Effect of Laser Power on Microstructure and Micro-Galvanic Corrosion Behavior of a 6061-T6 Aluminum Alloy Welding Joints
Abstract
:1. Introduction
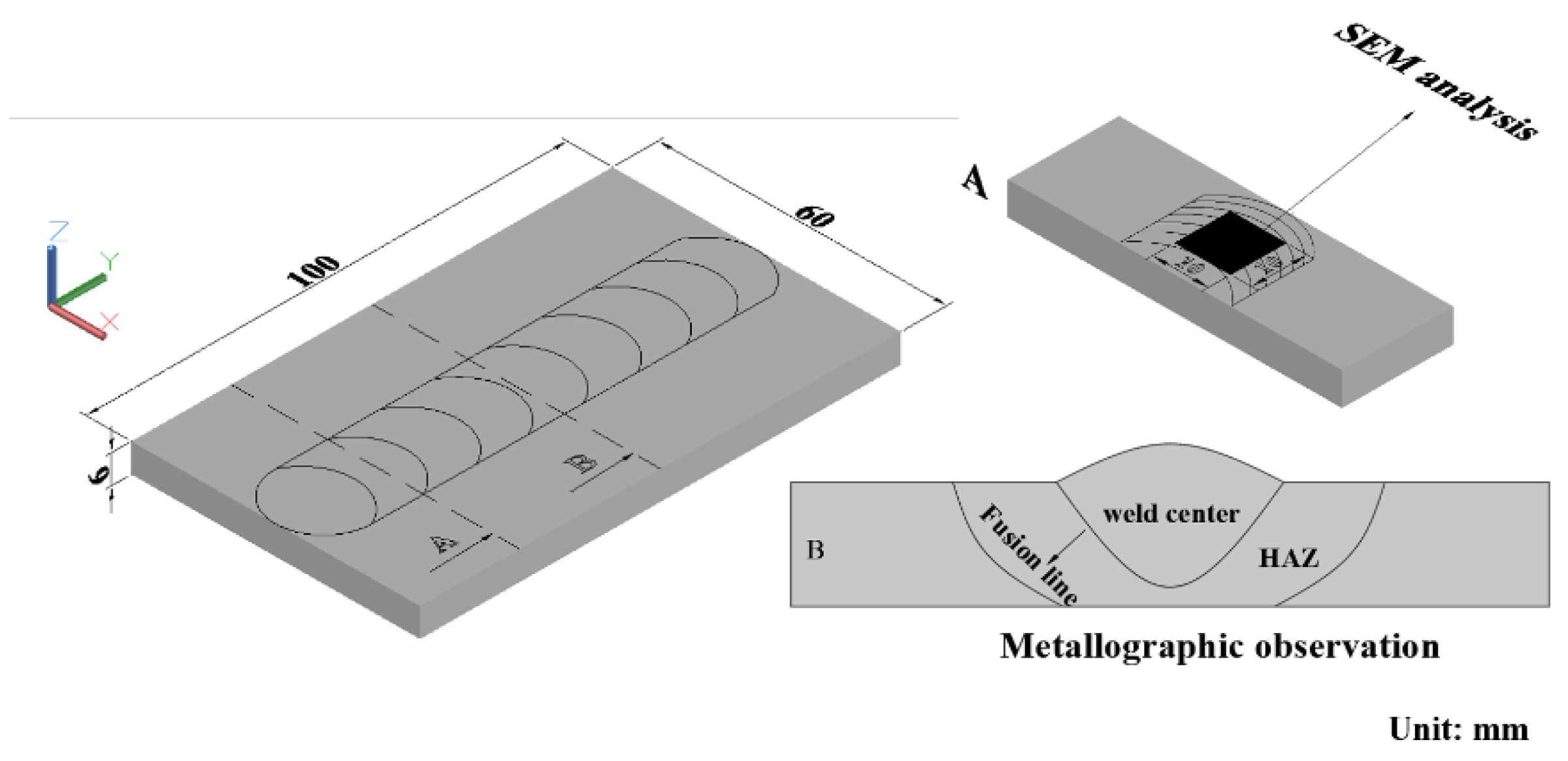
2. Experimental
3. Results and Discussion
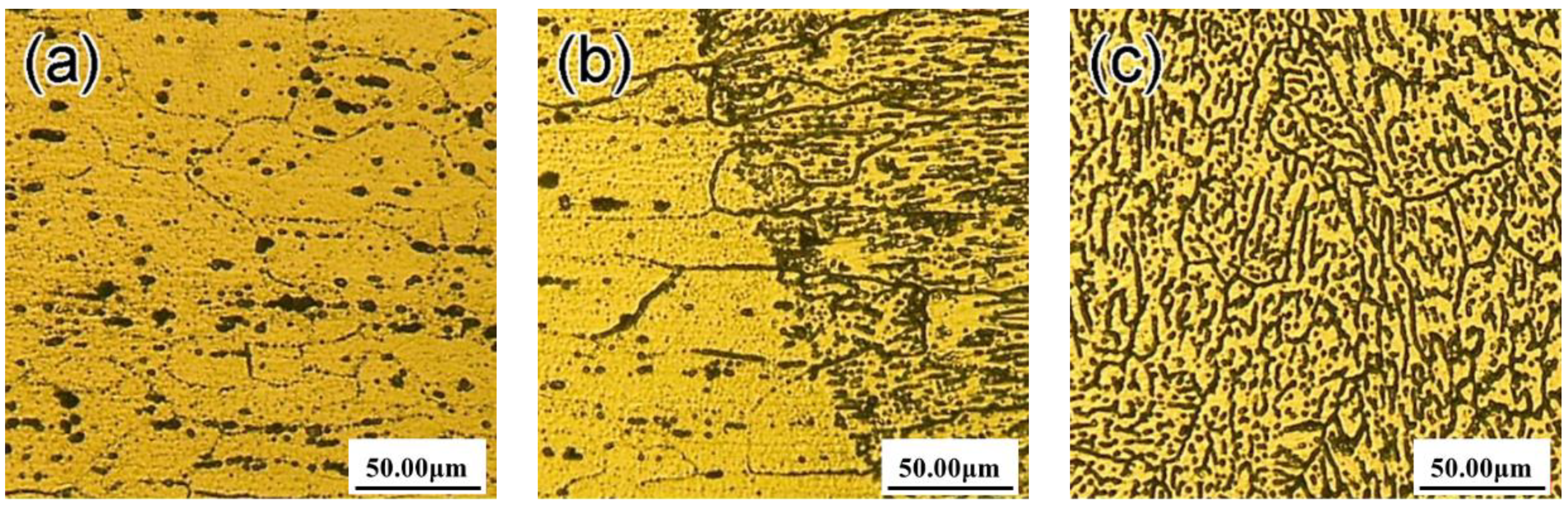
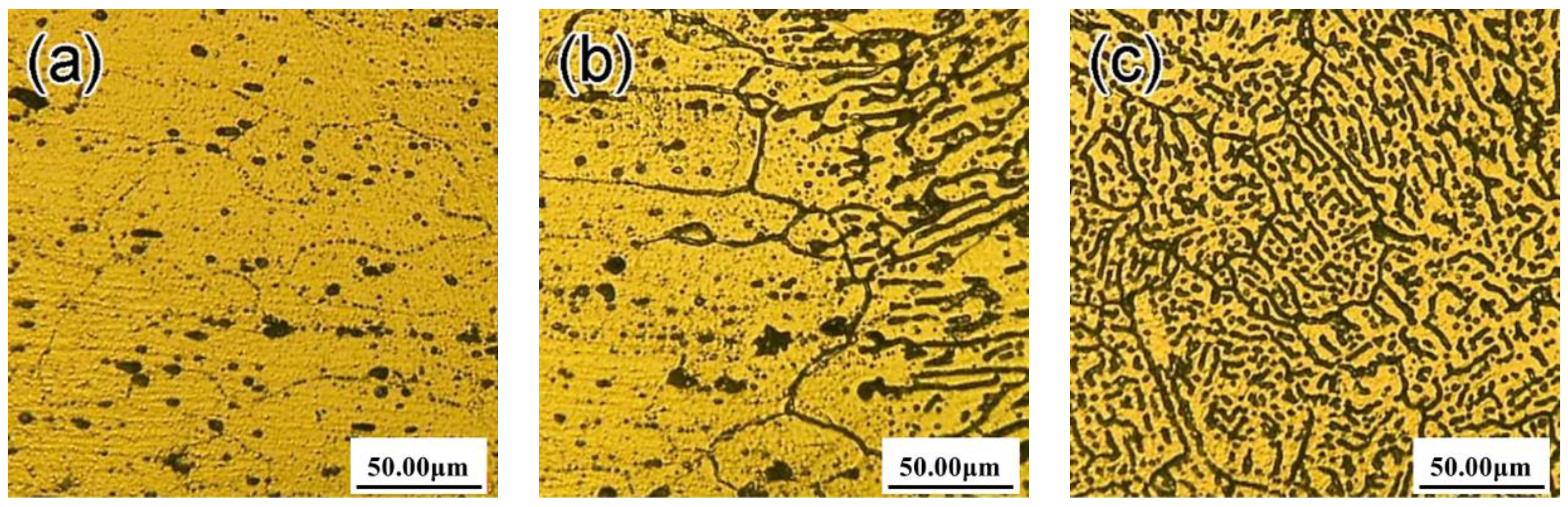
3.1. Microstructure of the Welding Joints
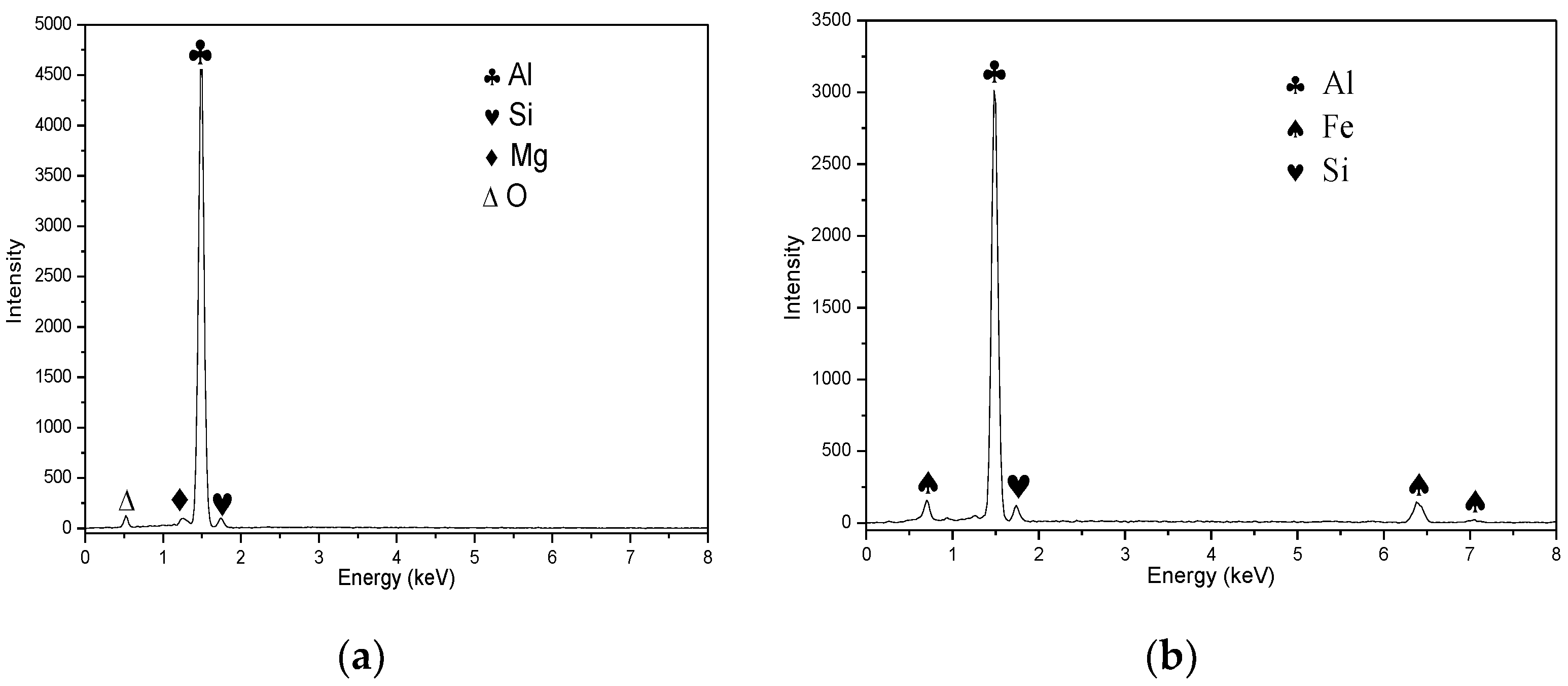
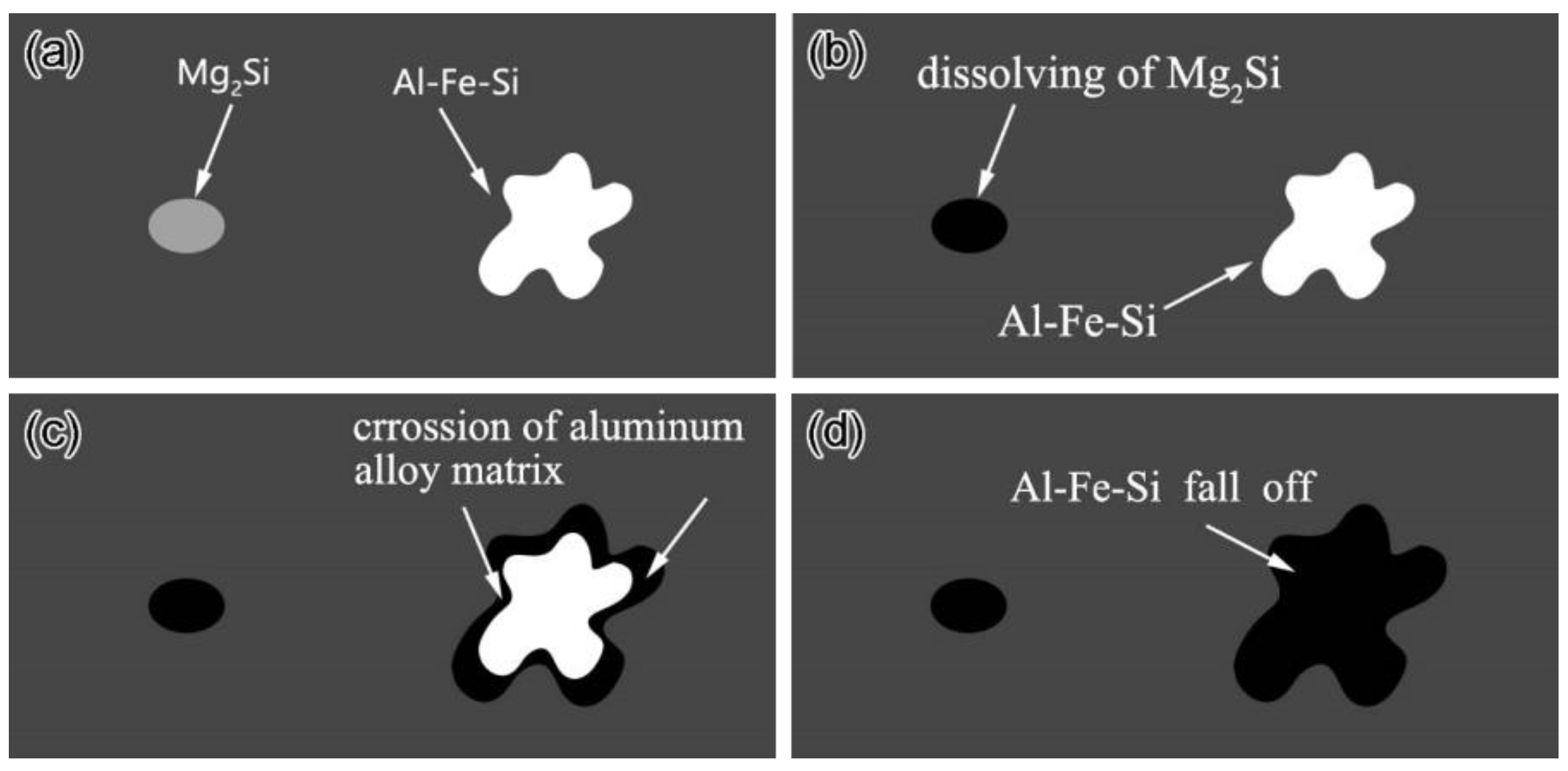
3.2. Corrosion Behavior of the Weld Center
4. Conclusions
- (1)
- With the increase of laser power, the segregation structure, equiaxed grain, and HAZ structure and columnar crystal at the fusion line are coarsening, and the dendrite gap increases.
- (2)
- The micro-galvanic corrosion in the 6061-T6 aluminum alloy welded joint is mainly induced by Mg2Si and Fe-Al-Si intermetallic particles.
- (3)
- The decrease in the corrosion pits is related to the lower density of intermetallic particles of intermetallic particles on the surface.
- (4)
- The volume of corrosion pits increases with the increase of laser power.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tucker, M.; Horstemeyer, M.F.; Whittington, W.; Solanki, K.; Gullett, P. The effect of varying strain rates and stress states on the plasticity, damage, and fracture of aluminum alloys. Mech. Mater. 2010, 42, 895–907. [Google Scholar] [CrossRef]
