Effect of Inclusions on the Corrosion Properties of the Nickel-Based Alloys 718 and EP718
Abstract
1. Introduction
2. Materials and Methods
3. Results
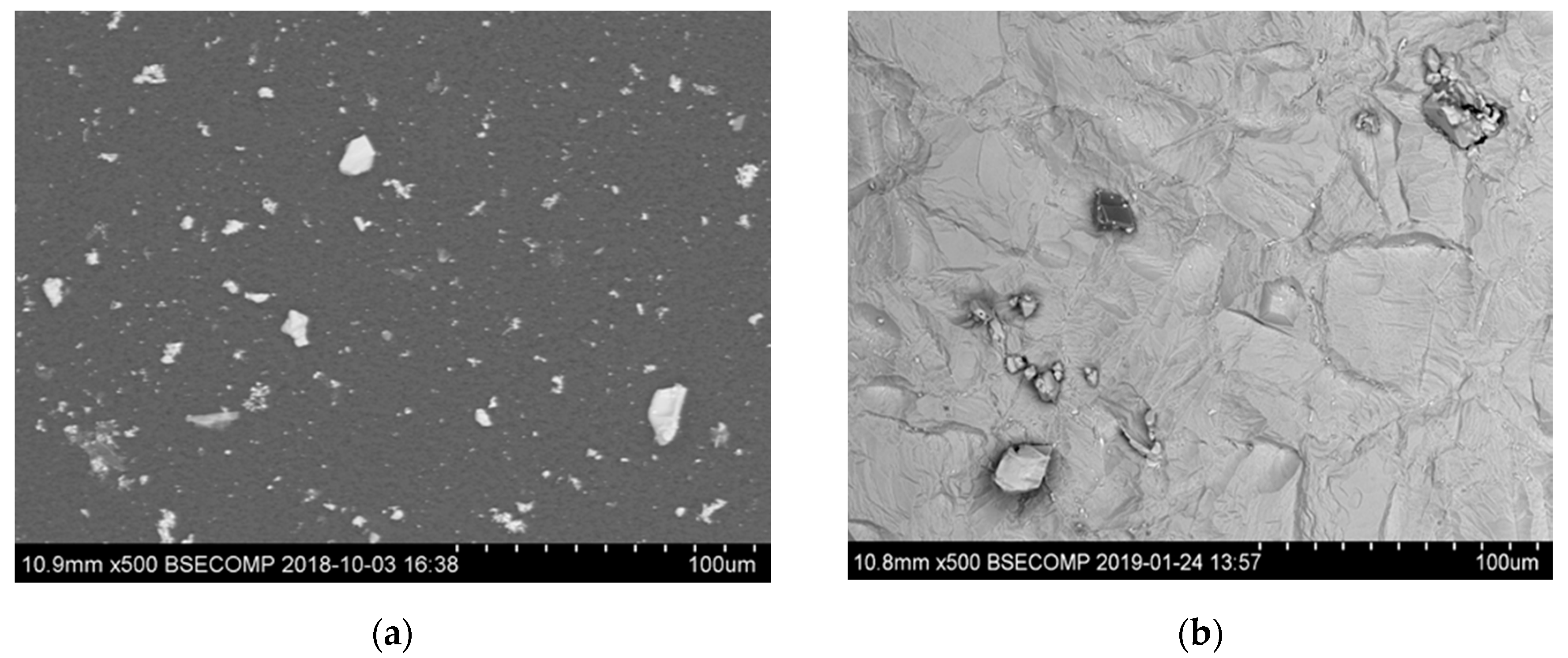
3.1. Characterization of Inclusions after Electrolytic Extraction of Metal Samples
- Irregular or regular carbides containing Nb and Ti as well as up to 1–6% of W (NbTi-C type) in the size range of 3–40 µm. The ratio of Nb and Ti of these carbides (RNb/Ti = %Nb/%Ti) in these inclusions varied from 1.2 up to 2.4 with an average value of 1.6 ± 0.2.
