Effects of Rare Earth (Ce and La) on Steel Corrosion Behaviors under Wet-Dry Cycle Immersion Conditions
Abstract
:1. Introduction
2. Experimental
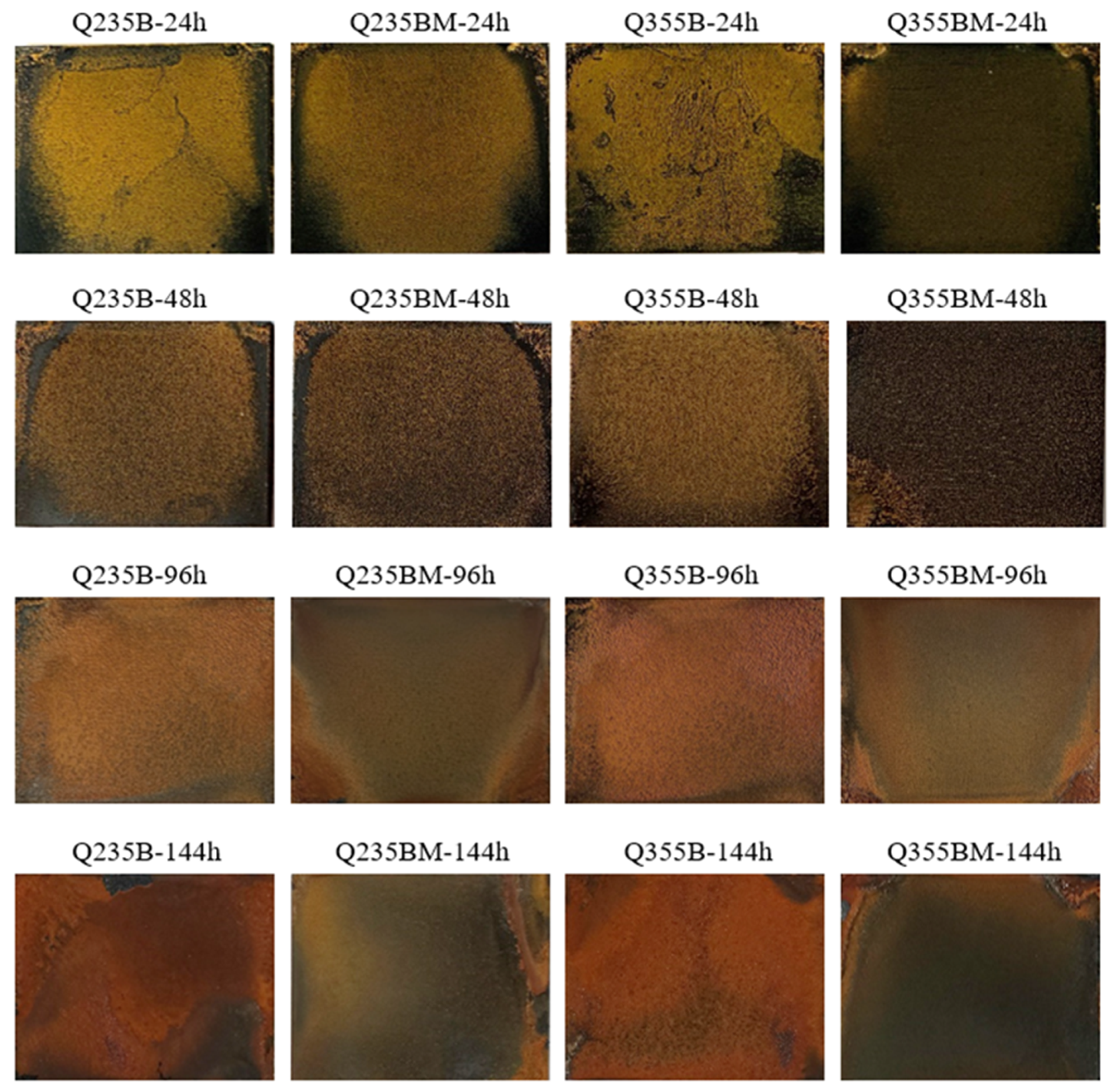
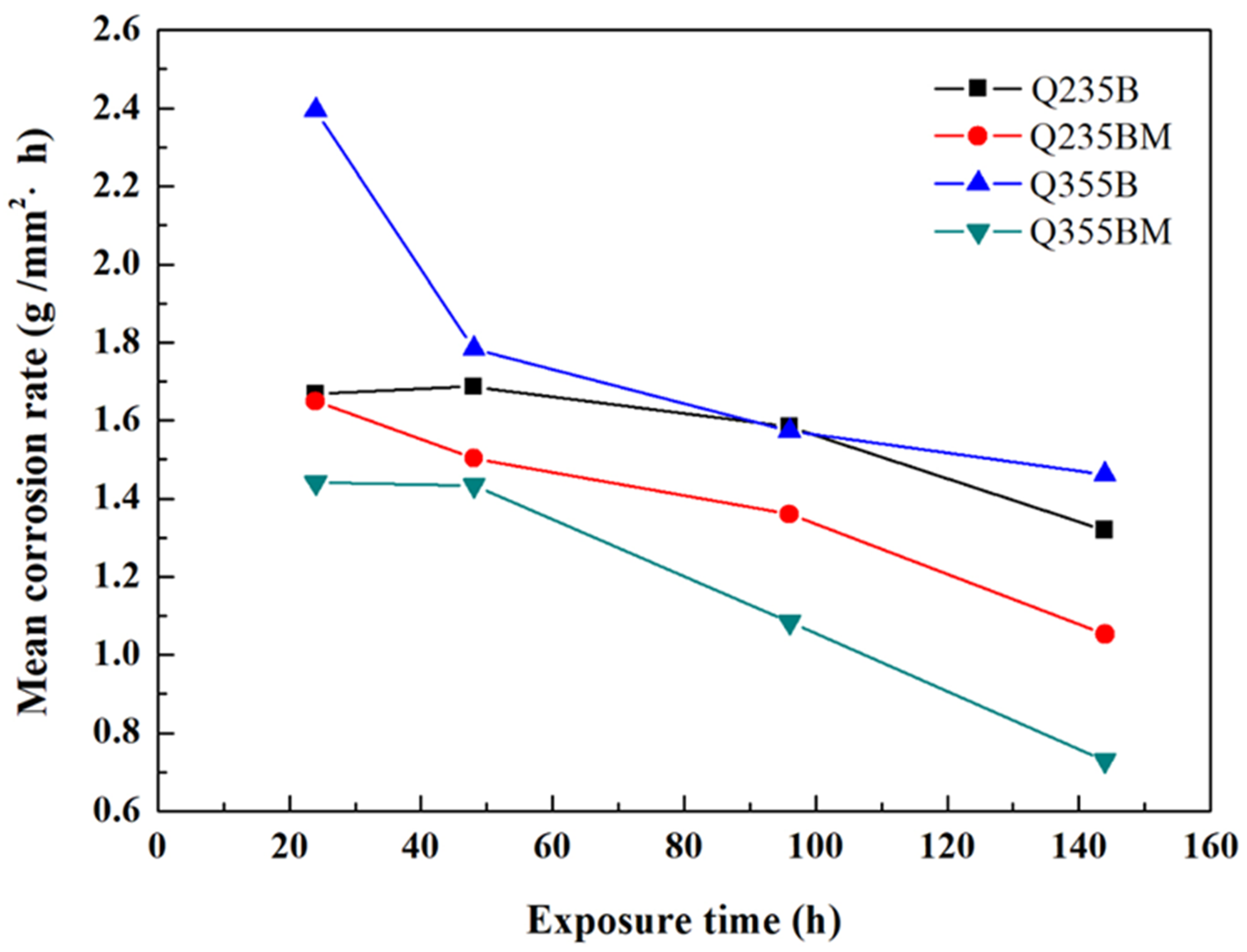
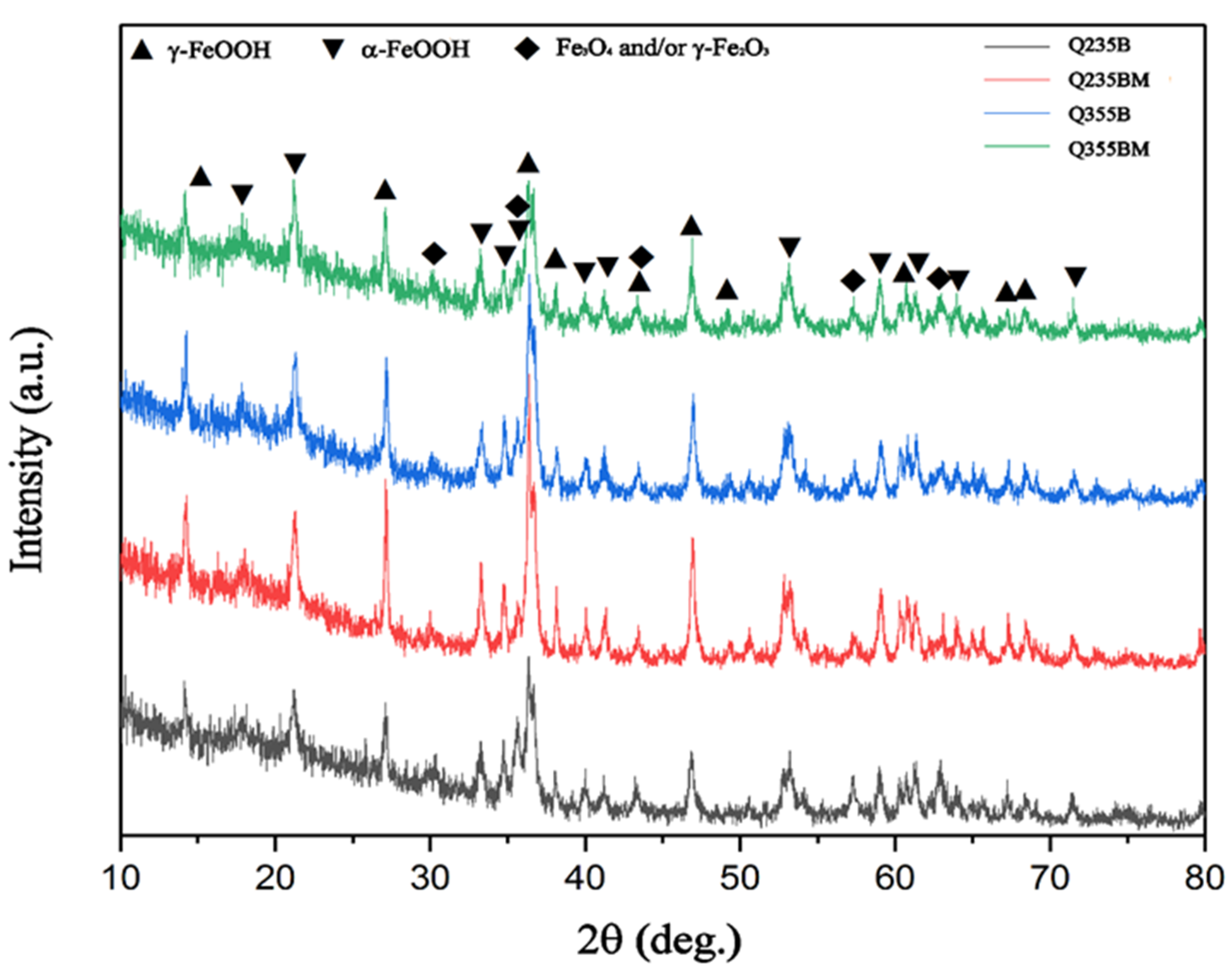
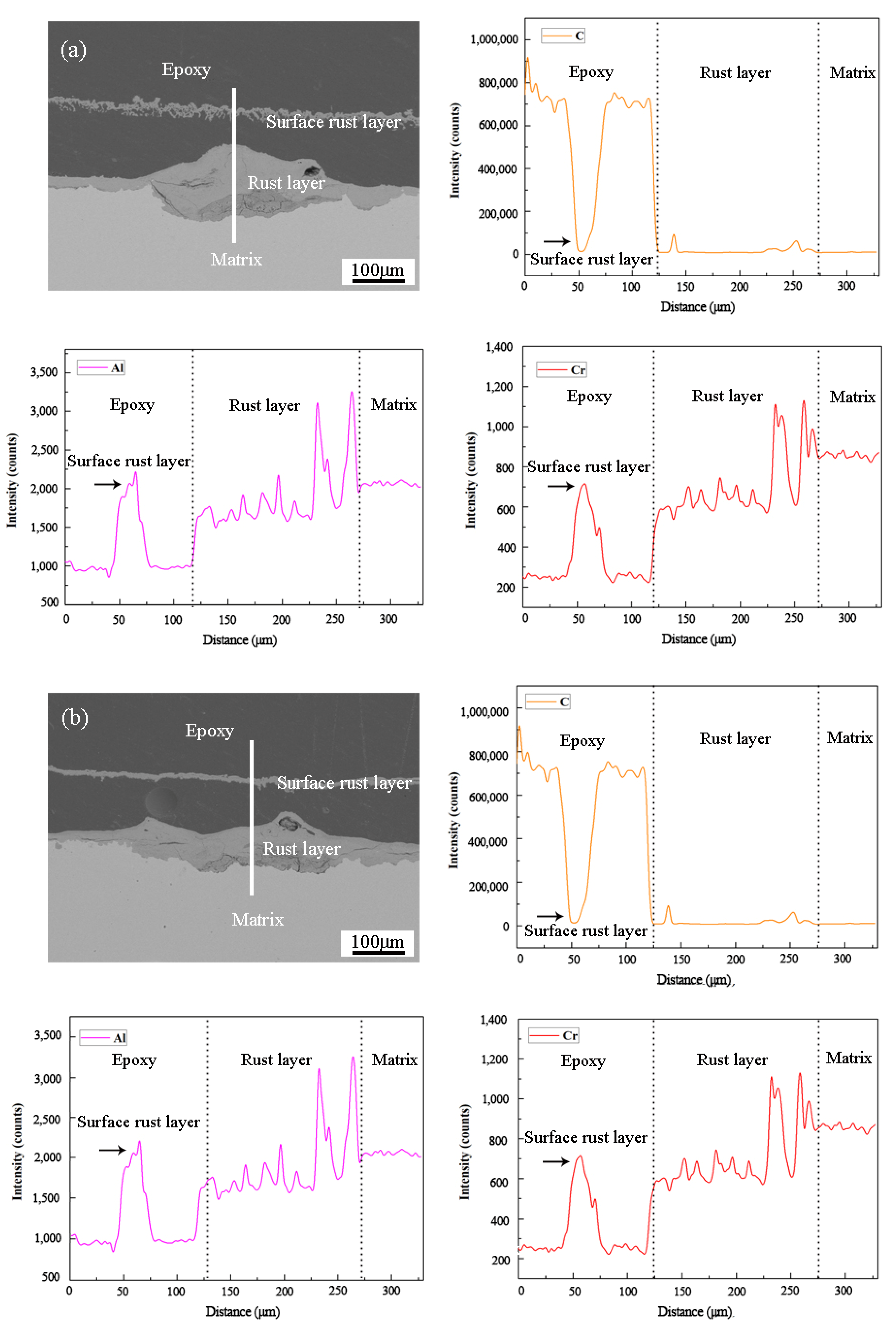
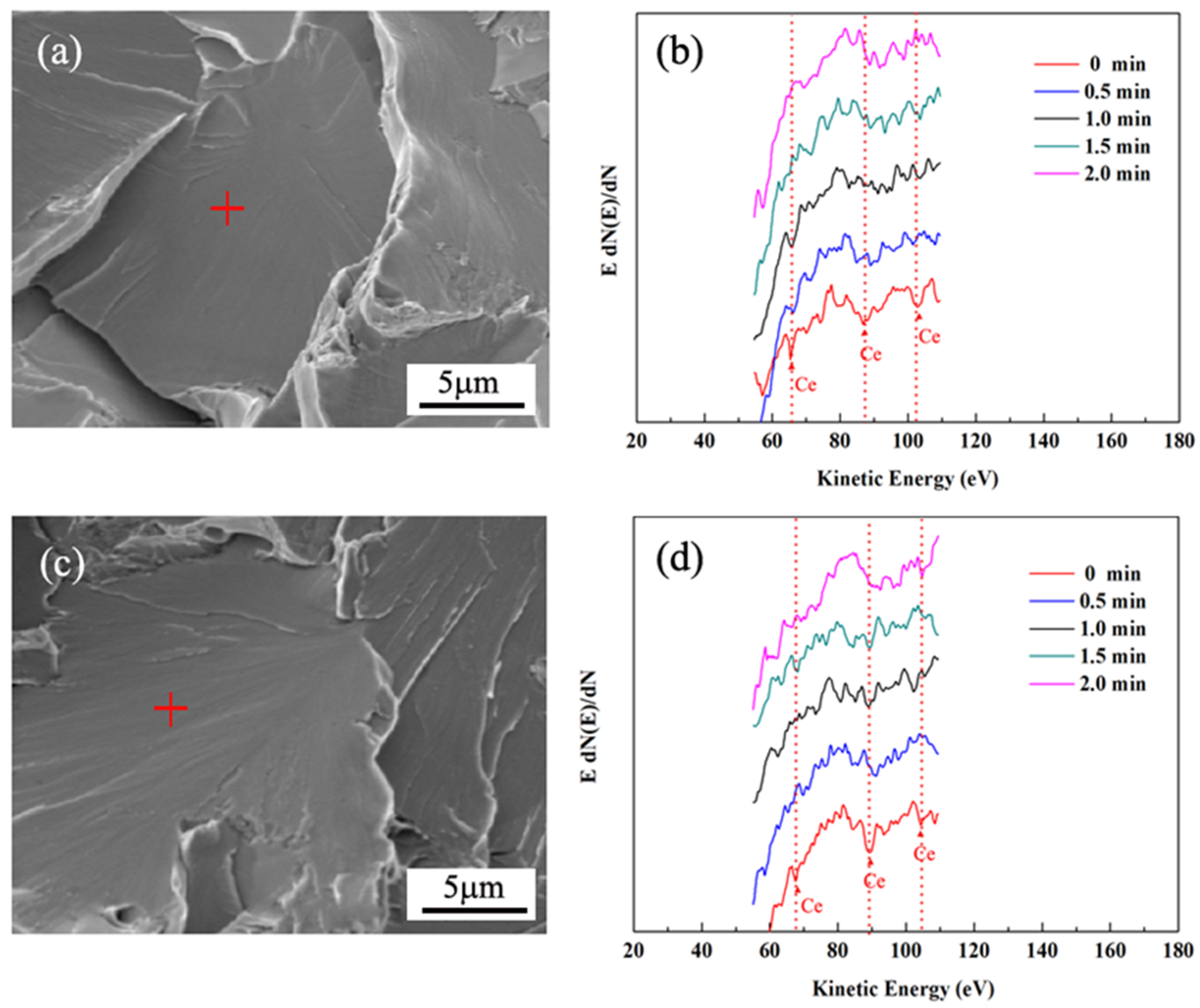
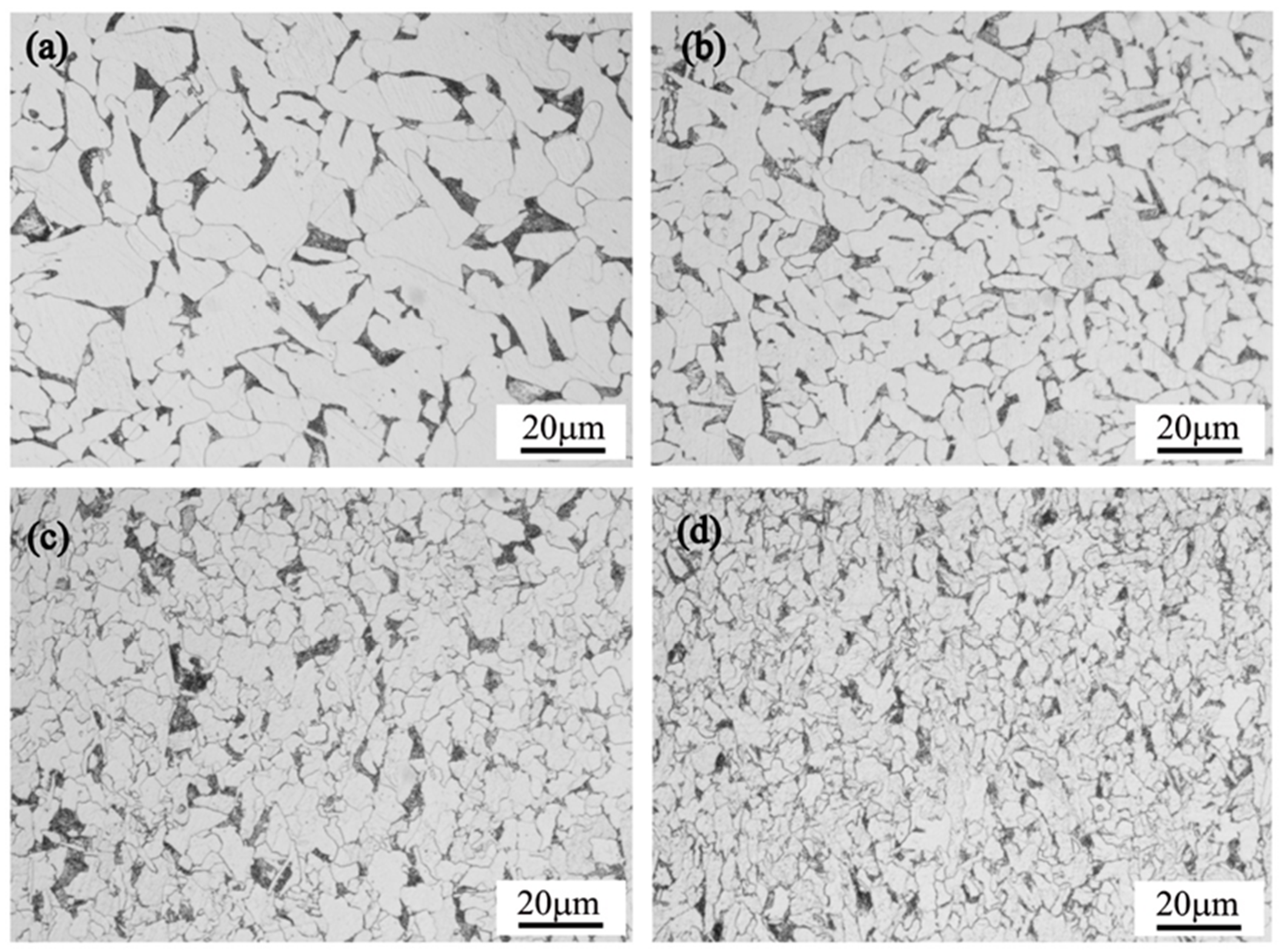
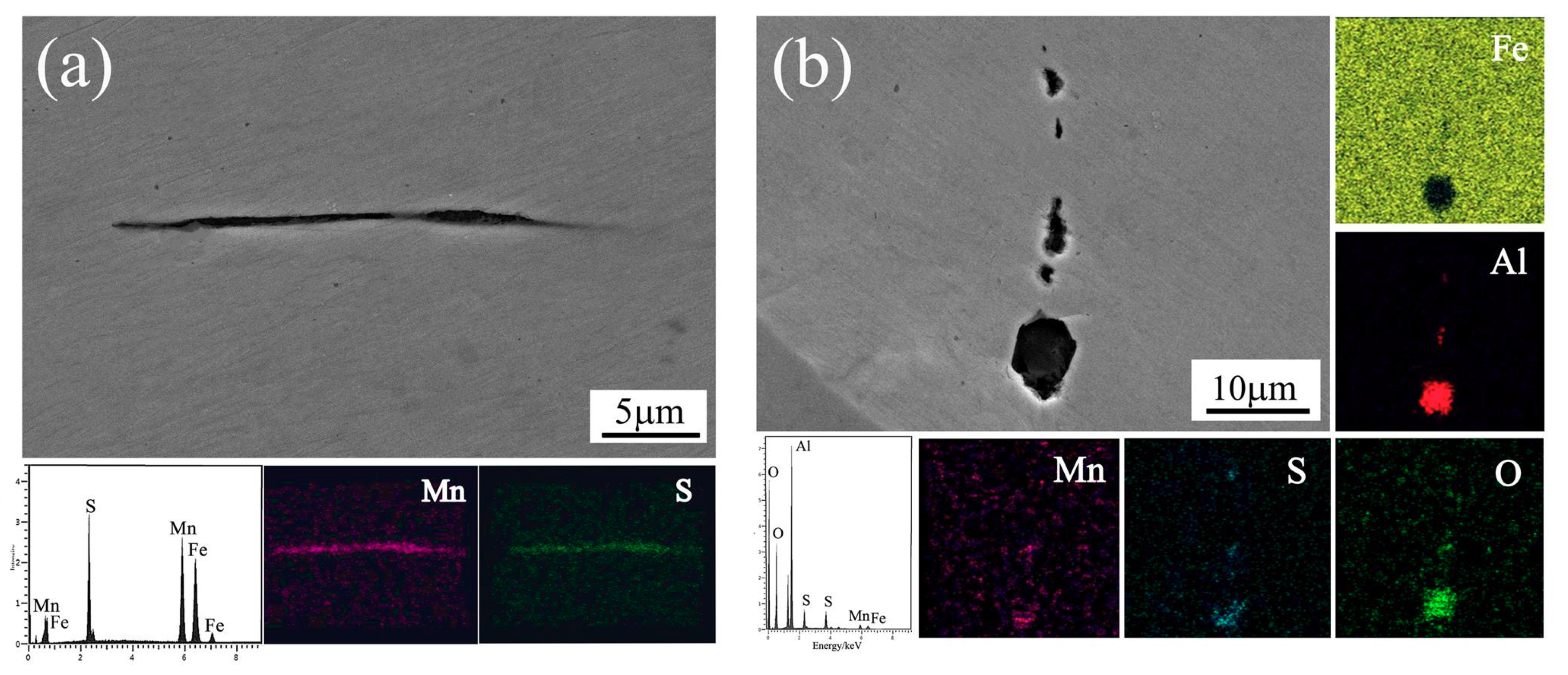
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seifert, H.P.; Hickling, J.; Lister, D. Corrosion and Environmentally-Assisted Cracking of Carbon and Low-Alloy Steels; Elsevier: Oxford, UK, 2012; pp. 105–142. [Google Scholar]
- Tait, W.S. Handbook of Environmental Degradation of Materials, 2nd ed.; William Andrew Publishing: New York, NY, USA, 2012; pp. 863–886. [Google Scholar]
- Zhou, Y.L.; Chen, Y.; Liu, Z.Y. Effects of Cr, Ni and Cu on the Corrosion Behavior of Low Carbon Microalloying Steel in a Cl− Containing Environment. J. Mater. Sci. Technol. 2013, 29, 168–174. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L. Improvement of corrosion resistance of Cu-based bulk metallic glasses by the microalloying of Mo. Intermetallics 2007, 15, 679–682. [Google Scholar] [CrossRef]
