Study on Enhancing the Thermoelectric Properties of Ti2CrSn Alloys
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
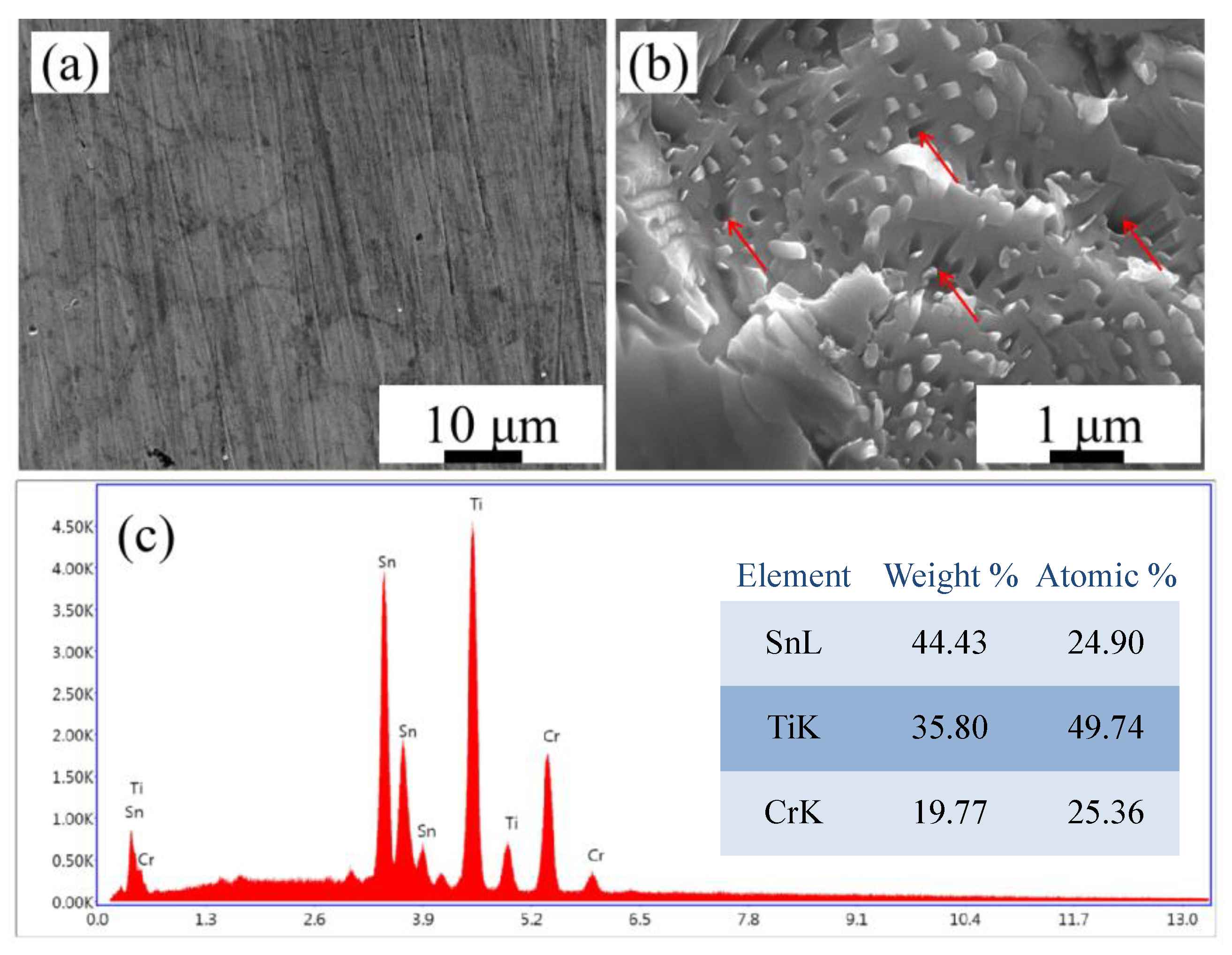
3.1. Structural Analysis
3.2. Analysis of Electrical Transport Properties
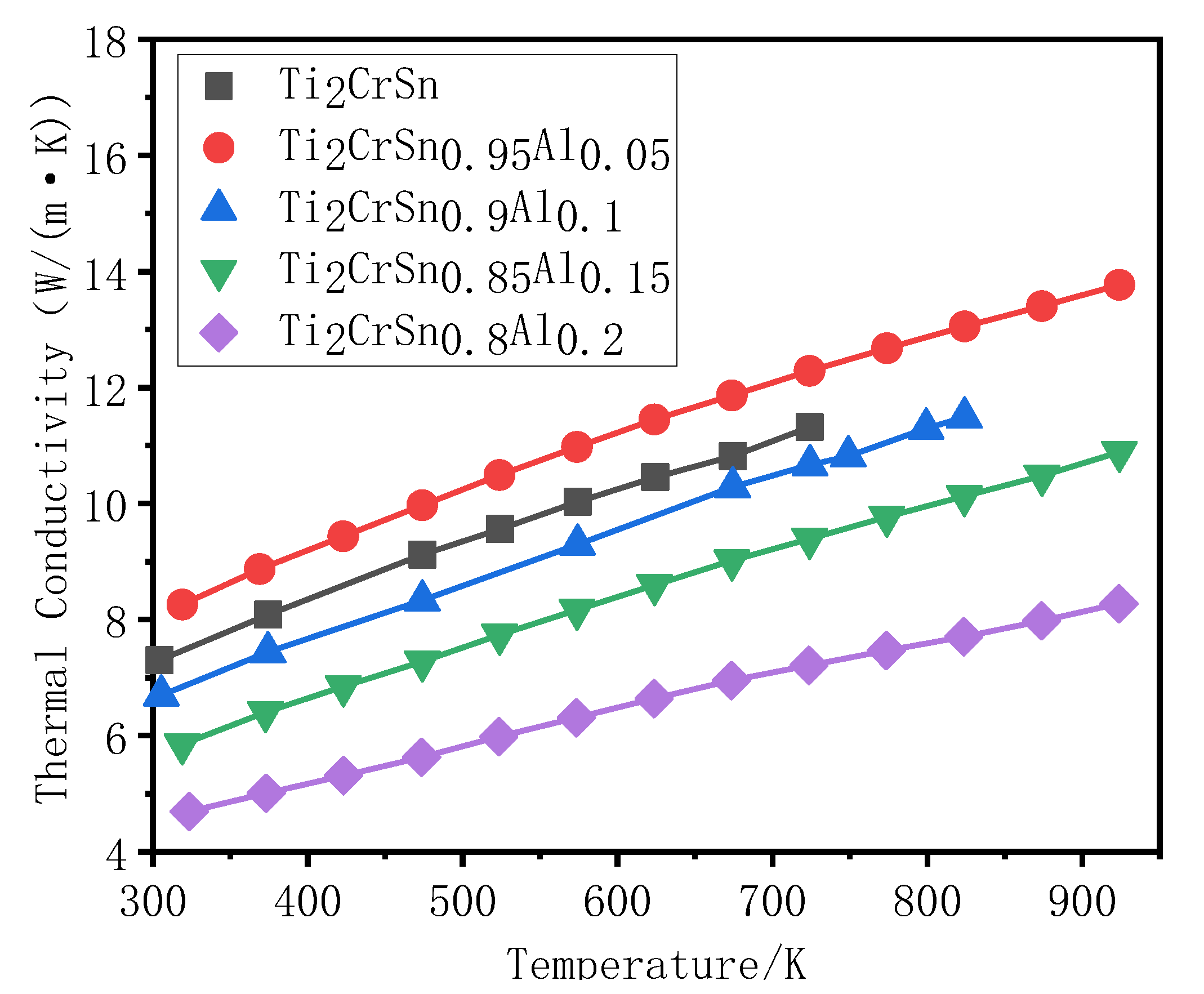
3.3. Analysis of Thermal Transport Properties
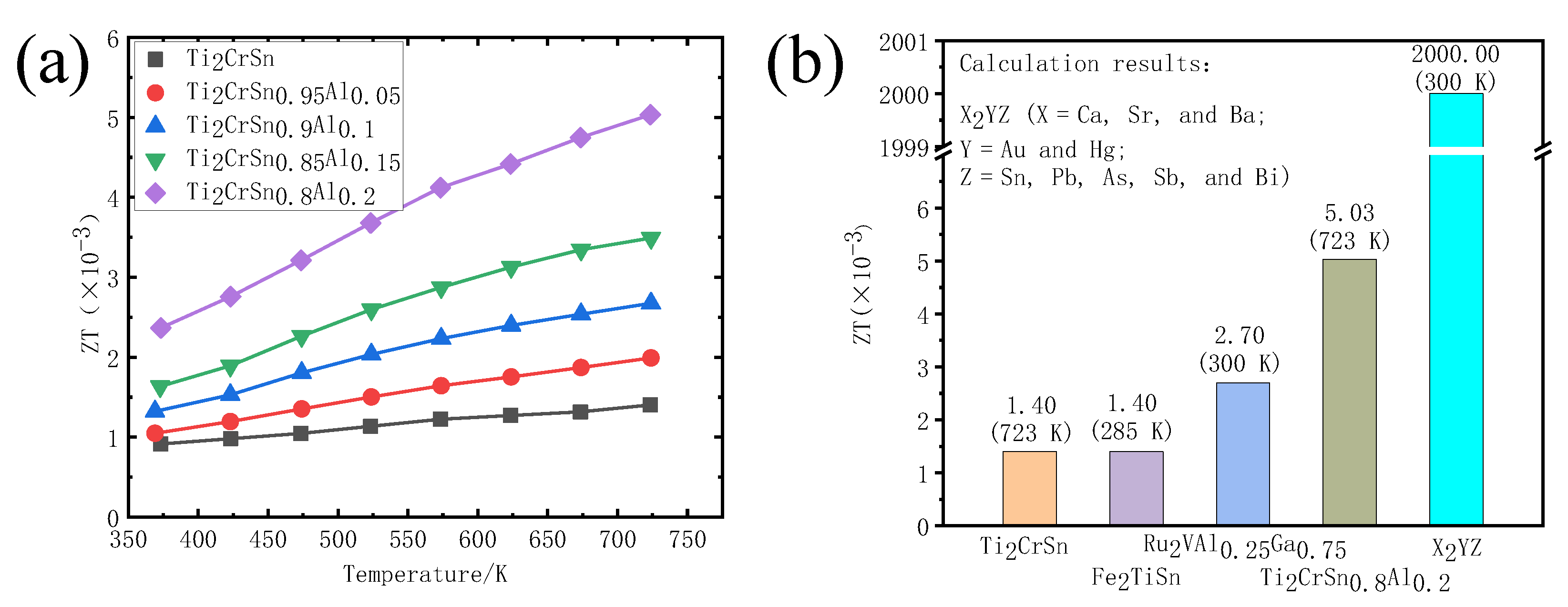
3.4. Thermoelectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Zhao, L.D. Thermoelectric materials: Energy conversion between heat and electricity. J. Mater. 2015, 1, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.L.; Zou, J.; Chen, Z.G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.M.; Zebarjadi, M.; He, J.; Tritt, T.M. Thermoelectric power factor: Enhancement mechanisms and strategies for higher performance thermoelectric materials. Mater. Sci. Eng. R Rep. 2015, 97, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Makongo, J.P.A.; Misra, D.K.; Zhou, X.; Pant, A.; Shabetai, M.R.; Su, X.; Uher, C.; Stokes, K.L.; Poudeu, P.F. Simultaneous Large Enhancements in Thermopower and Electrical Conductivity of Bulk Nanostructured Half-Heusler Alloys. J. Am. Chem. Soc. 2011, 133, 18843–18852. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef]
- Minnich, A.J.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Bulk nanostructured thermoelectric materials: Current research and future prospects. Energy Environ. Sci. 2009, 2, 466–479. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-Thermoelectric Performance of Nanostructured Bismuth Antimony Telluride Bulk Alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshwan, R.; Lei, Y.; Jin, Z.; Chen, Z.G. Eco-Friendly SnTe Thermoelectric Materials: Progress and Future Challenges. Adv. Funct. Mater. 2017, 27, 1703278.1–1703278.18. [Google Scholar] [CrossRef]