- Regular Ti and Nb nitrides (TiNb-N type) also contained up to 5% of W and had a size of 4–26 µm. Moreover, sometimes these inclusions contained small amounts of carbides of these elements as well. The values of a RNb/Ti in these nitrides varied from 0.2 up to 0.6 (0.3 ± 0.2 on average) depending on the fraction of NbTi-C inclusions precipitated on nitrides.
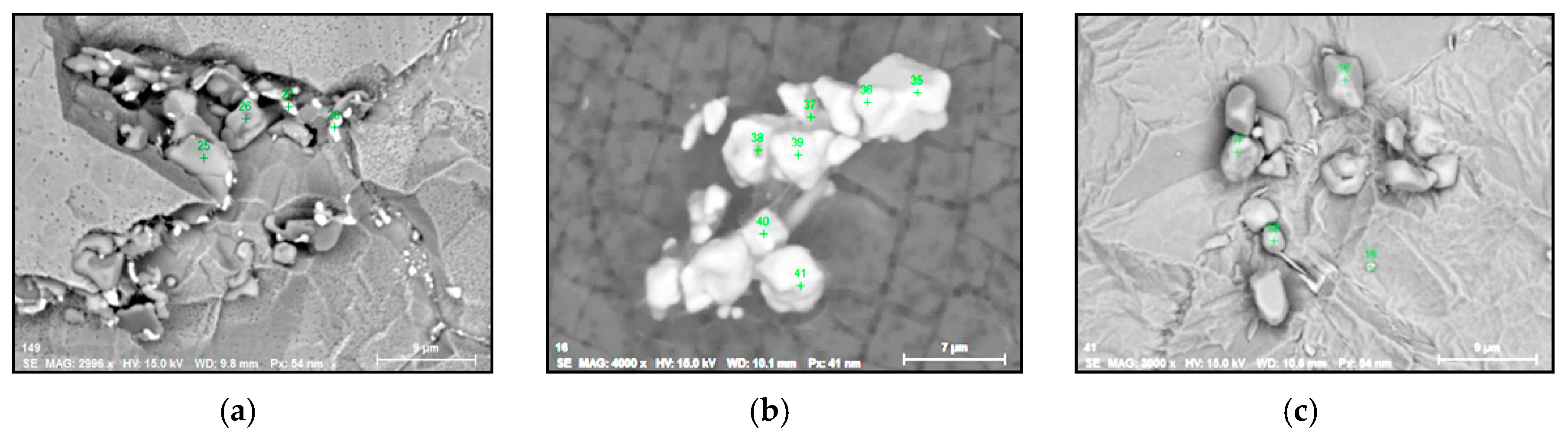
- Irregular Ti and Nb sulphides (TiNb-S type) also contained 7–21% of S, ≤ 4% of W and ≤ 3% of Mo. These sulfides are 2–37 µm in size and they were observed mostly as clusters on grain boundaries of a metal sample along the deformation direction in combination with carbides. The values of a RNb/Ti in these sulphides varied from 0.2 up to 0.7 (0.5 ± 0.2 on average) depending on the fraction of carbide inclusions. Moreover, it was found that these types of sulphides, that were detected as acicular inclusions by using a conventional 2D investigations on polished surface of metal samples, have a plate or petal-like shape with thickness 1–2 µm.
- Small size multicomponent carbides (0.5–4 µm) containing Nb, Ti, Mo, W and Cr and having different morphologies (such as spherical, irregular and acicular shapes). Length of acicular inclusions can be up to 12 µm. The contents of the main elements in these inclusions may vary (RNb/Ti = 1.4 ± 0.5). These types of inclusions were observed mostly on grain boundaries of the matrix and partially on surfaces of different inclusions. Based on the location, morphology and compositions, it can be safely assumed that these inclusions precipitated during solidification of a matrix during cooling and during heat treatment.