- Morozov, Y.; Calado, L.M.; Shakoor, R.A.; Raj, R.; Kahraman, R.; Taryba, M.G.; Montemor, M.F. Epoxy coatings modified with a new cerium phosphate inhibitor for smart corrosion protection of steel. Corros. Sci. 2019, 159, 108–128. [Google Scholar] [CrossRef]
- Xu, D.; Lou, C.; Huang, J.; Lu, X.; Xin, Z.; Zhou, C.L. Effect of inhibitor-loaded halloysite nanotubes on active corrosion protection of polybenzoxazine coatings on mild steel. Pro. Org. Coat. 2019, 134, 126–133. [Google Scholar] [CrossRef]
- Morcillo, M.; Dían, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part I: Basic concepts. Constr. Build. Mater. 2019, 213, 723–737. [Google Scholar] [CrossRef]
- Rodrigues, C.A.D.; Bandeira, R.M.; Duarte, B.B.; Tremiliosi-Filho, G.; Jorge, A.M., Jr. Effect of phosphorus content on the mechanical, microstructure and corrosion properties of supermartensitic stainless steel. Mat. Sci. Eng. A 2016, 650, 75–83. [Google Scholar] [CrossRef]
- Wang, L.M.; Lin, Q.; Ji, J.W.; Lan, D.N. New study concerning development of application of rare earth metals in steels. J. Alloys Compd. 2006, 408–412, 384–386. [Google Scholar] [CrossRef]
