Investigation on Direct-to-Blister Smelting of Chalcocite via Thermodynamics and Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
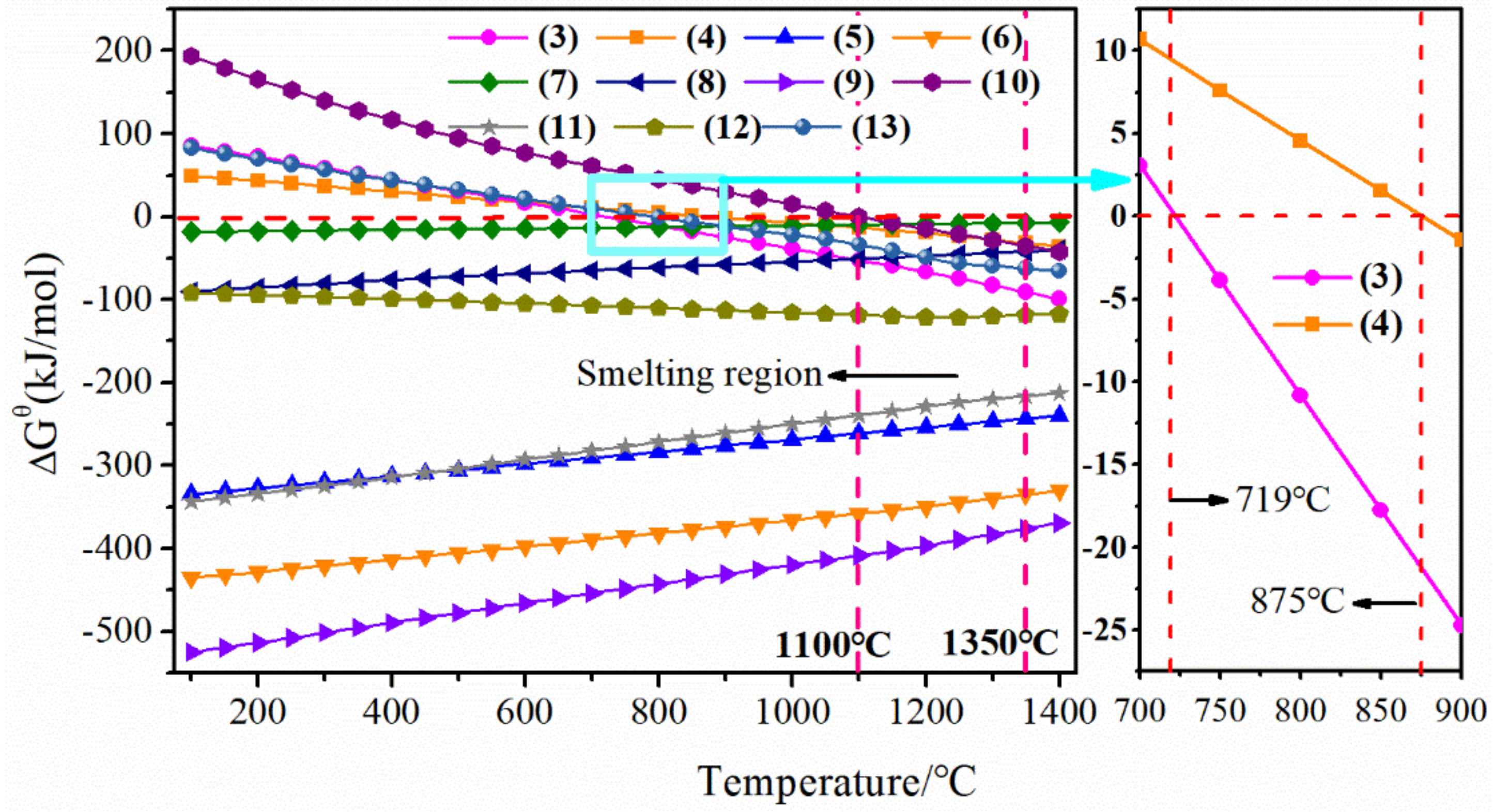
3.1. Thermodynamic Analysis
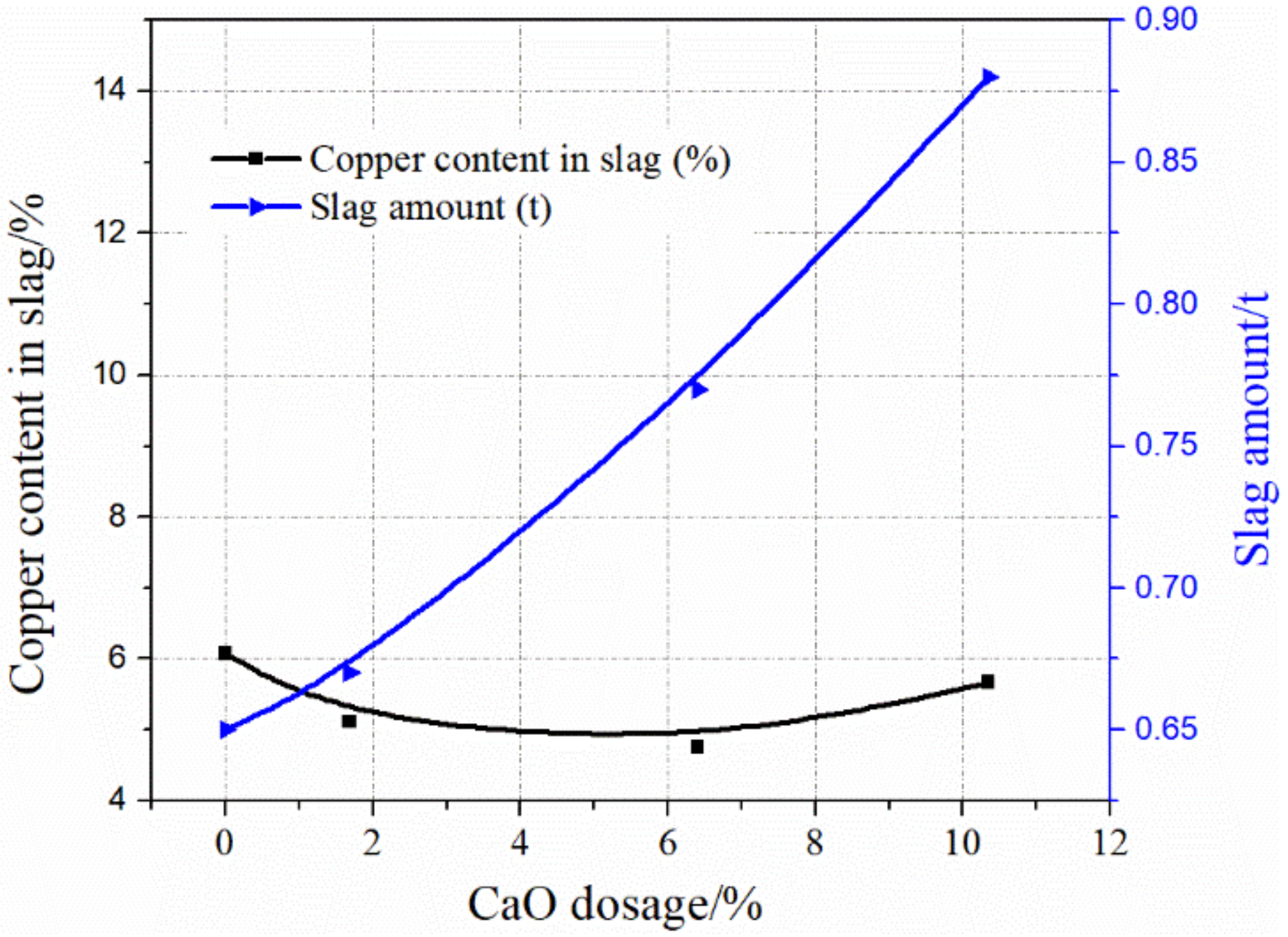
3.2. Slag Analysis
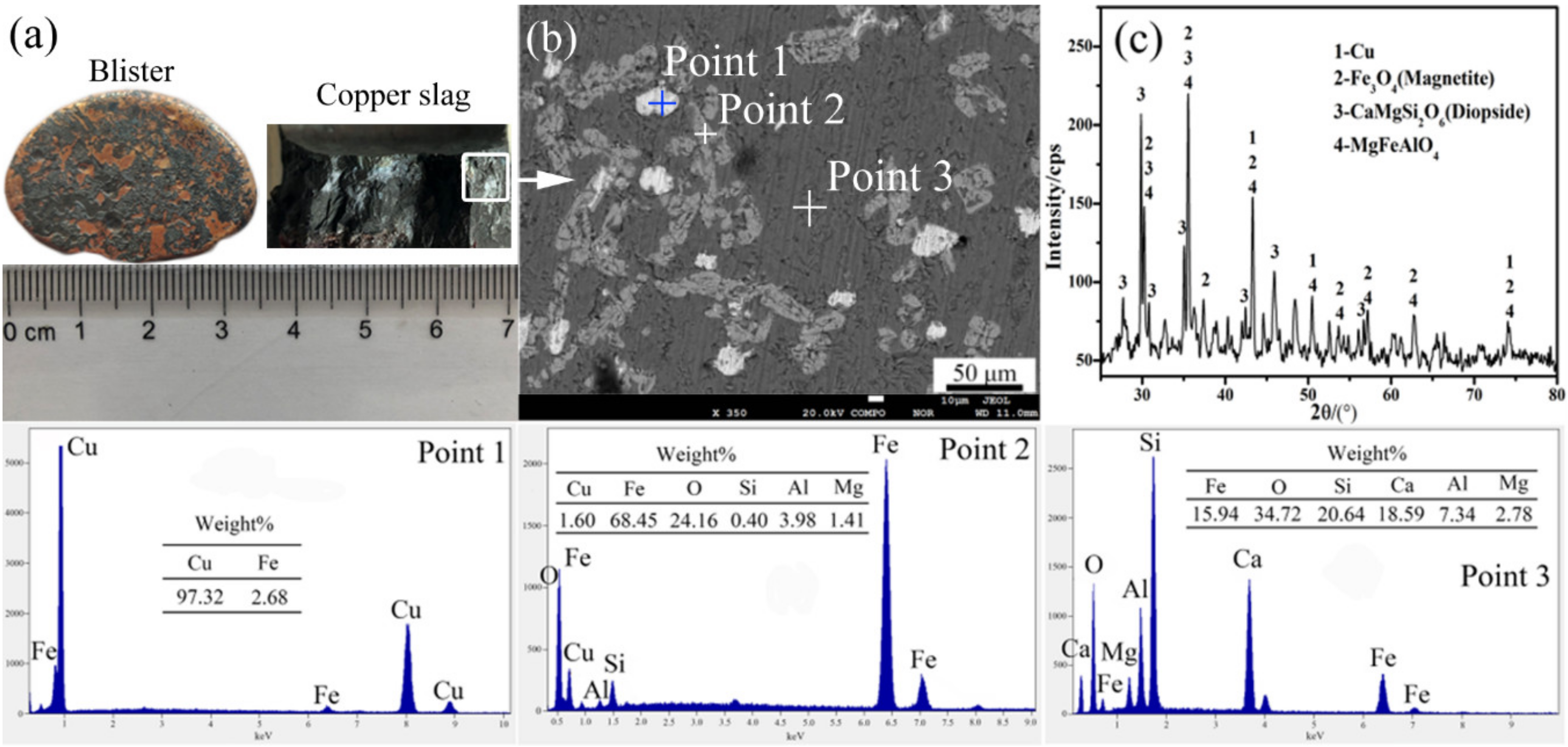
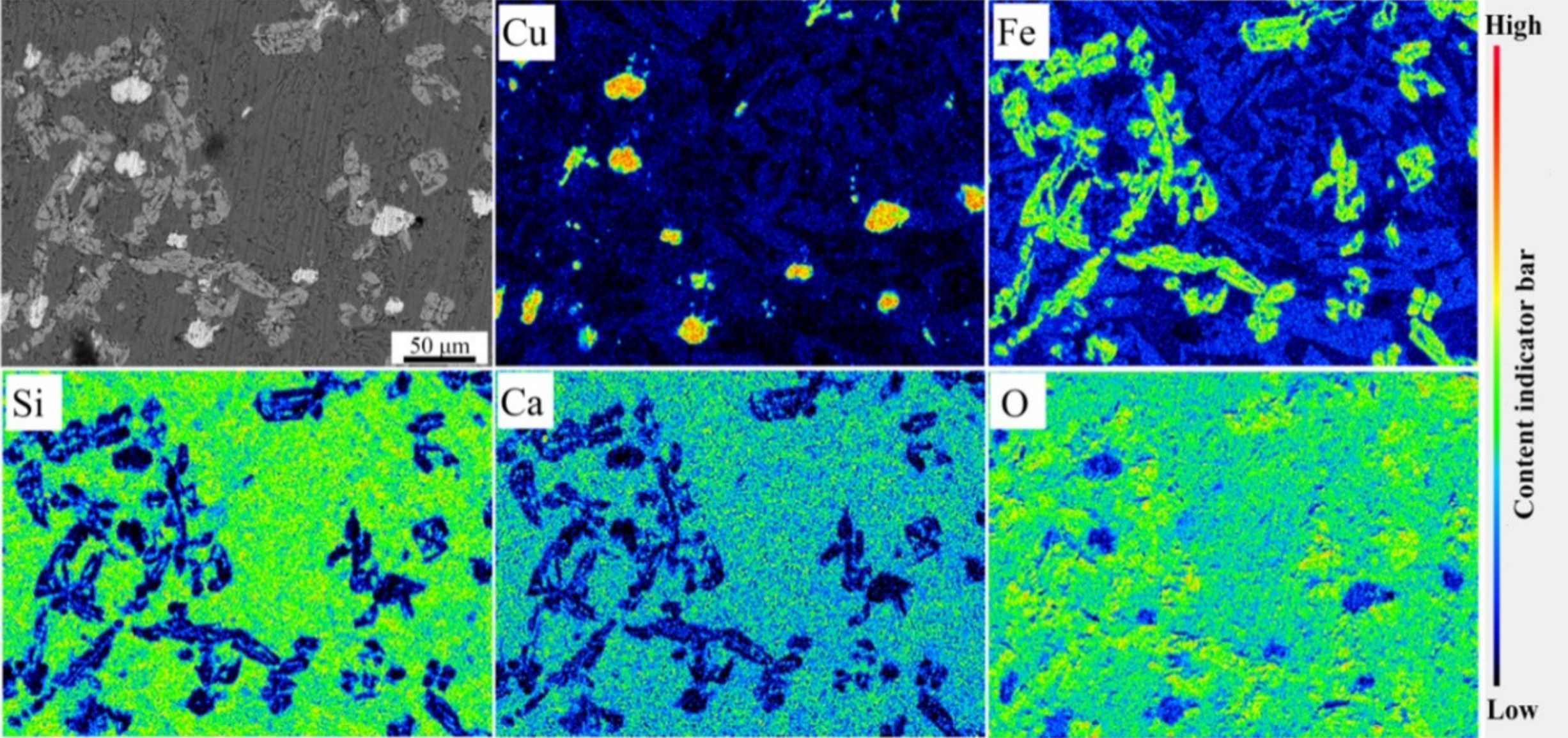
4. Verification of the Experiment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Degterov, S.A.; Pelton, A.D. A thermodynamic database for copper smelting and converting. Metall. Mater. Trans. B 1999, 30, 661–669. [Google Scholar] [CrossRef]
- Jarosz, P.; Kusiak, J.; Małecki, S.; Morkisz, P.; Oprocha, P.; Pietrucha, W.; Sztangret, Ł. Metamodeling and Optimization of a Blister Copper Two-Stage Production Process. JOM 2016, 68, 1535–1540. [Google Scholar] [CrossRef]
- Zhu, Z.Z. Current Copper Metall; Science Press: Beijing, China, 2003. [Google Scholar]
- Asteljoki, J.A.; Bailey, L.K.; George, D.B.; Rodolff, D.W. Flash Converting—Continuous Converting of Copper Mattes. JOM 1985, 37, 20–23. [Google Scholar] [CrossRef]
- Schmiedl, J.; Holeczy, J.; Sehnalek, F. Continuous production of copper from concentrates. Commun. Soil Plant Anal. 1969, 37, 2461–2469. [Google Scholar]