- Qiu, P.F.; Shi, X.; Chen, L.D. Cu-based thermoelectric materials. Energy Storage Mater. 2016, 3, 85–97. [Google Scholar] [CrossRef]
- Chen, L.D.; Huang, X.Y.; Zhou, M.; Shi, X.; Zhang, W.B. The high temperature thermoelectric performances of Zr0.5Hf0.5Ni0.8Pd0.2Sn0.99Sb0.01 alloy with nanophase inclusions. J. Appl. Phys. 2006, 99, 064305. [Google Scholar] [CrossRef]
- Sales, B.C.; Mandrus, D.; Williams, R.K. Filled Skutterudite Antimonides: A New Class of Thermoelectric Materials. Science 1996, 272, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Wang, X.Y.; Liu, Y.J.; Cao, F.; Zhao, L.D.; Zhang, Q. Development on thermoelectric materials. J. Chin. Ceram. Soc. 2018, 46, 288–305. [Google Scholar]
- Luo, H.; Xin, Y.; Liu, B.; Meng, F.; Liu, H.; Liu, E.; Wu, G. Competition of L21 and XA structural ordering in Heusler alloys X2CuAl (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni). J. Alloys Compd. 2016, 665, 180–185. [Google Scholar] [CrossRef]
- Li, J. First-Principles Theoretical Design of Heusler Alloy Thermoelectric Materials; Hebei University of Technology: Tianjin, China, 2017. [Google Scholar]
- Jiang, H.J. First-Principles Study on Electronic Structure, Magnetism and Semimetallic Properties of Half Heusler Alloy [D]; Yanbian University: Yanbian, China, 2008. [Google Scholar]