- Deng, X.X.; Jiang, M.; Wang, X.H. Mechanisms of inclusion evolution and intra-granular acicular ferrite formation in steels containing rare earth elements. Acta Metal. Sin Engl. 2012, 25, 241–248. [Google Scholar] [CrossRef]
- Williams, D.E.; Zhu, Y.Y. Explanation for initiation of pitting corrosion of stainless steels at sulfide inclusions. J. Electrochem. Soc. 2000, 147, 1763–1766. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Li, C.Y.; Qi, Y.M.; Chen, L.Q.; Chen, C.F. Mechanism of (Mg, Al, Ca)-oxide inclusion-induced pitting corrosion in 316L stainless steel exposed to Sulphur environments containing chloride ion. Corros. Sci. 2013, 67, 20–31. [Google Scholar] [CrossRef]
- Torkkeli, J.; Saukkonen, T.; Hänninen, H. Effect of MnS inclusion dissolution on carbon steel stress corrosion cracking in fuel-grade ethanol. Corros. Sci. 2015, 96, 14–22. [Google Scholar] [CrossRef]
- Shibaeva, T.V.; Laurinavichyute, V.K.; Tsirlina, G.A.; Arsenkin, A.M.; Grigorovich, K.V. The effect of microstructure and non-metallic inclusions on corrosion behavior of low carbon steel in chloride containing solutions. Corros. Sci. 2014, 80, 299–308. [Google Scholar] [CrossRef]