- Wilkomirsky, I.; Parra, R.; Parada, F.; Balladares, E. Continuous Converting of Copper Matte to Blister Copper in a High-Intensity Molten-Layer Reactor. JOM 2014, 66, 1687–1693. [Google Scholar] [CrossRef]
- Shibasaki, T.; Hayashi, M. Top-blown injection smelting and converting: The Mitsubishi process. JOM 1991, 43, 20–26. [Google Scholar] [CrossRef]
- Kucharski, M.; Sak, T.; Madej, P.; Wedrychowicz, M.; Mróz, W. A Study on the Copper Recovery from the Slag of the Outokumpu Direct-to-Copper Process. Metall. Mater. Trans. B 2014, 45, 590–602. [Google Scholar] [CrossRef]
- Mcbow, I.; Kongoli, F.; Tsymbulov, L. Composition and characteristics of slags from continuous converting of copper matte and concentrate. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2014, 123, 10–20. [Google Scholar]
- Nikolic, S.; Hayes, P.C.; Jak, E. Phase Equilibria in Ferrous Calcium Silicate Slags: Part IV. Liquidus Temperatures and Solubility of Copper in "Cu2O"-FeO-Fe2O3-CaO-SiO2 Slags at 1250 °C and 1300 °C at an Oxygen. Metall. Mater. Trans. B 2008, 39, 210–217. [Google Scholar] [CrossRef]
- Sridhar, R.; Toguri, J.M.; Simeonov, S. Copper losses and thermodynamic considerations in copper smelting. Metall. Mater. Trans. B 1997, 28, 191–200. [Google Scholar] [CrossRef]
