Selective Recovery of Tellurium from the Tellurium-Bearing Sodium Carbonate Slag by Sodium Sulfide Leaching Followed by Cyclone Electrowinning
Abstract
1. Introduction
2. Experiments
2.1. Reagents and Materials
2.2. Experimental Equipment and Procedure
2.2.1. Sodium Sulfide Leaching
2.2.2. Cyclone Electrowinning
2.3. Calculation Method
2.4. Characterization and Analyses
3. Results and Discussion
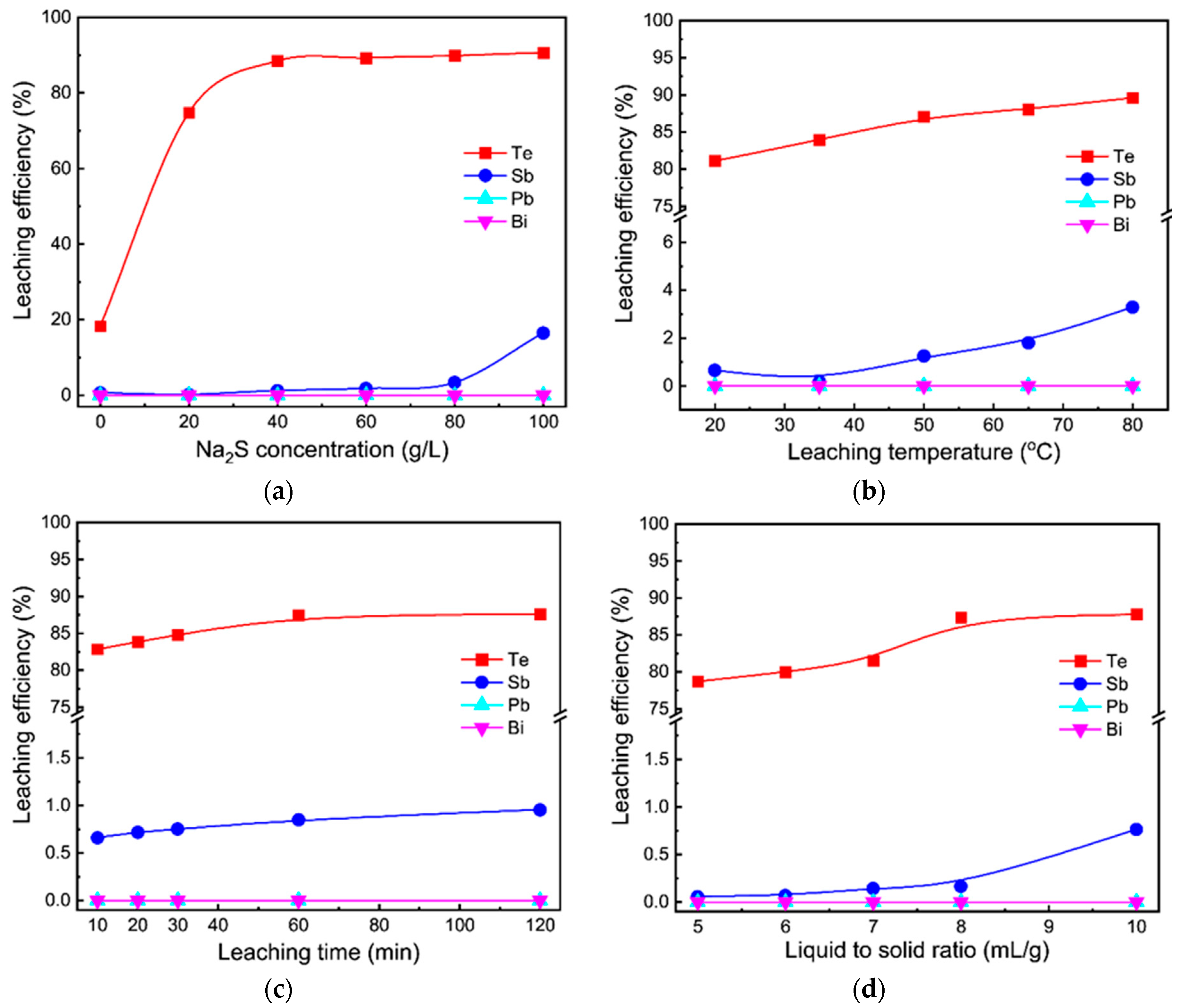
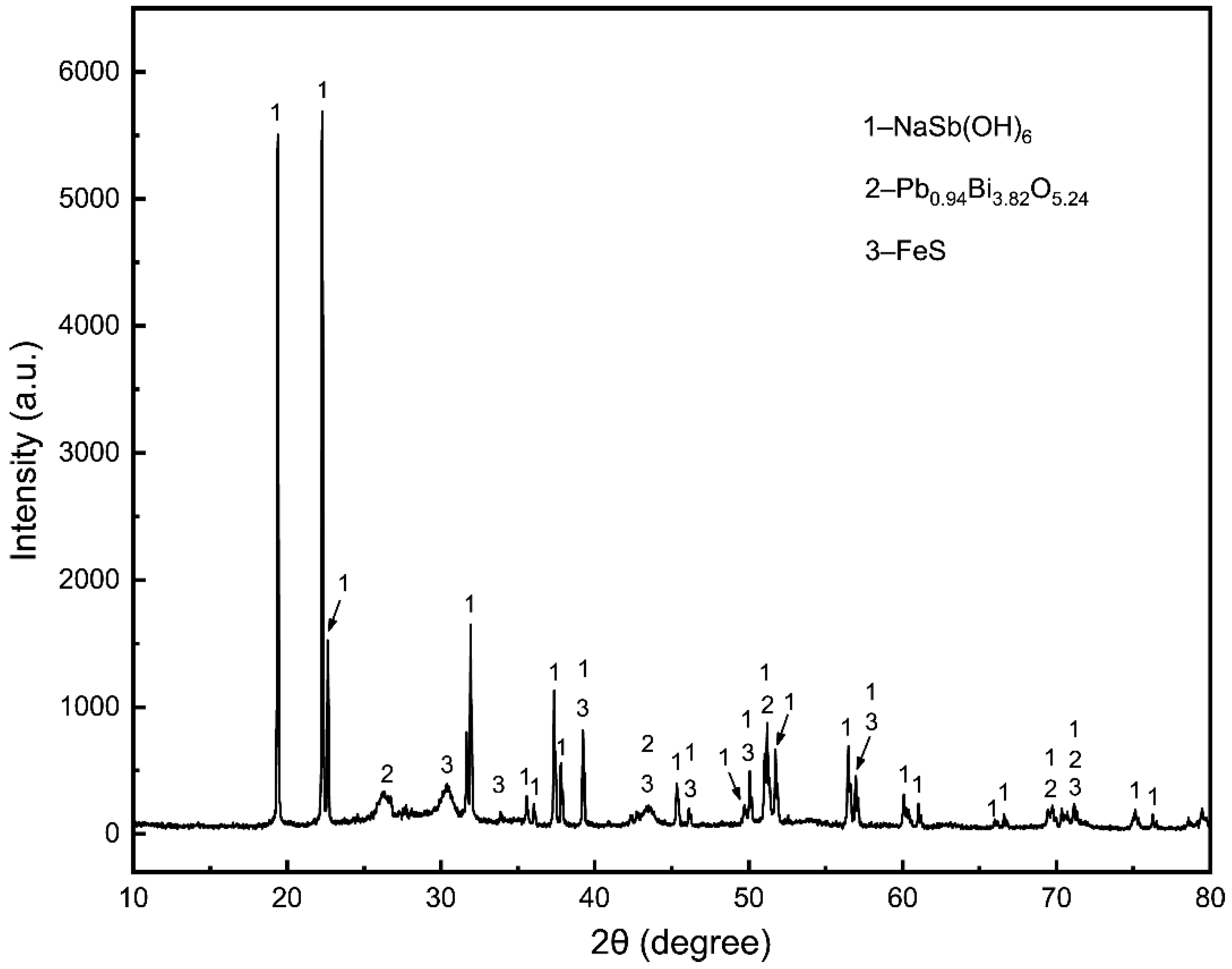
3.1. Sodium Sulfide Leaching
3.1.1. Thermodynamic Analyses
3.1.2. Different Effects on Sodium Sulfide Leaching of TSCS
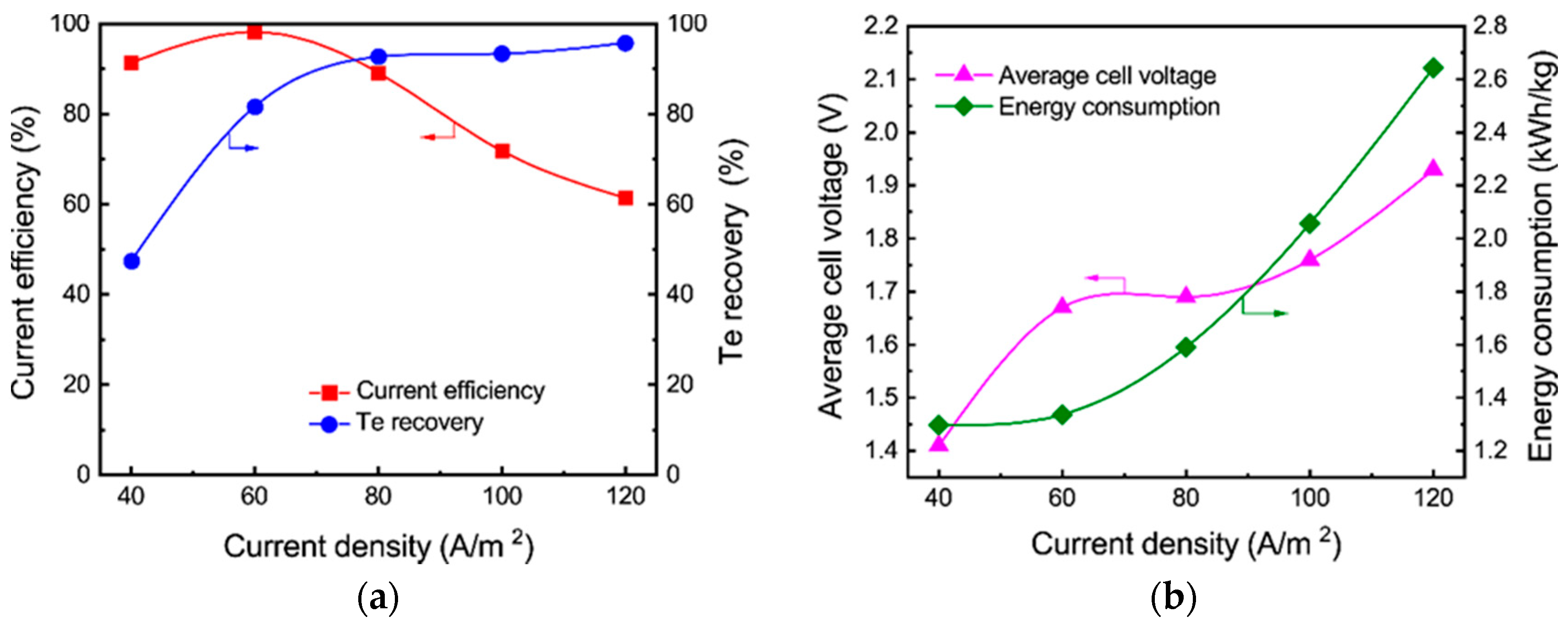