- Sheng, J.; Huang, S.; Zhou, J.; Lu, J.; Xu, S.; Zhang, H. Effect of laser peening with different energies on fatigue fracture evolution of 6061-T6 aluminum alloy. Opt. Laser Technol. 2016, 77, 169–176. [Google Scholar] [CrossRef]
- Abúndez, A.; Pereyra, I.; Campillo, B.; Serna, S.; Alcudia, E.; Molina, A.; Blanco, A.; Mayen, J. Improvement of ultimate tensile strength by artificial ageing and retrogression treatment of aluminium alloy 6061. Mater. Sci. Eng. A 2016, 668, 201–207. [Google Scholar] [CrossRef]
- Chanyathunyaroj, K.; Phetchcrai, S.; Laungsopapun, G.; Rengsomboon, A. Fatigue characteristics of 6061 aluminum alloy subject to 3.5% NaCl environment. Int. J. Fatigue 2020, 133, 105420. [Google Scholar] [CrossRef]
- Bardel, D.; Fontaine, M.; Chaise, T.; Perez, M.; Nelias, D.; Bourlier, F.; Garnier, J. Integrated modelling of a 6061-T6 weld joint: From microstructure to mechanical properties. Acta Mater. 2016, 117, 81–90. [Google Scholar] [CrossRef]
- Liang, W.; Rometsch, P.; Cao, L.; Birbilis, N. General aspects related to the corrosion of 6xxx series aluminium alloys: Exploring the influence of Mg/Si ratio and Cu. Corros. Sci. 2013, 76, 119–128. [Google Scholar] [CrossRef]
- Liang, M.; Melchers, R.; Chaves, I. Corrosion and pitting of 6060 series aluminium after 2 years exposure in seawater splash, tidal and immersion zones. Corros. Sci. 2018, 140, 286–296. [Google Scholar] [CrossRef]
- Gungor, B.; Kaluc, E.; Taban, E.; Sik, A. Mechanical, fatigue and microstructural properties of friction stir welded 5083-H111 and 6082-T651 aluminum alloys. Mater. Des. 2014, 56, 84–90. [Google Scholar] [CrossRef]