- Swinbourne, D.R.; West, R.C.; Reed, M.E.; Sheeran, A. Computational thermodynamic modelling of direct to blister copper smelting. Miner. Process. Extr. Metall. 2011, 120, 1–9. [Google Scholar] [CrossRef]
- Stefanova, V.; Trifonov, Y. Phase composition of spinel melts obtained during flash smelting of the mineral chalcopyrite. Russ. J. Non-Ferr. Met. 2008, 49, 148–155. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, M.; Cui, Z.; Contreras, L.; Zhao, B. Phase Equilibrium Studies of Iron Silicate Slag Under Direct to Blister Copper-Making Condition. Metall. Mater. Trans. B 2019, 51, 1–5. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, M.; Cui, Z.; Contreras, L.; Zhao, B. Development of Ferrous-Calcium Silicate Slag for the Direct to Blister Copper-Making Process and the Equilibria Investigation. Metall. Mater. Trans. B 2020, 51, 973–984. [Google Scholar] [CrossRef]
- Kongoli, F.; Mcbow, I.; Yazawa, A.; Takeda, Y.; Yamaguchi, K.; Budd, R.; Llubani, S. Liquidus relationships of calcium ferrite and ferrous calcium silicate slag in continuous copper converting. Miner. Process. Extr. Metall. 2008, 117, 67–76. [Google Scholar] [CrossRef]
- Yazawa, A. Thermodynamic considerations of copper smelting. Can. Metall. Q. 1974, 13, 443–453. [Google Scholar] [CrossRef]