3.2. Tellurium Cyclone Electrowinning
3.2.1. Effect of Current Density
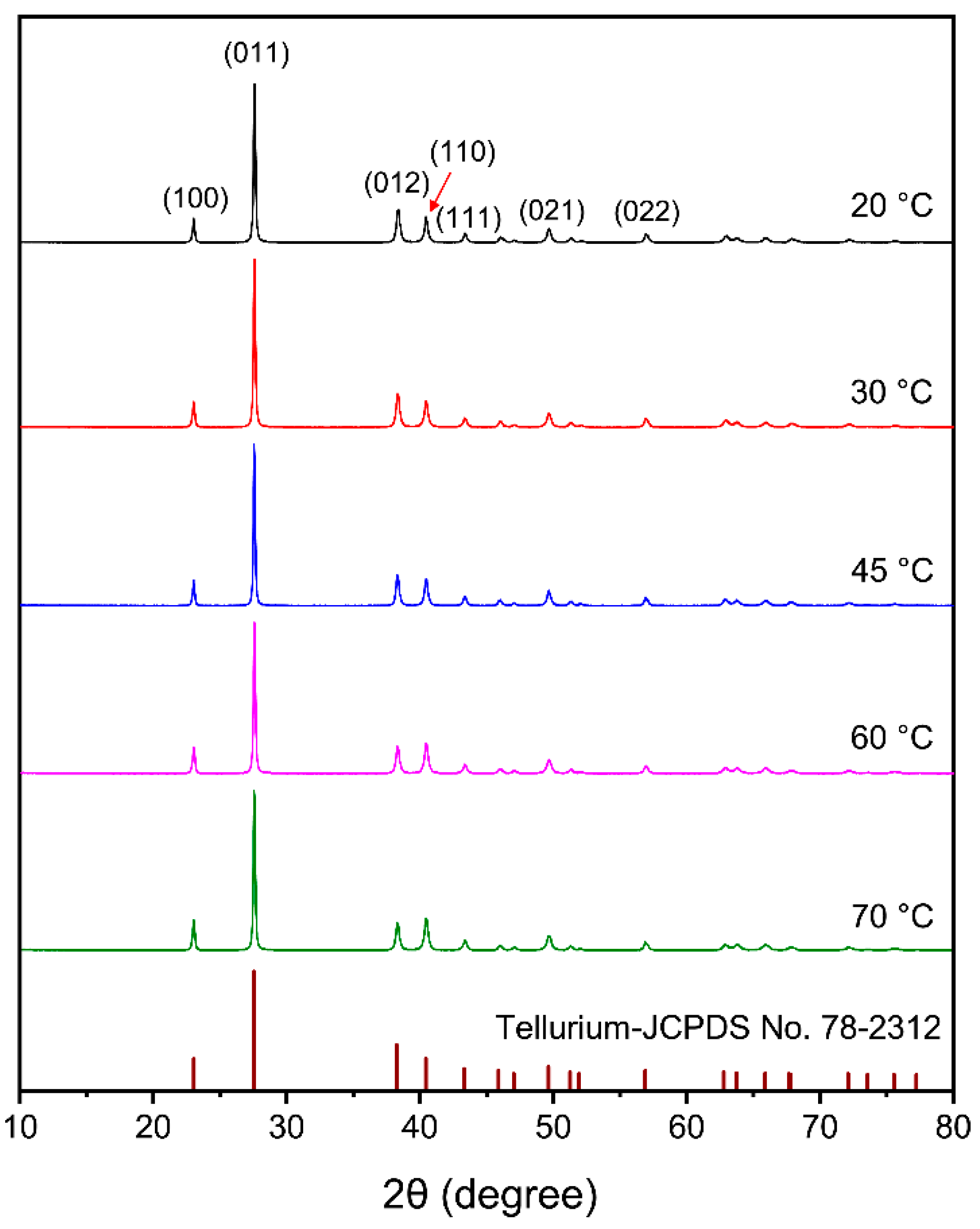
3.2.2. Effect of Electrolyte Temperature
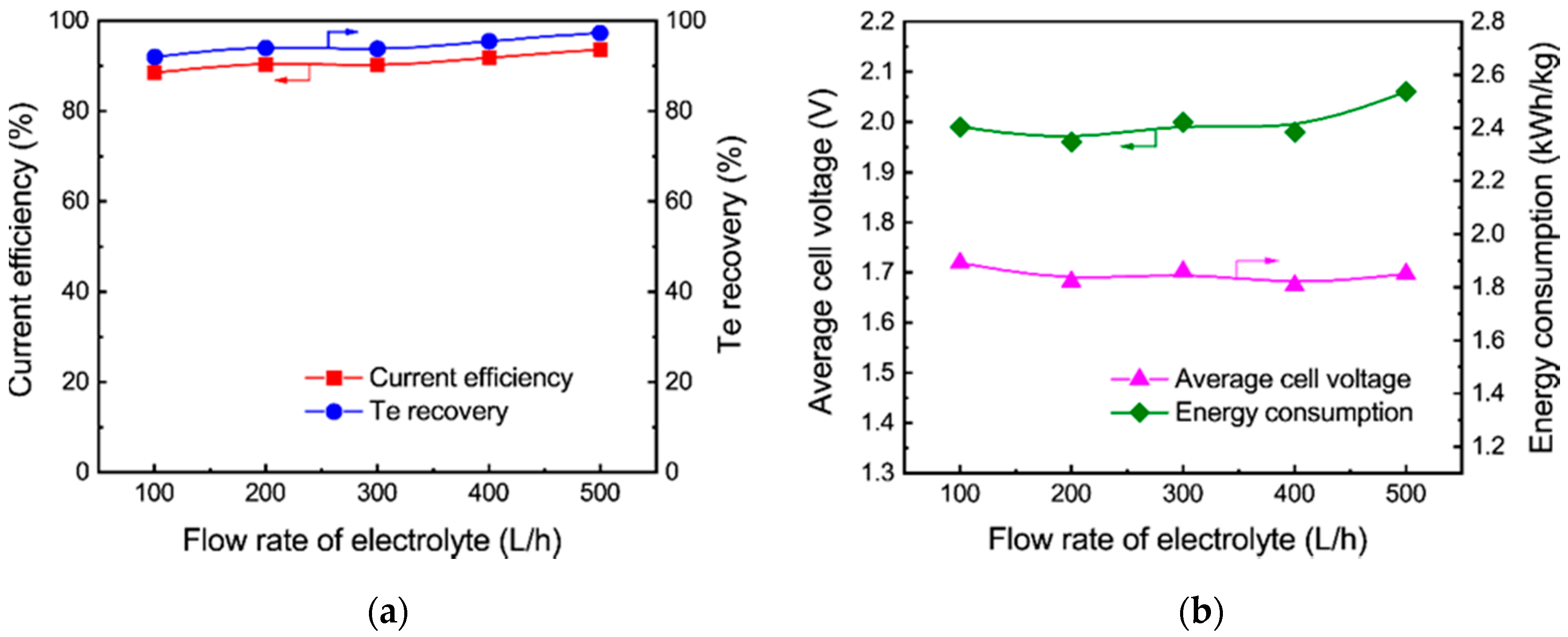
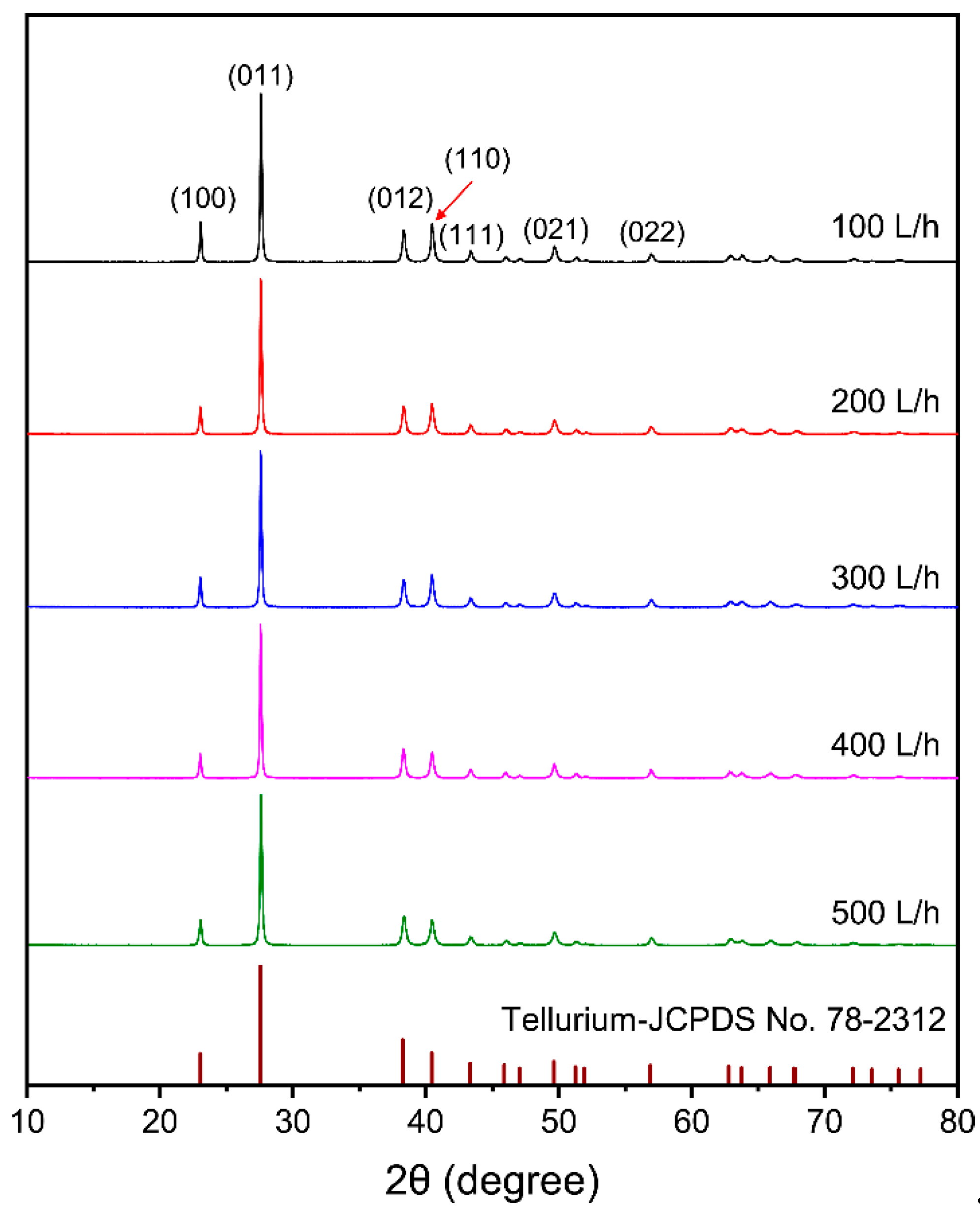
3.2.3. Effect of Flow Rate of the Electrolyte
4. Conclusions
- (1)
- In this study, an efficient and selective process was proposed to recover tellurium from TSCS by sodium sulfide leaching followed by cyclone electrowinning;
- (2)
- A total of 88% of tellurium was selectively extracted in 40 g/L Na2S solution at 50 °C for 60 min with liquid to solid ratio of 8:1 mL/g, while antimony, lead and bismuth were enriched in the leaching residue;