- Regular and irregular carbides containing Nb and Ti (NbTi-C type) and also containing 0–7% of Cr and having 2–30 µm sizes. The ratio of the Nb and Ti contents of these carbides (RNb/Ti) in these inclusions varied in a wide range (from 3.4 up to 23) and had an average value of 9.9 ± 3.7. These NbTi-C inclusions were observed on film filters and metal surfaces as separate particles as well as clusters or groups of particles located very close to each other, as shown in Figure 2.
- Large size irregular nitrides containing Ti and Nb (TiNb-N type) having sizes ranging from 9 to 27 µm in size. The value of the RNb/Ti ratio in these nitrides varied from 0.2 up to 0.9 µm (0.3 ± 0.2 on average). These nitride inclusions were usually located in the matrix as separate particles.
- Small size carbides (0.5–8 µm) containing mostly Nb, Ti and 1–3% Cr (NbTi-C) and having spherical, irregular or acicular shapes, as shown in Figure 2. The value of the RNb/Ti ratio in these nitrides varied from 5.6 up to 14.9 µm (8.6 ± 2.1 µm on the average). These small size carbides were usually located on grain boundaries of the matrix and sometimes on surfaces of different inclusions. It was assumed that these small carbides precipitated during solidification of in a solidified the matrix as well as during heat treatment.
3.2. Effect of Inclusions on Corrosion Resistance
4. Discussion
- Titanium sulphides have the most negative effect on the matrix dissolution;
- Carbides, especially at the boundaries, containing chromium, molybdenum and tungsten in their composition cause a significant dissolution of the matrix around the inclusions;
- Large (more than 10 µm) carbides and nitrides of titanium and niobium are also able to reduce the corrosion resistance.
5. Conclusions
- The electrolytic extraction technique can successfully be applied for three-dimensional (3D) investigations of different inclusions on film filters and surfaces of metal samples after extraction of Ni-based alloys. It was shown that the morphology of inclusions is much more complicated than what can be determined on a flat section. For instance, a thin plate-like sulphides of Ti and Nb (with a thickness of 1–2 µm) that were detected in clusters (up to 37 µm) are located mostly on grain boundaries in the EP718 alloy. However, these which were detected only as separate acicular sulphides by using conventional two-dimensional (2D) investigations on polished surfaces of this metal sample.