- Atabaki, M.M.; Ma, J.; Yang, G.; Kovacevic, R. Hybrid laser/arc welding of advanced high strength steel in different butt joint configurations. Mater. Des. 2014, 64, 573–587. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, M.; Zeng, X. Effect of microstructural characteristics on high cycle fatigue properties of laser-arc hybrid welded AA6082 aluminum alloy. J. Mater. Process. Technol. 2016, 231, 479–487. [Google Scholar] [CrossRef]
- Arunkumar, S.; Kannan, T.D.B.; Sathiya, P. Optimization, characterization and heat treatment of TIG-welded AA2219-T87 alloy. Emerg. Mater. Res. 2019, 8, 387–393. [Google Scholar] [CrossRef]
- Shanavas, S.; Dhas, J.E.R. Parametric optimization of friction stir welding parameters of marine grade aluminium alloy using response surface methodology. Trans. Nonferrous Met. Soc. China 2017, 27, 2334–2344. [Google Scholar] [CrossRef]
- Liu, T.; Mu, Z.; Hu, R.; Pang, S. Sinusoidal oscillating laser welding of 7075 aluminum alloy: Hydrodynamics, porosity formation and optimization. Int. J. Heat Mass Transf. 2019, 140, 346–358. [Google Scholar] [CrossRef]
- Xu, G.; Zheng, Z.; Cao, Q.; Hu, Q.; Li, L.; Guo, Q.; Du, B. Numerical and experimental investigation on weld formation during laser+MIG hybrid fillet welding of aluminum alloy in horizontal position. Int. J. Adv. Manuf. Technol. 2019, 102, 2683–2694. [Google Scholar] [CrossRef]