- (3)
- Tellurium in the sodium sulfide leach liquor of the TSCS was directly electrowon by cyclone electrowinning. The optimized experimental conditions were found to be 80 A/m2 of current density, 45 °C of electrolyte temperature and 400 L/h of electrolyte flow rate. Under these conditions, the tellurium concentration in the leach liquor decreased from 12.81 to 0.62 g/L. A total of 91.81% of current efficiency and 95.47% of tellurium recovery were achieved. The average cell voltage and energy consumption were 1.98 V and 1.81 kWh/kg, respectively;
- (4)
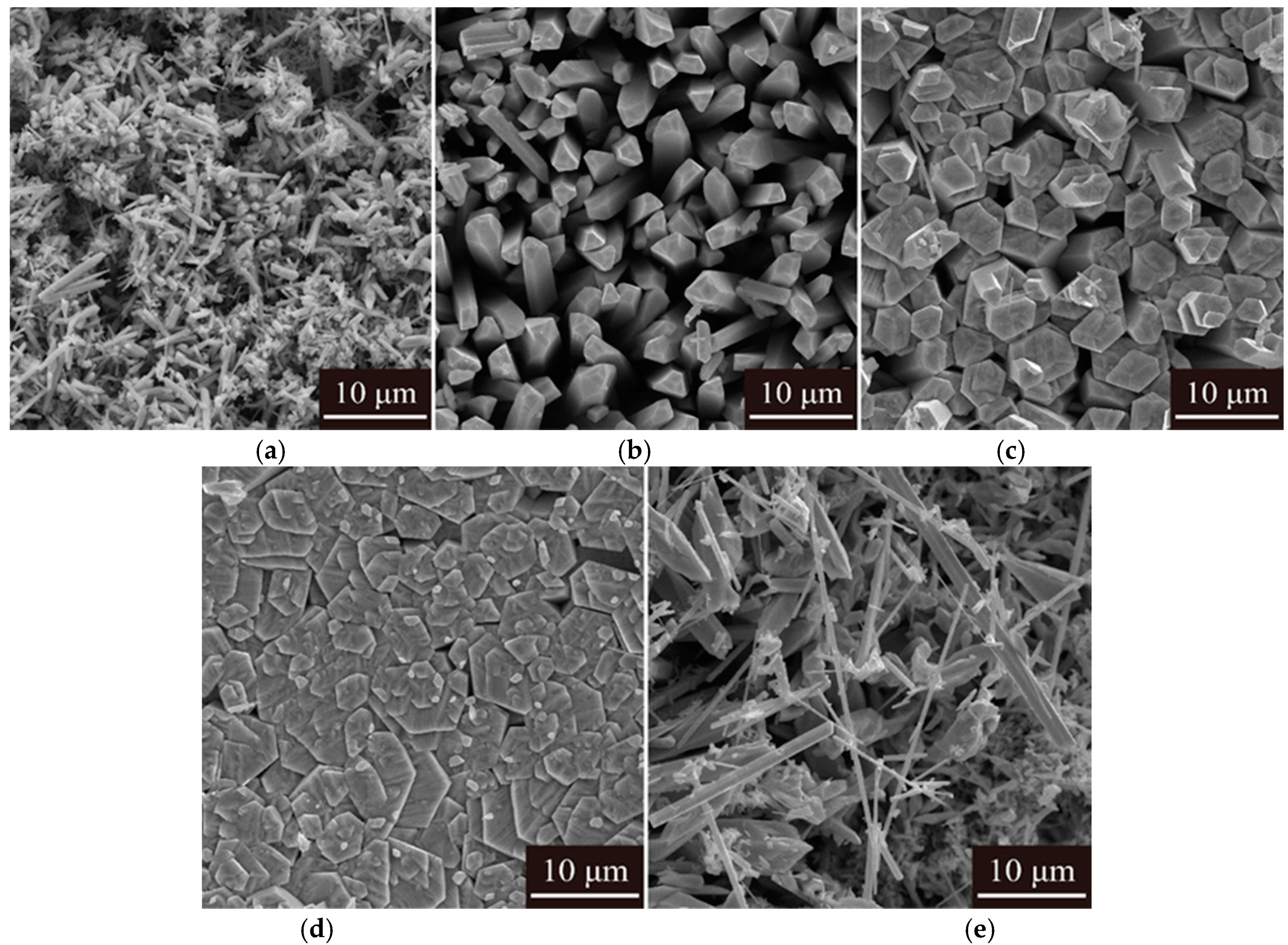