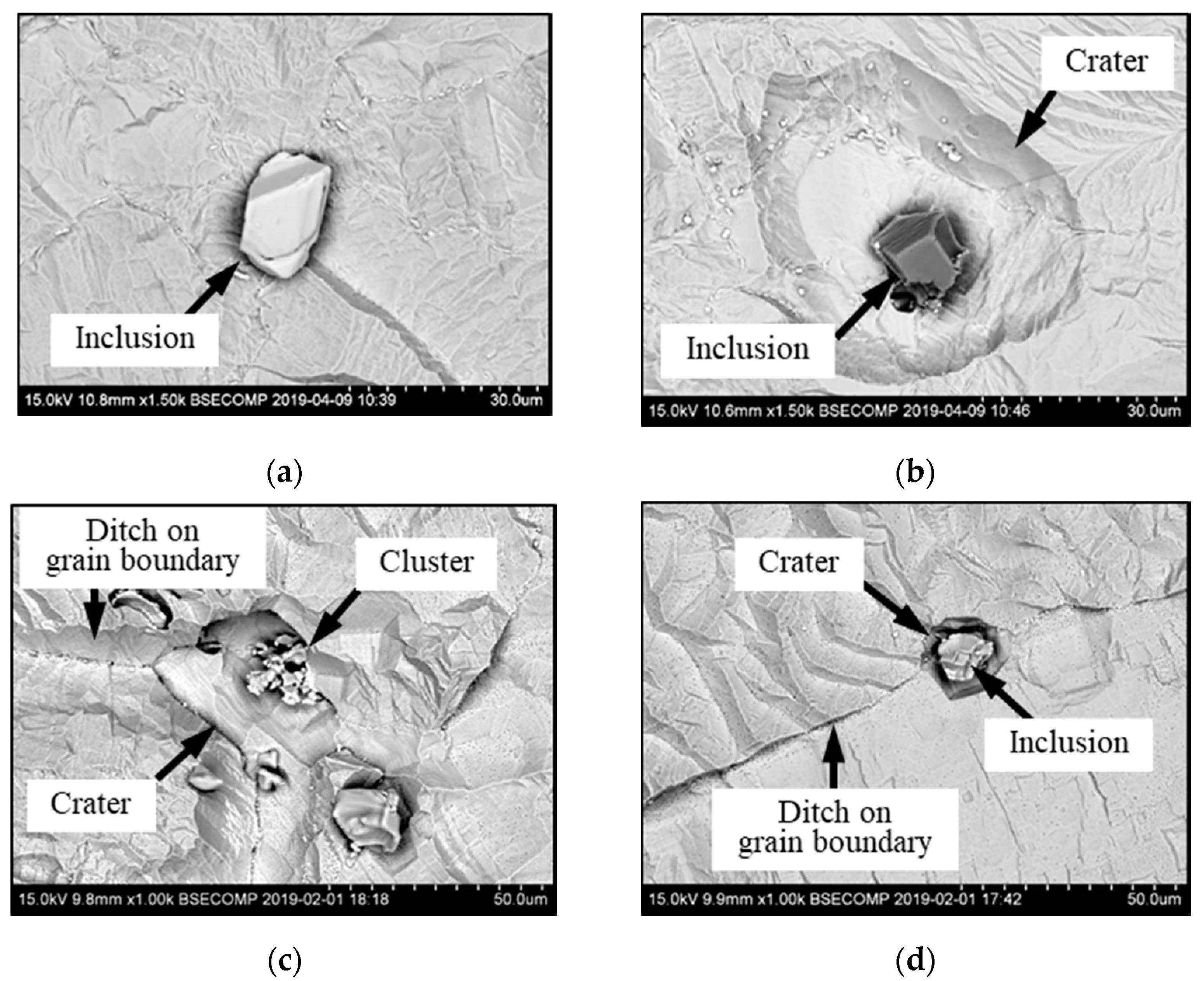
- An evaluation of different extents of dissolution of the matrix around different inclusions after EE by determination of equivalent diameter of “crater” (Dcr) and relative coefficient of the matrix dissolution (KD) makes it possible to estimate a metal weakening around investigated inclusions, which correlates to a corrosion resistance of metal.
- In the EP718 alloy, four types of inclusions were found. These are listed according to the higher degree of harmful influence on the corrosion resistance of the alloy: TiNb-S sulphides, NbTi-C carbides, small size multicomponent carbides (NbTiMoWCr-C) and TiNb-N nitrides. Three types of typical inclusions were found in alloy 718, namely NbTi-C carbides, TiNb-N nitrides and small size carbides (NbTiCr-C). In addition to separate inclusions, clusters (up to 40 µm) consisting of different inclusions were found in both alloys.
- The most harmful effects of inclusions on dissolution of the matrix were found to occur for the sulphides (TiNb-S) and small carbides (NbTiMoWCr-C) located on grain boundaries. The large carbides (NbTi-C) and nitrides (TiNb-N) located as on grain boundaries as well inside of grains have less harmful influence. The nitride inclusions (TiNb-N) having sizes larger than 10 µm can also significantly reduce the corrosion resistance of Ni-based alloys, although in the literature they are described as the most neutral with respect to the influence on the matrix.
- In addition to the composition of the inclusion, their location (at the boundary or in the grain) and size also affect the corrosion resistance and a pitting propagation. Inclusions located at the grain boundaries reduce the corrosion resistance the most. For instance, the dissolution parameters (KD and Dcr) for inclusions located on grain boundaries are from 2.1 to 2.7 times larger than those for inclusions located inside the grains for both investigated alloys. Large inclusions of more than 10 µm affect the corrosion resistance more significantly even if they mostly are neutral nitrides inclusions.