- Ha, H.Y.; Park, C.J.; Kwon, H.S. Effects of misch metal on the formation of non-metallic inclusions and the associated resistance to pitting corrosion in 25% Cr duplex stainless steels. Scr. Mater. 2006, 55, 991–994. [Google Scholar] [CrossRef]
- Park, I.J.; Lee, S.M.; Kang, M.; Lee, S.; Lee, S.; Lee, Y.K. Pitting corrosion behavior in advanced high strength steels. J. Alloys Compd. 2015, 619, 205–210. [Google Scholar] [CrossRef]
- Lu, W.; Liu, H.; Hsu, T.Y.; Xu, Z.Y. Segregation of rare earth during isothermal transformation in low carbon steels. Scripta Metal. Mater. 1993, 29, 273–274. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, S.F.; Song, S.H. Effect of minor rare earth cerium addition on the hot ductility of a reactor pressure vessel steel. Results Phys. 2019, 15, 102746. [Google Scholar] [CrossRef]
- Song, S.H.; Xu, Y.W.; Chen, X.M.; Jiang, X. Effect of rare earth cerium and impurity tin on the hot ductility of a Cr-Mo low alloy steel. J. Rare Earths 2016, 34, 1062–1068. [Google Scholar] [CrossRef]
- Barrett, C.D.; Imandoust, A.; Kadiri, H.E. The effect of rare earth element segregation on grain boundary energy and mobility in magnesium and ensuing texture weakening. Scr. Mater. 2018, 146, 46–50. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, W.Z.; Cheng, L.; Liu, J.; Wu, K.M.; Liu, M. Effects of niobium and rare earth elements on microstructure and initial marine corrosion behavior of low-alloy steels. Appl. Surf. Sci. 2019, 475, 83–93. [Google Scholar] [CrossRef]
- Qian, Y.H.; Ma, C.H.; Niu, D.; Xu, J.J.; Li, M.S. Influence of alloyed chromium on the atmospheric corrosion resistance of weathering steels. Corros. Sci. 2013, 74, 424–429. [Google Scholar] [CrossRef]
- Liu, Z.; Lian, X.T.; Liu, T.S.; Yang, Y.D.; Zhu, J.N.; Dong, H. Effects of rare earth elements on corrosion behaviors of low-carbon steels and weathering steels. Mater. Corros. 2020, 71, 258–266. [Google Scholar] [CrossRef]
- Yu, J.X.; Wang, H.K.; Yu, Y.; Luo, Z.; Liu, W.D.; Wang, C.M. Corrosion behavior of X65 pipeline steel: Comparison of wet–Dry cycle and full immersion. Corros. Sci. 2018, 133, 276–287. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Tzeng, H.J.; Wei, L.I.; Wang, L.H.; Oung, J.C.; Shih, H.C. Corrosion resistance and mechanical properties of low-alloy steels under atmospheric conditions. Corros. Sci. 2005, 47, 1001–1021. [Google Scholar] [CrossRef]
- Wang, Z.F.; Liu, J.R.; Wu, L.X.; Han, R.D.; Sun, Y.Q. Study of the corrosion behavior of weathering steels in atmospheric environments. Corros. Sci. 2013, 67, 1–10. [Google Scholar] [CrossRef]
- McGuire, G.E. Auger Electron Spectroscopy Reference Manual; Springer: Boston, MA, USA, 1979; pp. 114–116. [Google Scholar] [CrossRef]
- Sánchez-Amaya, J.M.; Blanco, G.; Garcia-Garcia, F.J.; Bethencourt, M.; Botana, F.J. XPS and AES analyses of cerium conversion coatings generated on AA5083 by thermal activation. Surf. Coat. Technol. 2012, 213, 105–116. [Google Scholar] [CrossRef]
- Yue, L.J.; Wang, L.M.; Han, J.S. Effects of rare earth on inclusions and corrosion resistance of 10PCuRE weathering steel. J. Rare Earths 2010, 28, 952–956. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Liu, Z.Y.; Zhang, D.W.; Li, X.G.; Terryna, H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Szummer, A.; Janik-Czacho, M.; Hofmann, S. Discontinuity of the passivating film at nonmetallic inclusions in stainless steels. Mater. Chem. Phys. 1993, 34, 181–183. [Google Scholar] [CrossRef]
- Aghuy, A.A.; Zakeri, M.; Moayed, M.H.; Mazinani, M. Effect of grain size on pitting corrosion of 304L austenitic stainless steel. Corros. Sci. 2015, 94, 368–376. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005–075013. [Google Scholar] [CrossRef]
- Hadzima, B.; Janeček, M.; Estrin, Y.; Kim, H.S. Microstructure and corrosion properties of ultrafine-grained interstitial free steel. Mater. Sci. Eng. A. 2007, 462, 243–247. [Google Scholar] [CrossRef]
- Avci, R.; Davis, B.H.; Wolfenden, M.L.; Beech, I.B.; Lucas, K.; Paul, D. Mechanism of MnS-mediated pit initiation and propagation in carbon steel in an anaerobic sulfidogenic media. Corros. Sci. 2013, 76, 267–274. [Google Scholar] [CrossRef]




| Sample | C | Si | Mn | P | S | Cr | Al | Cu | Ce | La |
|---|---|---|---|---|---|---|---|---|---|---|
| Q235B | 0.12 | 0.09 | 0.34 | 0.019 | 0.009 | 0.024 | 0.022 | 0.023 | - | - |
| Q235BM | 0.12 | 0.08 | 0.34 | 0.020 | 0.010 | 0.023 | 0.022 | 0.023 | 0.018 | 0.0065 |