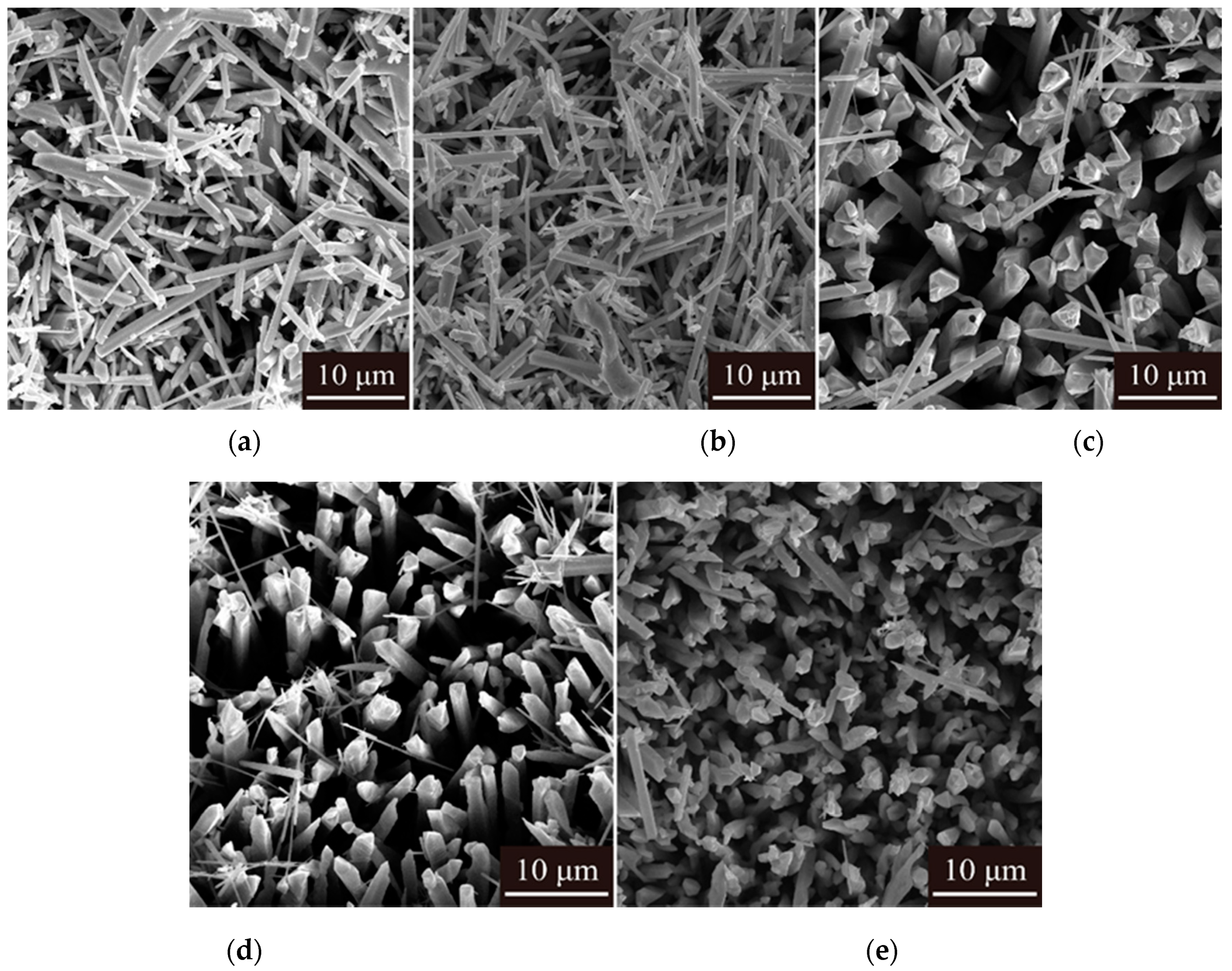
- The purity of tellurium deposits reached 99.38%. Smooth and uniform tellurium deposits with rod-like crystal were obtained. The tellurium deposits showed a strong preferred orientation of (010). By increasing the current density and electrolyte flow rate and decreasing the temperature, the growth of (110) crystal planes was suppressed.
Author Contributions
Funding
Conflicts of Interest
References
- Chivers, T.; Laitinen, R.S. Tellurium: A maverick among the chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yang, Q.; Zhang, D.; Yang, S.; Fu, J. The effect of tellurium on the formation of MnTe-MnS