Author Contributions
Funding
Conflicts of Interest
References
- Kazakov, A.A. Nonmetallic Inclusions in Steel—Origin, Estimation, Interpretation and Control. Microsc. Microanal. 2016, 22, 1938–1939. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, Y. Inclusions in Stainless Steels—A Review. Steel Res. Int. 2017, 88, 1700130. [Google Scholar] [CrossRef]
- Ånmark, N.; Karasev, A.; Jönsson, P.G. The Influence of Microstructure and Non-Metallic Inclusions on the Machinability of Clean Steels. Steel Res. Int. 2016, 88, 1600111. [Google Scholar] [CrossRef]
- Amezhnov, A.V.; Rodionova, I.G.; Zaitsev, A.I.; Mogutnov, B.M.; Baklanova, O.N. Influence of the chemical composition of non-metallic inclusions on corrosion resistance of carbon and low-alloy steels in aqueous media typical for service conditions of oil-field pipelines. Probl. Ferr. Metal. Mater. Sci. 2018, 3, 81–90. [Google Scholar]
- Cyril, N.; Fatemi, A.; Cryderman, B. Effects of Sulfur Level and Anisotropy of Sulfide Inclusions on Tensile, Impact, and Fatigue Properties of SAE 4140 Steel. SAE Int. J. Mater. Manuf. 2008, 1, 218–227. [Google Scholar] [CrossRef]
- Kazakov, A.A.; Zhitenev, A.; Kovalev, P. Distribution Pattern of Nonmetallic Inclusions on a Cross Section of Continuous-Cast Steel Billets for Rails. Microsc. Microanal. 2015, 21, 1751–1752. [Google Scholar] [CrossRef]
- Bettini, E.; Kivisäkk, U.; Leygraf, C.; Pan, J. Study of corrosion behavior of a 22% Cr duplex stainless steel: Influence of nano-sized chromium nitrides and exposure temperature. Electrochim. Acta 2013, 113, 280–289. [Google Scholar] [CrossRef]
- Knyazeva, M.; Pohl, M. Duplex Steels. Part II: Carbides and Nitrides. Met. Microstruct. Anal. 2013, 2, 343–351. [Google Scholar] [CrossRef]
- Muto, I.; Ito, D.; Hara, N. Microelectrochemical Investigation on Pit Initiation at Sulfide and Oxide Inclusions in Type 304 Stainless Steel. J. Electrochem. Soc. 2009, 156, C55. [Google Scholar] [CrossRef]
- Shakhmatov, A.V.; Badrak, R.P.; Kolesov, S.S.; Kharkov, A.A. Influence of structure on the corrosion properties of high manganese high nitrogen stainless steels. In Proceedings of the European Corrosion Congress 2015 (EUROCORR’ 2015), Graz, Austria, 6–10 September 2015. [Google Scholar]
- Matsuura, H.; Choi, W.; Kamimura, G. Evolution of Non-metallic Inclusions in Solid Fe–Al–Ti–N Alloy during Heating. In Proceedings of the 8th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 26 February–2 March 2017; Hwang, J.Y., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Urbano, M.F.; Coda, A.; Beretta, S.; Cadelli, A.; Sczerzenie, F. The Effect of Inclusions on Fatigue Properties for Nitinol. In Fatigue and Fracture Metallic Medical Materials and Devices; Mitchell, M., Smith, S., Woods, T., Berg, B., Eds.; ASTM International: West Conshohocken, PA, USA, 2013; pp. 18–34. [Google Scholar] [CrossRef]