| Q355B | 0.14 | 0.23 | 1.15 | 0.020 | 0.007 | 0.023 | 0.039 | 0.020 | - | - |
| Q355BM | 0.13 | 0.23 | 1.15 | 0.020 | 0.007 | 0.023 | 0.039 | 0.020 | 0.011 | 0.0041 |
| Sample | α-FeOOH | γ-FeOOH | Fe3O4 or/and γ-Fe2O3 |
|---|---|---|---|
| Q235B | 28% | 53% | 19% |
| Q235BM | 35% | 34% | 31% |
| Q355B | 17% | 63% | 20% |
| Q355BM | 44% | 23% | 33% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, X.; Zhu, J.; Wang, R.; Liu, T.; Xu, J.; Xu, D.; Dong, H. Effects of Rare Earth (Ce and La) on Steel Corrosion Behaviors under Wet-Dry Cycle Immersion Conditions. Metals 2020, 10, 1174. https://doi.org/10.3390/met10091174
Lian X, Zhu J, Wang R, Liu T, Xu J, Xu D, Dong H. Effects of Rare Earth (Ce and La) on Steel Corrosion Behaviors under Wet-Dry Cycle Immersion Conditions. Metals. 2020; 10(9):1174. https://doi.org/10.3390/met10091174
Chicago/Turabian StyleLian, Xintong, Jianan Zhu, Ruiqian Wang, Tengshi Liu, Jing Xu, Dexiang Xu, and Han Dong. 2020. "Effects of Rare Earth (Ce and La) on Steel Corrosion Behaviors under Wet-Dry Cycle Immersion Conditions" Metals 10, no. 9: 1174. https://doi.org/10.3390/met10091174