composite inclusions in non-quenched and tempered steel. Metals 2018, 8, 639. [Google Scholar] [CrossRef]
- Huang, R.; Jiao, L.; Li, M.; Zhu, D. Effect of dilute tellurium and selenium additions on the high-temperature oxidation resistance of copper alloys. Oxid. Met. 2018, 89, 141–149. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E. Dynamic analysis of the global metals flows and stocks in electricity generation technologies. J. Clean Prod. 2013, 59, 260–273. [Google Scholar] [CrossRef]
- Salim, H.K.; Stewart, R.A.; Sahin, O.; Dudley, M. Drivers, barriers and enablers to end-of-life management of solar photovoltaic and battery energy storage systems: A systematic literature review. J. Clean Prod. 2019, 211, 537–554. [Google Scholar] [CrossRef]
- Kavlak, G.; Graedel, T.E. Global anthropogenic tellurium cycles for 1940–2010. Resour. Conserv. Recycl. 2013, 76, 21–26. [Google Scholar] [CrossRef]
- Ahmad, K.; Afzaal, M.; Ritch, J.S.; Chivers, T.; O’Brien, P. Epitaxial CdTe rods on Au/Si Islands from a molecular compound. J. Am. Chem. Soc. 2010, 132, 5964–5965. [Google Scholar] [CrossRef]
- Bauer, D.; Diamond, D.; Li, J.; McKittrick, M.; Sandalow, D.; Telleen, P.U.S. Department of Energy Critical Materials Strategy; Department of Energy: Washington, DC, USA, 2011.