- Prillhofer, B.; Antrekowitsch, H.; Böttcher, H.; Enright, P. Nonmetallic inclusions in the secondary aluminium industry for the production of aerospace alloys. In Proceedings of the TMS Light Metal Conference, New Orleans, LA, USA, 9–13 March 2008. [Google Scholar]
- Xu, J.; Wiese, G.; John, H.; Liu, X. Oil-Grade Alloy 718 in Oil Field Drilling Applications. In Proceedings of the 7th International Symposium on Superalloy 718 and Derivatives TMS (The Minerals, Metals & Materials Society), Pittsburgh, PA, USA, 10–13 October 2010; pp. 923–932. [Google Scholar]
- Karasev, A.; Alekseeva, E.; Lukianov, A.; Jönsson, P.G. Characterization of non-metallic inclusions in corrosion-resistance nickel-based EP718 and 718 alloys by using electrolytic extraction method E3S Web Conf., 121. In Proceedings of the I International Conference “Corrosion in the Oil and Gas Industry”, Saint-Petersburg, Russia, 20–22 May 2019. [Google Scholar] [CrossRef]
- Lackner, R.; Mori, G.; Prohaska, M.; Egger, R.; Tichler, G. UNS 06626: Precipitation and sensitization. In Proceedings of the NACE Corrosion Conference 2013, Houston, TX, USA, 7–12 March 2013; pp. 24–40. [Google Scholar]
- Obasi, G.C.; Zhang, Z.; Sampath, D.; Morana, R.; Akid, R.; Preuss, M. Effect of Microstructure and Alloy Chemistry on Hydrogen Embrittlement of Precipitation-Hardened Ni-Based Alloys. Met. Mater. Trans. A 2018, 49, 1167–1181. [Google Scholar] [CrossRef]
- Pahlavanyali, S.; Wood, M.; Marchant, G. The Effect of Carbide Decomposition And Reformation on Rupture Lives of In738lc During Multiple Reheat Treatment And Degradation Cycles. In Proceedings of the TMS Conference, Orlando, FL, USA, 11–15 March 2012; pp. 463–471. [Google Scholar]
- Tormoen, G.; Sridhar, N.; Anderko, A. Localized corrosion of heat treated alloys part 1—Repassivation potential of alloy 600 as function of solution chemistry and thermal aging. Corrosion engineering. Sci. Technol. 2010, 45, 155–162. [Google Scholar]
- Garfias-Mesias, F.; Klapper, H.; Klower, J. Determination of Precursor Sites for Pitting Corrosion of UNS N07718 in Chloride Environments—Part 2. In Proceedings of the NACE Corrosion Conference 2018, Phoenix, AZ, USA, 15–19 April 2018. [Google Scholar]
- Klöwer, J.; Tarzimoghadam, Z.; Gosheva, O.; Klapper, H.S. Effect of Microstructural Particularities on the Corrosion Resistance of Nickel Alloy UNS N07718—What Really Makes the Difference? In Proceedings of the NACE Corrosion Conference 2017, New Orleans, LA, USA, 26–30 March 2017; p. 9068. [Google Scholar]
- Klapper, H.S.; Zadorozne, N.S.; Rebak, R.B. Localized corrosion of nickel alloys: Review. Acta Metall. Sin. 2017, 30, 296–305. [Google Scholar] [CrossRef]
- Mishra, A.; Richesin, D.; Rebak, R.B. Localized corrosion study of Ni-Cr-Mo alloys for oil and gas applications. In Proceedings of the NACE—International Corrosion Conference Series 2015, Dallas, TX, USA, 15–19 March 2015; p. 5802. [Google Scholar]