- Yazawa, A.; Takeda, Y.; Nakazawa, S. Ferrous Calcium Silicate Slag to be Used for Copper Smelting and Converting. Proc. Copp. 1999, 6, 587–599. [Google Scholar]
- Yazawa, A.; Takeda, Y.; Waseda, Y. Thermodynamic Properties and Structure of Ferrite Slags and Their Process Implications. Can. Metall. Q. 2013, 20, 129–134. [Google Scholar] [CrossRef]
- Somerville, M.; Chen, C.; Alvear, G.R.F.F.; Nikolic, S. Fluxing Strategies for the Direct to Blister Smelting of High Silica and Low Iron Copper Concentrates. In Advances in Molten Slags, Fluxes, and Salts, Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts, Washington, DC, USA, 22–25 May 2016; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2016; pp. 667–675. [Google Scholar]
- Voigt, P.; Burrows, A.; Somerville, M.; Chen, C. Direct-to-Blister Copper Smelting with the ISASMELT™ Process. In 8th International Symposium on High-Temperature Metallurgical Processing; Springer: Cham, Switzerland, 2017; pp. 261–267. [Google Scholar]
- Taskinen, P. Direct-to-blister smelting of copper concentrates: The slag fluxing chemistry. Miner. Process. Extr. Metall. 2011, 120, 240–246. [Google Scholar] [CrossRef]