- Guo, X.Y.; Xu, Z.P.; Li, D.; Tian, Q.H.; Xu, R.Z.; Zhang, Z. Recovery of tellurium from high tellurium-bearing materials by alkaline sulfide leaching followed by sodium sulfite precipitation. Hydrometallurgy 2017, 171, 355–361. [Google Scholar] [CrossRef]
- Shao, L.; Diao, J.; Ji, C.; Li, G. A novel and clean process for extracting tellurium and bismuth from Dashuigou tellurium ore by oxidizing leaching. Hydrometallurgy 2020, 191, 105205. [Google Scholar] [CrossRef]
- Lee, J.C.; Kurniawan, K.; Chung, K.W.; Kim, S. Metallurgical process for total recovery of all constituent metals from copper anode slimes: A review of established technologies and current progress. Met. Mater. Int. 2020. [Google Scholar] [CrossRef]
- Ludvigsson, B.M.; Larsson, S.R. Anode slimes treatment: The Boliden experience. JOM 2003, 55, 41–44. [Google Scholar] [CrossRef]
- Halli, P.; Wilson, B.P.; Hailemariam, T.; Latostenmaa, P.; Yliniemi, K.; Lundstroem, M. Electrochemical recovery of tellurium from metallurgical industrial waste. J. Appl. Electrochem. 2020, 50, 1–14. [Google Scholar] [CrossRef]
- Bonificio, W.D.; Clarke, D.R. Bacterial recovery and recycling of tellurium from tellurium-containing compounds by Pseudoalteromonas sp EPR3. J. Appl. Microbiol. 2014, 117, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.C.; Sohn, H.J.; Jeong, G.J.; Lee, C.K.; Rhee, K.I. Electrowinning of tellurium from alkaline leach liquor of cemented Te. J. Appl. Electrochem. 2000, 30, 315–322. [Google Scholar] [CrossRef]
- Rhee, K.I.; Lee, C.K.; Ha, Y.C.; Jeong, G.J.; Kim, H.S.; Sohn, H.J. Tellurium recovery from cemented tellurium with minimum waste disposal. Hydrometallurgy 1999, 53, 189–201. [Google Scholar] [CrossRef]
- Shuva, M.A.H.; Rhamdhani, M.A.; Brooks, G.A.; Masood, S.; Reuter, M.A. Thermodynamics data of valuable elements relevant to e-waste processing through primary and secondary copper production: A review. J. Clean Prod. 2016, 131, 795–809. [Google Scholar] [CrossRef]
- Sun, Z.M.; Zheng, Y.J. Preparation of high pure tellurium from raw tellurium containing Cu and Se by chemical method. Trans. Nonferrous Met. Soc. China 2011, 21, 665–672. [Google Scholar] [CrossRef]
- Awe, S.A.; Sundkvist, J.E.; Bolin, N.J.; Sandstrom, A. Process flowsheet development for recovering antimony from Sb-bearing copper concentrates. Miner. Eng. 2013, 49, 45–53. [Google Scholar] [CrossRef]
- Celep, O.; Alp, İ.; Deveci, H. Improved gold and silver extraction from a refractory antimony ore by pretreatment with alkaline sulphide leach. Hydrometallurgy 2011, 105, 234–239. [Google Scholar] [CrossRef]
- Barr, N.; De Denus, R.N.; Treasure, P.A. Mineral recovery apparatus. U.S. Patent 5,529,672, 25 June 1996. [Google Scholar]
- Jin, W.; Hu, M.Q.; Hu, J.G. Selective and efficient electrochemical recovery of dilute copper and tellurium from acidic chloride solutions. ACS Sustain. Chem. Eng. 2018, 6, 13378–13384. [Google Scholar] [CrossRef]
- Hong, J.B.; Xu, Z.P.; Li, D.; Guo, X.Y.; Yu, D.W.; Tian, Q.H. A pilot study: Efficient electrowinning of tellurium from alkaline solution by cyclone electrowinning technology. Hydrometallurgy 2020, 196, 105429. [Google Scholar] [CrossRef]
- Guo, X.Y.; Qin, H.; Tian, Q.H.; Li, D. Recovery of metals from waste printed circuit boards by selective leaching combined with cyclone electrowinning process. J. Hazard. Mater. 2020, 384, 121355. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Xue, Y.D.; Su, J.L.; Zheng, S.L.; Lei, H.; Cai, W.Q.; Jin, W. Efficient electrochemical recovery of dilute selenium by cyclone electrowinning. Hydrometallurgy 2018, 179, 232–237. [Google Scholar] [CrossRef]
- Jin, W.; Su, J.L.; Chen, S.F.; Li, P.; Moats, M.S.; Maduraiveeran, G.; Lei, H. Efficient electrochemical recovery of fine tellurium powder from hydrochloric acid media via mass transfer enhancement. Sep. Purif. Technol. 2018, 203, 117–123. [Google Scholar] [CrossRef]
- Xu, Z.P.; Guo, X.Y.; Tian, Q.H.; Li, D.; Zhang, Z.; Zhu, L. Electrodeposition of tellurium from alkaline solution by cyclone electrowinning. Hydrometallurgy 2020, 193, 105316. [Google Scholar] [CrossRef]
- Benmadani, Y.; Kermaoui, A.; Chalal, M.; Khemici, W.; Kellou, A.; Pellé, F. Erbium doped tellurite glasses with improved thermal properties as promising candidates for laser action and amplification. Opt. Mater. 2013, 35, 2234–2240. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, C.; Feng, Y.; Sun, L.; Ni, Y.; Xu, Z. Effects of GeO2 on the thermal stability and optical properties of Er3+/Yb3+-codoped oxyfluoride tellurite glasses. Mater. Chem. Phys. 2011, 126, 786–790. [Google Scholar] [CrossRef]
- Christie, A.B.; Sutherland, I.; Walls, J.M. Studies of the composition, ion-induced reduction and preferential sputtering of anodic oxide films on Hg0.8Cd0.2Te by XPS. Surf. Sci. 1983, 135, 225–242. [Google Scholar] [CrossRef]
- Wagner, C.D. Chemical shifts of auger lines, and the auger parameter. Faraday Discuss. Chem. Soc. 1975, 60, 291–300. [Google Scholar] [CrossRef]
- Anderson, C.G. The metallurgy of antimony. Chem. Erde Geochem. 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Feigl, F. Chemistry of Specific, Selective and Sensitive Reactions; Academic Press: New York, NY, USA, 1949. [Google Scholar]