- Khar’Kov, A.A.; Shakhmatov, A.V.; Gyulikhandanov, E.L.; Alekseeva, E.L. Comparative Analysis of Corrosion-Resistant Alloys Inconel 718 and ÉP. Chem. Pet. Eng. 2019, 54, 771–778. [Google Scholar] [CrossRef]
- Gyulikhandanov, E.L.; Alekseeva, E.L.; Shakhmatov, A.V.; Loshachenko, A.S.; Lapechenkov, A.A. Structure and properties of nickel-based alloy EP718 in the process of manufacturing. Vopr. Mater. 2020, 4, 42–52. [Google Scholar] [CrossRef]
- Aghajani, A.; Tewes, J.; Parsa, A.B.; Hoffmann, T.; Kostka, A.; Kloewer, J. Identification of Mo-Rich M23C6 Carbides in Alloy. Met. Mater. Trans. A 2016, 47, 4382–4392. [Google Scholar] [CrossRef]
- Golenishcheva, O.; Klöwer, J.; Aghajani, A.; Oechsner, M.; Andersohn, G. Influence of Delta-phase Precipitation on the Pitting Performance of UNS N07718. In Proceedings of the NACE Corrosion Conference Proceedings 2014, Houston, TX, USA, 9–13 March 2014; p. 3895. [Google Scholar]
- Saleem, B.; Dong, H. Phase Characterization of CRA Fastener Inconel 718 in Relation of Hydrogen Assisted Cracking. In Proceedings of the Materials Today, Swindon, UK, 10–11 July 2015; pp. 383–392. [Google Scholar] [CrossRef]
- Liu, L.; Tanaka, K.; Hirose, A.; Kobayashi, K.F. Effects of precipitation phases on the hydrogen embrittlement sensitivity of Inconel. Sci. Technol. Adv. Mater. 2002, 3, 335–344. [Google Scholar] [CrossRef]
- Badrak, R.; Howie, W.; Delacruz, A.; Kolesov, S. Characterization of direct metal laser sintered alloy 718 in the as-fabricated and heat treated condition. In Proceedings of the NACE—International Corrosion 2018 Conference, Phoenix, AZ, USA, 15–19 April 2018; p. 11297. [Google Scholar]
- Labre, C.; Pinto, A.; Solorzano, I. Microstructure Evolution of Ni-base Superalloy 625: From Conventional Thermomechanical Processed to Selective Laser Melting Processed. Microsc. Microanal. 2017, 23 (Suppl. 1), 2250–2251. [Google Scholar] [CrossRef][Green Version]
- Inoue, R.; Kiyokawa, K.; Tomoda, K.; Ueda, S.; Ariyama, T. Three-Dimensional Estimation of Multi-Component Inclusion Particle in Steel. In Proceedings of the 8th International Workshop on Progress in Analytical Chemistry and Materials Characterisation in the Steel and Metal Industries (CETAS-2011), Luxembourg, 17–19 May 2011; pp. 118–125. [Google Scholar]
- Sims, T.; Stoloff, N.S.; Hagel, W.C. Superalloys II; John Wiley & Sons: New York, NY, USA, 1987; p. 640. [Google Scholar]
- Davis, J.R. (Ed.) ASM Specialty Handbook, Nickel, Cobalt and Their Alloys; ASM International: Materials Park, PH, USA, 2000; p. 442. [Google Scholar]


| Alloy | C Max | Ni | Cr | Mo | Nb | Ti | W | Al | P Max | S Max |
|---|---|---|---|---|---|---|---|---|---|---|
| EP718 | 0.1 | 43–47 | 14–16 | 4.0–5.2 | 0.8–1.5 | 1.8–2.4 | 2.5–3.5 | 0.9–1.4 | 0.01 | 0.015 |