- Xu, G.; Wu, C.; Ma, X.; Wang, X. Numerical analysis of welding residual stress and distortion in laser+GMAW hybrid welding of aluminum alloy T-joint. Acta Met. Sin. Engl. Lett. 2013, 26, 352–360. [Google Scholar] [CrossRef]
- Gao, X.; Wu, C.; Goecke, S.; Kügler, H. Numerical simulation of temperature field, fluid flow and weld bead formation in oscillating single mode laser-GMA hybrid welding. J. Mater. Process. Technol. 2017, 242, 147–159. [Google Scholar] [CrossRef]
- Atabaki, M.M.; Nikodinovski, M.; Chenier, P.; Ma, J.; Liu, W.; Kovacevic, R. Experimental and numerical investigations of hybrid laser arc welding of aluminum alloys in the thick T-joint configuration. Opt. Laser Technol. 2014, 59, 68–92. [Google Scholar] [CrossRef]
- Ahmad, R.; Bakar, M. Effect of a post-weld heat treatment on the mechanical and microstructure properties of AA6061 joints welded by the gas metal arc welding cold metal transfer method. Mater. Des. 2011, 32, 5120–5126. [Google Scholar] [CrossRef]
- Xu, G.; Li, P.; Li, L.; Hu, Q.; Zhu, J.; Gu, X.; Du, B. Influence of Arc Power on Keyhole-Induced Porosity in Laser + GMAW Hybrid Welding of Aluminum Alloy: Numerical and Experimental Studies. Materials 2019, 12, 1328. [Google Scholar] [CrossRef] [Green Version]
- Chu, Q.; Bai, R.; Jian, H.; Lei, Z.; Hu, N.; Yan, C. Microstructure, texture and mechanical properties of 6061 aluminum laser beam welded joints. Mater. Charact. 2018, 137, 269–276. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, X.; Gao, L.-X.; Joo, H.G.; Lee, K.Y. Effect of laser–arc hybrid welding on fracture and corrosion behaviour of AA6061-T6 alloy. Mater. Sci. Eng. A 2011, 528, 2748–2754. [Google Scholar] [CrossRef]