- Li, B.; Wang, X.; Wei, Y.; Wang, H.; Barati, M. Extraction of copper from copper and cadmium residues of zinc hydrometallurgy by oxidation acid leaching and cyclone electrowinning. Miner. Eng. 2018, 128, 247–253. [Google Scholar] [CrossRef]
- Rudnik, E. Investigation of industrial waste materials for hydrometallurgical recovery of zinc. Miner. Eng. 2019, 139, 105871. [Google Scholar] [CrossRef]
- Handle, B.; Broderick, G.; Paschen, P. A statistical response surface study of the tellurium electrowinning process. Hydrometallurgy 1997, 46, 105–120. [Google Scholar] [CrossRef]
- Zhao, T.C. The Metallurgy of Antimony; Central South University of Technology Press: Changsha, China, 1988. [Google Scholar]
- Awe, S.A.; Sandström, Å. Electrowinning of antimony from model sulphide alkaline solutions. Hydrometallurgy 2013, 137, 60–67. [Google Scholar] [CrossRef]
- Cooper, W.C. Tellurium; Van Nostrand Reinhold: New York, NY, USA, 1971. [Google Scholar]
- Zhang, R.L.; Huang, A.D.; Guo, H.; Lu, Q.Y.; Ju, D.C.; Yang, Z.B. Preparation of zinc powders by acid electrowinning. Russ. J. Nonferrous Metals 2019, 60, 173–178. [Google Scholar] [CrossRef]
- Youbi, B.; Lghazi, Y.; Ait Himi, M.; Bimaghra, I. Nucleation and growth mechanism of tellurium electrodeposited on tin-doped indium oxide substrate. J. Appl. Electrochem. 2020, 50, 159–168. [Google Scholar] [CrossRef]
- Dobrosz-Gomez, I.; Ramos Garcia, B.D.; GilPavas, E.; Gomez Garcia, M.A. Kinetic study on HCN volatilization in gold leaching tailing ponds. Miner. Eng. 2017, 110, 185–194. [Google Scholar] [CrossRef]
- Wu, T.J.; Zhang, M.L.; Lee, K.H.; Lee, C.M.; Lee, H.K.; Choa, Y.; Myung, N.V. Electrodeposition of compact tellurium thick films from alkaline baths. J. Electrochem. Soc. 2017, 164, D82–D87. [Google Scholar] [CrossRef]
- Peng, X.; Fall, M.; Haruna, S. Sulphate induced changes of rheological properties of cemented paste backfill. Miner. Eng. 2019, 141, 105849. [Google Scholar]
- Li, R.; Huang, H.; Wang, J.J.; Liang, W.; Gao, P.; Zhang, Z.; Xiao, R.; Zhou, B.; Zhang, X. Conversion of Cu(II)-polluted biomass into an environmentally benign Cu nanoparticles-embedded biochar composite and its potential use on cyanobacteria inhibition. J. Clean Prod. 2019, 216, 25–32. [Google Scholar] [CrossRef]
- Kalliomaki, T.; Wilson, B.P.; Aromaa, J.; Lundstrom, M. Diffusion coefficient of cupric ion in a copper electrorefining electrolyte containing nickel and arsenic. Miner. Eng. 2019, 134, 381–389. [Google Scholar] [CrossRef]
- Carissimi, E.; Rubio, J. Polymer-bridging flocculation performance using turbulent pipe flow. Miner. Eng. 2015, 70, 20–25. [Google Scholar] [CrossRef]
- Carotenuto, G.; Palomba, M.; De Nicola, S.; Ambrosone, G.; Coscia, U. Structural and Photoconductivity Properties of Tellurium/PMMA Films. Nanoscale Res. Lett. 2015, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Fernando Perez, J.; Llanos, J.; Saez, C.; Lopez, C.; Canzares, P.; Andres Rodrigo, M. Towards the scale up of a pressurized-jet microfluidic flow-through reactor for cost-effective electro-generation of H2O2. J. Clean Prod. 2019, 211, 1259–1267. [Google Scholar] [CrossRef]





| Element | Sb | Te | Pb | Na | Bi | Zn | Si | Fe | Al | Se | Cu |
| Wt.% | 23.60 | 11.60 | 11.50 | 9.74 | 5.21 | 1.71 | 2.29 | 2.16 | 1.49 | 0.97 | 0.95 |
| Species | Na2TeO4 | NaSb(OH)6 | |||
| (kJ/mol) | −392.98 | −980.85 | −1487.51 | 85.66 | −157.32 |
| Species | H2O | ||||
| (kJ/mol) | −110.91 | −45.87 | −36.19 | −237.14 | −261.88 |
| Element | Sb | Pb | Na | Bi | S | Fe | Te | Si | Zn | Al | Cu | Se |
| Wt.% | 25.52 | 12.64 | 8.06 | 6.04 | 4.40 | 3.34 | 2.80 | 2.12 | 1.91 | 1.51 | 1.03 | 0.56 |
| Element | Te | Se | Cu 1 | As 1 | Sb 1 | Fe 1 | Pb 1 | Ni 1 |
| Concentration (g/L) | 12.82 | 0.47 | 22.02 | 23.34 | 12.71 | 1.56 | 0.16 | 0.13 |
| Element | Te | Se | Cu | S | Na 2 | Zn 2 | Sb 2 | Pb 2 | Ni 2 |
| wt (%) | 99.38 | 0.51 | 0.033 | 0.060 | 48.08 | 36.90 | 7.04 | 5.65 | 11.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Li, Z.; Li, D.; Guo, X.; Yang, Y.; Tian, Q.; Li, J. Selective Recovery of Tellurium from the Tellurium-Bearing Sodium Carbonate Slag by Sodium Sulfide Leaching Followed by Cyclone Electrowinning. Metals 2020, 10, 1176. https://doi.org/10.3390/met10091176
Xu Z, Li Z, Li D, Guo X, Yang Y, Tian Q, Li J. Selective Recovery of Tellurium from the Tellurium-Bearing Sodium Carbonate Slag by Sodium Sulfide Leaching Followed by Cyclone Electrowinning. Metals. 2020; 10(9):1176. https://doi.org/10.3390/met10091176
Chicago/Turabian StyleXu, Zhipeng, Zoujiang Li, Dong Li, Xueyi Guo, Ying Yang, Qinghua Tian, and Jun Li. 2020. "Selective Recovery of Tellurium from the Tellurium-Bearing Sodium Carbonate Slag by Sodium Sulfide Leaching Followed by Cyclone Electrowinning" Metals 10, no. 9: 1176. https://doi.org/10.3390/met10091176
APA StyleXu, Z., Li, Z., Li, D., Guo, X., Yang, Y., Tian, Q., & Li, J. (2020). Selective Recovery of Tellurium from the Tellurium-Bearing Sodium Carbonate Slag by Sodium Sulfide Leaching Followed by Cyclone Electrowinning. Metals, 10(9), 1176. https://doi.org/10.3390/met10091176