- Taskinen, P.; Kojo, I. Fluxing options in the direct-to-blister copper smelting. In Proceedings of the Molten 2009: VIII International Conference on Molton Slags, Fluxes, and Salts, Santiago, Chile, 18–21 January 2009; pp. 1139–1151. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Duffy, J.A.; Ingram, M.D.; Sommerville, I.D. Acid–base properties of molten oxides and metallurgical slags. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Ph. 1978, 74, 1410–1419. [Google Scholar] [CrossRef]
- Fallah-Mehrjardi, A.; Hayes, P.C.; Jak, E. The Effect of CaO on Gas/Slag/Matte/Tridymite Equilibria in Fayalite-Based Copper Smelting Slags at 1473 K (1200 °C) and P (SO2) = 0.25 Atm. Metall. Mater. Trans. 2018, 49, 602–609. [Google Scholar] [CrossRef]
- Kim, H.G.; Sohn, H.Y. Effects of CaO, Al2O3, and MgO additions on the copper solubility, ferric/ferrous ratio, and minor-element behavior of iron-silicate slags. Metall. Mater. Trans. B 1998, 29, 583–590. [Google Scholar] [CrossRef]





| Mineral | Concentrate Composition (wt.%) | Fe/SiO2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Fe | S | SiO2 | CaO | MgO | Al2O3 | ||
| Chalcocite | 53.55 | 4.23 | 15.26 | 14.37 | 0.26 | 1.15 | 3.55 | 0.29 |
| Chalcopyrite | 30.00 | 29.00 | 30.00 | 5.00 | 0.50 | 0.48 | 0.50 | 5.80 |
| Cu | Fe | S | SiO2 | CaO | MgO | Al2O3 | Fe/SiO2 |