- Rahman, A.B.M.; Kumar, S.; Gerson, A. Galvanic corrosion of laser weldments of AA6061 aluminium alloy. Corros. Sci. 2007, 49, 4339–4351. [Google Scholar] [CrossRef]
- Gharavi, F.; Matori, K.A.; Yunus, R.; Othman, N.K.; Fadaeifard, F. Corrosion behavior of Al6061 alloy weldment produced by friction stir welding process. J. Mater. Res. Technol. 2015, 4, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Fu, F.; Dai, Z.; Qiao, Y.; Chen, J.; Yang, L.; Liu, W. Effect of Laser Power on Hybrid Laser-Gas Metal Arc Welding (GMAW) of a 6061 Aluminum Alloy. J. Korean Phys. Soc. 2020, 77, 991–996. [Google Scholar] [CrossRef]
- Qiao, Y.; Tian, Z.; Cai, X.; Chen, J.; Wang, Y.; Song, Q.; Li, H. Cavitation Erosion Behaviors of a Nickel-Free High-Nitrogen Stainless Steel. Tribol. Lett. 2019, 67, 1. [Google Scholar] [CrossRef]
- Liu, S.Y.; Li, Y.Q.; Liu, F.D.; Zhang, H.; Ding, H.T. Effects of relative positioning of energy sources on weld integrity for hybrid laser arc welding. Opt. Lasers Eng. 2016, 81, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Zhan, X.; Zhao, Y.; Liu, Z.; Gao, Q.; Bu, H. Microstructure and porosity characteristics of 5A06 aluminum alloy joints using laser-MIG hybrid welding. J. Manuf. Process. 2018, 35, 437–445. [Google Scholar] [CrossRef]
