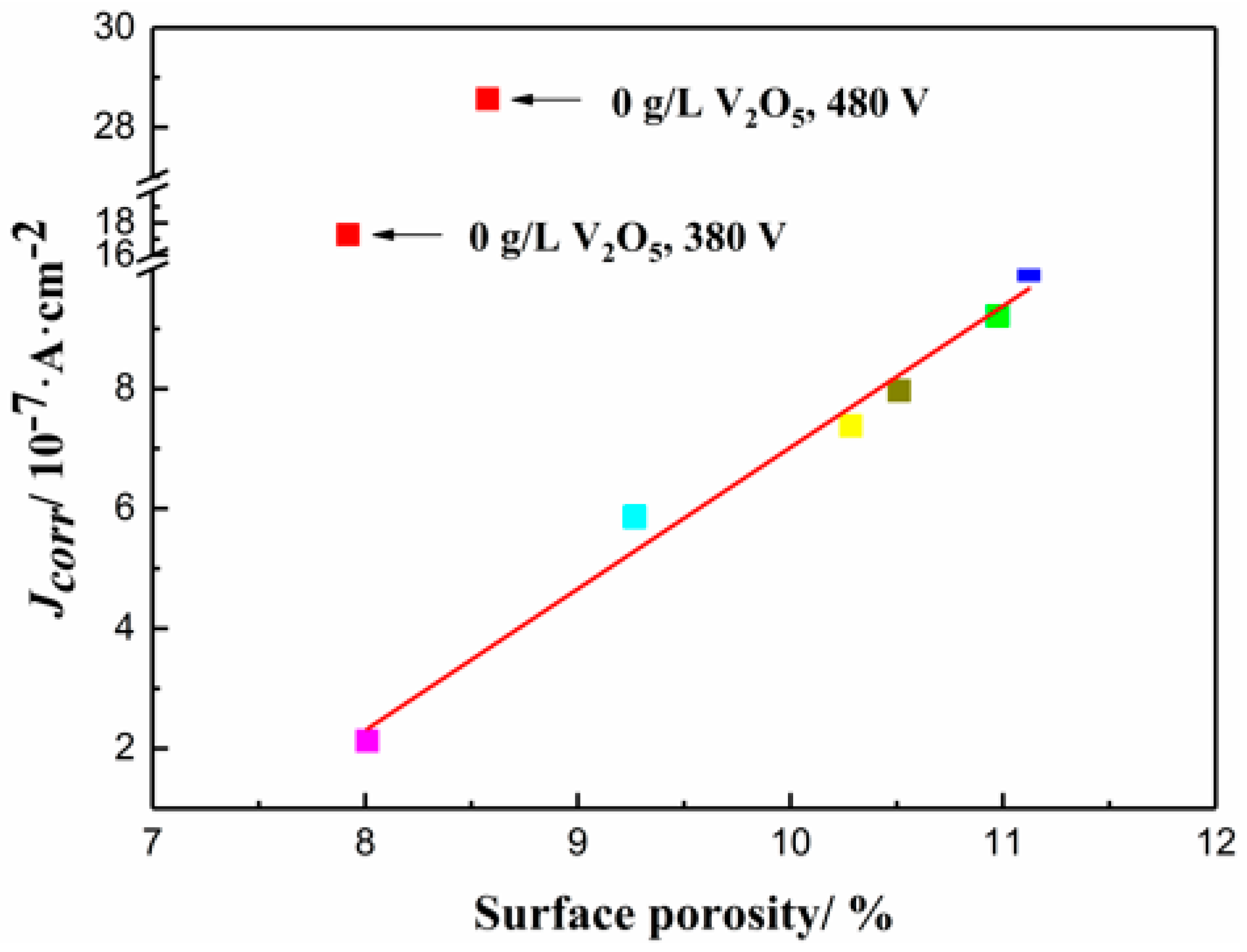
| Electrolyte | 0 g/L V2O5 | 0.2 g/L V2O5 | 0.5 g/L V2O5 | 0.8 g/L V2O5 |
|---|---|---|---|---|
| pH | 12.34 | 12.29 | 12.20 | 12.09 |
| Conductivity/mS·cm−1 | 25.0 | 24.2 | 23.3 | 22.6 |
| Element | Mg | Al | Zn | O | Si | F | V |
|---|---|---|---|---|---|---|---|
| Wt% | 68.84 | 9.58 | 2.00 | 11.45 | 3.66 | 3.70 | 0.78 |
| At% | 66.27 | 8.31 | 0.72 | 16.75 | 3.05 | 4.56 | 0.36 |
| Parameter | Ecorr/mV | Jcorr/A·cm−2 | RP/kΩ·cm2 | |
|---|---|---|---|---|
| AZ91D Substrate | −1541 ± 12 | (3.091 ± 1.53) × 10−5 | (7.093 ± 0.34) × 102 | |
| 380 V | 0 g/L | −1453 ± 23 | (1.728 ± 0.95) × 10−6 | (1.499 ± 0.21) × 104 |
| 0.2 g/L | −1492 ± 6 | (2.129 ± 0.84) × 10−7 | (1.989 ± 1.18) × 105 | |
| 0.5 g/L | −1590 ± 14 | (7.386 ± 1.91) × 10−7 | (6.187 ± 1.49) × 104 | |
| 0.8 g/L | −1559 ± 15 | (7.967 ± 2.33) × 10−7 | (5.592 ± 1.52) × 104 | |
| 480 V | 0 g/L | −1532 ± 18 | (2.857 ± 0.27) × 10−6 | (1.421 ± 0.62) × 104 |
| 0.2 g/L | −1526 ± 17 | (9.215 ± 2.65) × 10−7 | (4.313 ± 1.87) × 104 | |
| 0.5 g/L | −1527 ± 21 | (9.976 ± 1.76) × 10−7 | (4.416 ± 1.36) × 104 | |
| 0.8 g/L | −1571 ± 9 | (5.862 ± 0.79) × 10−7 | (7.252 ± 0.29) × 104 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Ma, Y.; Wang, Y. Effect of V2O5 Additive on Micro-Arc Oxidation Coatings Fabricated on Magnesium Alloys with Different Loading Voltages. Metals 2020, 10, 1146. https://doi.org/10.3390/met10091146
Wang Z, Ma Y, Wang Y. Effect of V2O5 Additive on Micro-Arc Oxidation Coatings Fabricated on Magnesium Alloys with Different Loading Voltages. Metals. 2020; 10(9):1146. https://doi.org/10.3390/met10091146
Chicago/Turabian StyleWang, Zhanying, Ying Ma, and Yushun Wang. 2020. "Effect of V2O5 Additive on Micro-Arc Oxidation Coatings Fabricated on Magnesium Alloys with Different Loading Voltages" Metals 10, no. 9: 1146. https://doi.org/10.3390/met10091146