|---|---|---|---|---|---|---|---|
| 50.15 | 7.81 | 17.39 | 13.01 | 0.29 | 1.05 | 3.11 | 0.6 |
| Oxygen Consumption | Cu Content in Slag | S Content in Blister | Viscosity | Slag Amount for Production of 1 t Blister |
|---|---|---|---|---|
| 152.51 m3 | 4.76% | 1.45% | 0.48 Pa s | 0.77 t |
| 157.69 m3 | 6.46% | 0.5% | 0.49 Pa s | 0.81 t |
| Smelting Parameters | Copper Direct Yield Rate (%) | Copper Content in Slag (wt.%) | Blister Copper Grade (wt.%) | S in Blister Copper (wt.%) |
|---|---|---|---|---|
| Theoretical value | 93.33 | 6.46 | 99.25 | 0.50 |
| Experimental value | 82.12 | 17.17 | 99.09 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Guo, X.; Tian, B.; Li, B.; Wei, Y. Investigation on Direct-to-Blister Smelting of Chalcocite via Thermodynamics and Experiment. Metals 2021, 11, 19. https://doi.org/10.3390/met11010019
Zhou S, Guo X, Tian B, Li B, Wei Y. Investigation on Direct-to-Blister Smelting of Chalcocite via Thermodynamics and Experiment. Metals. 2021; 11(1):19. https://doi.org/10.3390/met11010019
Chicago/Turabian StyleZhou, Shiwei, Xiang Guo, Bo Tian, Bo Li, and Yonggang Wei. 2021. "Investigation on Direct-to-Blister Smelting of Chalcocite via Thermodynamics and Experiment" Metals 11, no. 1: 19. https://doi.org/10.3390/met11010019