- Lei, J.; Shi, C.; Zhou, S.; Gu, Z.; Zhang, L.-C. Enhanced corrosion and wear resistance properties of carbon fiber reinforced Ni-based composite coating by laser cladding. Surf. Coat. Technol. 2018, 334, 274–285. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Rao, Z.; Liao, S.; Wang, P.-C. Modeling of transport phenomena and solidification cracking in laser spot bead-on-plate welding of AA6063-T6 alloy. Part II—Simulation results and experimental validation. Int. J. Adv. Manuf. Technol. 2014, 74, 285–296. [Google Scholar] [CrossRef]
- Heard, D.W.; Gauvin, R.; Brochu, M. Non-equilibrium solute partitioning in a laser re-melted Al–Li–Cu alloy. Acta Mater. 2013, 61, 7432–7436. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.; Marcos, M.; Sánchez-Amaya, J.M. Influence of the degree of polishing of alloy AA 5083 on its behaviour against localised alkaline corrosion. Corros. Sci. 2004, 46, 1909–1920. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, B.; Zhao, Y.; Liu, J. Corrosion and electrochemical behaviors of 7A09 Al–Zn–Mg–Cu alloy in chloride aqueous solution. Trans. Nonferrous Met. Soc. China 2015, 25, 2509–2515. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Huang, W.; Thompson, G.; Zhang, X.; Luo, C.; Sun, Z. Localized corrosion in AA2099-T83 aluminum–lithium alloy: The role of intermetallic particles. Mater. Chem. Phys. 2015, 161, 201–210. [Google Scholar] [CrossRef]