| Alloy 718 | 0.045 | 50–55 | 17–21 | 2.8–3.3 | 4.8–5.2 | 0.8–1.2 | - | 0.4–0.6 | 0.01 | 0.01 |
| Type | SEM Image on Film Filter | SEM Image on Metal Surface | Composition (Mass. %) | Size (µm) |
|---|---|---|---|---|
| NbTi-C (inclusions and clusters) | 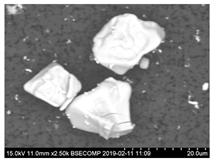 |  | 50–70% Nb, 24–45% Ti, 1–6% W | 3–40 |
| TiNb-N, TiNb-NC (inclusions and clusters) |  |  | 48–83% Ti, 2–42% Nb, 0–5% W | 4–26 |
| TiNb-S, TiNb-SC (inclusions and clusters) | 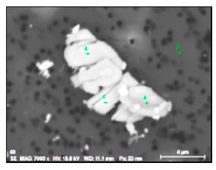 |  | 40–71% Ti, 14–33% Nb, 0–4% W, 0–3% Mo, 7–21% S | 2–37 |
| NbTiMoWCr-C | 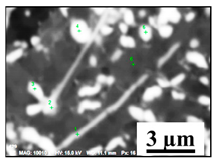 |  | 8–57% Nb, 3–42% Ti, 0–38% Mo, 0–26% W, 3–25% Cr | 0.5–12 |
| Type | SEM Image on Film Filter | SEM Image on Metal Surface | Composition (Mass. %) | Size (µm) |
|---|---|---|---|---|
| NbTi-C (inclusions and clusters) | 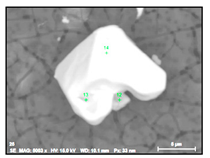 |  | 72–96% Nb, 2–16% Ti, 0–7% Cr | 2–30 |
| TiNb-N | 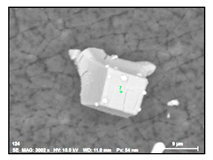 | 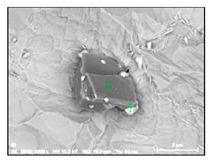 | 59–79% Ti, 9–39% Nb | 9–27 |
| NbTiCr-C | 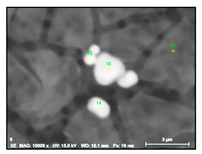 | 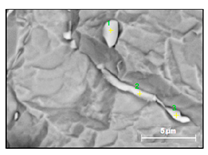 | 78–93% Nb, 6–15% Ti, 1–3% Cr | 0.5–8 |
| Alloy | Type of Inclusion | Average RNb/Ti (= %Nb/%Ti) | Size Range of Inclusions (µm) | Size of Crater, Dcr (µm) | Average KD |
|---|---|---|---|---|---|
| EP718 | NbTi-C | 1.6 ± 0.2 | 3–40 | 6–24 (16–40) * | 2.4 ± 1.1 (4.9 ± 3.7) * |
| TiNb-N | 0.3 ± 0.2 | 4–26 | 9–10 (-) | 2.8 ± 0.9 (-) | |
| TiNb-S | 0.3 ± 0.2 | 2–37 (-) | - (15–40) | - (8.8 ± 3.6) | |
| NbTiMoWCr-C | 1.4 ± 0.5 | 0.5–12 | - (8–12) | - (21.3 ± 7.7) | |
| Alloy 718 | NbTi-C | 9.9 ± 3.7 | 2–30 | 6–21 (11–40) | 2.2 ± 0.8 (5.1 ± 2.7) |
| TiNb-N | 0.3 ± 0.2 | 9–27 | 17–26 (24–36) | 2.4 ± 0.1 (6.5 ± 5.5) | |
| NbTiCr-C | 8.6 ± 2.1 | 0.5–8 | - (2–7) | - (5.2 ± 3.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alekseeva, E.; Karasev, A.; Jönsson, P.G.; Alkhimenko, A. Effect of Inclusions on the Corrosion Properties of the Nickel-Based Alloys 718 and EP718. Metals 2020, 10, 1177. https://doi.org/10.3390/met10091177
Alekseeva E, Karasev A, Jönsson PG, Alkhimenko A. Effect of Inclusions on the Corrosion Properties of the Nickel-Based Alloys 718 and EP718. Metals. 2020; 10(9):1177. https://doi.org/10.3390/met10091177
Chicago/Turabian StyleAlekseeva, Ekaterina, Andrey Karasev, Pär G. Jönsson, and Aleksey Alkhimenko. 2020. "Effect of Inclusions on the Corrosion Properties of the Nickel-Based Alloys 718 and EP718" Metals 10, no. 9: 1177. https://doi.org/10.3390/met10091177
APA StyleAlekseeva, E., Karasev, A., Jönsson, P. G., & Alkhimenko, A. (2020). Effect of Inclusions on the Corrosion Properties of the Nickel-Based Alloys 718 and EP718. Metals, 10(9), 1177. https://doi.org/10.3390/met10091177