- Chen, M.-A.; Ou, Y.-C.; Fu, Y.-H.; Li, Z.-H.; Li, J.-M.; Liu, S.-D. Effect of friction stirred Al-Fe-Si particles in 6061 aluminum alloy on structure and corrosion performance of MAO coating. Surf. Coat. Technol. 2016, 304, 85–97. [Google Scholar] [CrossRef]
- Fahimpour, V.; Sadrnezhaad, S.; Karimzadeh, F. Corrosion behavior of aluminum 6061 alloy joined by friction stir welding and gas tungsten arc welding methods. Mater. Des. 2012, 39, 329–333. [Google Scholar] [CrossRef]
- Sun, F.; Li, X.; Lu, L.; Cheng, X.; Dong, C.; Gao, J. Corrosion behavior of 5052 and 6061 aluminum ALLOYS in deep ocean environment of south China sea. Acta Met. Sin. 2013, 49, 1219. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.F.; Zhang, D.W.; Cheng, M.; Sun, X.G.; Xiao, K.; Thee, C.; Li, X.G. Effect of Alloying Elements on Initial Corrosion Behavior of Aluminum Alloy in Bangkok, Thailand. Acta Metall. Sin. 2020, 56, 119–128. [Google Scholar]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Tan, L.; Allen, T.R. Effect of thermomechanical treatment on the corrosion of AA5083. Corros. Sci. 2010, 52, 548–554. [Google Scholar] [CrossRef]
- Qin, Q.D.; Zhao, H.L.; Li, J.; Zhang, Y.; Zhang, B.R.; Su, X.D. Microstructures and mechanical properties of TIG welded Al-Mg2Si alloy joints. J. Manuf. Process. 2020, 56, 941–949. [Google Scholar] [CrossRef]
- Du, P.; Li, Y.L.; Yan, X.D.; Zhang, Z.H. Study on homogenization process for high quality 6061 alloy. Mater. Heat Treat. 2010, 39, 161–165. [Google Scholar]


| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al | |
|---|---|---|---|---|---|---|---|---|---|
| 6061 aluminum alloy | 0.50 | 0.7 | 0.45 | 0.15 | 1.0 | 0.25 | 0.25 | 0.15 | Bal. |
| ER5356 | 0.25 | 0.4 | 0.1 | 0.11 | 4.9 | 0.065 | 0.1 | 0.11 | Bal. |
| Mg | Al | Si | Mn | Fe | |
|---|---|---|---|---|---|
| area A | 2.22 | 95.17 | 2.61 | - | - |
| area B | 2.22 | 94.41 | 1.58 | - | 1.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Fu, F.; Dai, Z.; Qiao, Y.; Chen, J.; Liu, W. Effect of Laser Power on Microstructure and Micro-Galvanic Corrosion Behavior of a 6061-T6 Aluminum Alloy Welding Joints. Metals 2021, 11, 3. https://doi.org/10.3390/met11010003
Zhou H, Fu F, Dai Z, Qiao Y, Chen J, Liu W. Effect of Laser Power on Microstructure and Micro-Galvanic Corrosion Behavior of a 6061-T6 Aluminum Alloy Welding Joints. Metals. 2021; 11(1):3. https://doi.org/10.3390/met11010003
Chicago/Turabian StyleZhou, Huiling, Fanglian Fu, Zhixin Dai, Yanxin Qiao, Jian Chen, and Wen Liu. 2021. "Effect of Laser Power on Microstructure and Micro-Galvanic Corrosion Behavior of a 6061-T6 Aluminum Alloy Welding Joints" Metals 11, no. 1: 3. https://doi.org/10.3390/met11010003
APA StyleZhou, H., Fu, F., Dai, Z., Qiao, Y., Chen, J., & Liu, W. (2021). Effect of Laser Power on Microstructure and Micro-Galvanic Corrosion Behavior of a 6061-T6 Aluminum Alloy Welding Joints. Metals, 11(1), 3. https://doi.org/10.3390/met11010003