- Bhat, I.H.; Bhat, T.M.; Gupta, D.C. Magneto-electronic and thermoelectric properties of some Fe-based Heusler alloys. J. Phys. Chem. Solids 2018, 119, 251–257. [Google Scholar] [CrossRef]
- Bhat, T.M.; Nabi, M.; Gupta, D.C. Structural, elastic, thermodynamic and thermoelectric properties of Fe2TiSn Heusler alloy: High pressure study. Results Phys. 2019, 12, 15–20. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Mao, X.; Liu, Z.; Xu, X.; Liu, Y.; Teng, S.; Yin, F.; Congcong, L.; Xiujuan, M.; et al. Improved thermoelectric performance of p-doped half-Heusler Ti0.5Zr0.5CoSb0.5P0.5, Ti0.5Hf0.5CoSb0.5P0.5, and Zr0.5Hf0.5CoSb0.5P0.5 compounds. Mater. Res. Express 2019, 6, 126305. [Google Scholar] [CrossRef]
- Mikami, M.; Kinemuchi, Y.; Ozaki, K.; Terazawa, Y.; Takeuchi, T. Thermoelectric properties of tungsten-substituted Heusler Fe2VAl alloy. J. Appl. Phys. 2012, 111, 093710. [Google Scholar] [CrossRef]
- Huang, X.Y.; Xu, Z.; Chen, L.D. Half Heusler thermoelectric semiconductor material. Inorg. Mater. 2004, 19, 25–30. [Google Scholar]
- Li, J.; Yang, G.; Yang, Y.M.; Zhang, Q.; Zhang, Z.; Fang, W.; Yin, F.; Li, J. Electronic and thermoelectric properties of nonmagnetic inverse Heusler semiconductors Sc2FeSi and Sc2FeGe. J. Magn. Magn. Mater. 2017, 442, 371–376. [Google Scholar] [CrossRef]
- Ma, H.R.; Li, J.; Yang, G.; Yang, Y.; Mao, X.; Li, C.; Yin, F. Improved thermoelectric property of Ti0.75HfMo0.25CrGe by doping Ti2CrGe Heusler alloy with Hf and Mo: Confirmation of entropy "gene" in thermoelectric materials design. J. Appl. Phys. 2018, 124, 235104. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Zhang, Q.; Zhang, Z.; Yang, G.; Ma, H.; Lu, Z.; Fang, W.; Xie, H.; Liang, C.; et al. The thermoelectric properties of predicted semiconducting Ti2CrGe and Ti2CrSn: A first principles study. Comput. Mater. Sci. 2016, 125, 183–187. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Li, J.; Yang, G.; Ma, H.; Zhou, G.; Lu, Z.; Fang, W.; Xie, H.; Liang, C.; et al. A new stable antiferromagnetic semiconductor: The case of inverse Heusler compound Ti2CrSn. Intermetallics 2017, 85, 149–155. [Google Scholar] [CrossRef]
- Yin, M.; Nash, P. Standard enthalpies of formation of selected Ru2YZ Heusler compounds. J. Alloys Compd. 2015, 634, 70–74. [Google Scholar] [CrossRef]
- Yu, B.L.; Tang, X.F.; Qi, Q.; Zhang, Q.J. Preparation and heat transport properties of CoSb3 nanothermal materials. Acta Phys. Sin. 2004, 9, 3131. [Google Scholar]
- Taranova, A.I.; Novitskii, A.P.; Voronin, A.I.; Taskaev, S.V.; Khovaylo, V.V. Influence of V Doping on the Thermoelectric Properties of Fe2Ti1–xVxSn Heusler Alloys. Semiconductors 2019, 53, 768–771. [Google Scholar] [CrossRef]
- Nishino, Y.; Deguchi, S.; Mizutani, U. Thermal and transport properties of the Heusler-type Fe2VAl1−xGex (0 ≤ x ≤ 0.20) alloys: Effect of doping on lattice thermal conductivity, electrical resistivity, and Seebeck coefficient. Phys. Rev. B 2006, 74, 115115–1–115115–6. [Google Scholar] [CrossRef]
- Nakatsugawa, H.; Ozaki, T.; Kishimura, H.; Okamoto, Y. Thermoelectric Properties of Heusler Fe2TiSn Alloys. J. Electron. Mater. 2020, 49, 2802–2812. [Google Scholar] [CrossRef]
- Pedersen, S.V.; Croteau, J.R.; Kempf, N.; Zhang, Y.; Butt, D.P.; Jaques, B.J. Novel synthesis and processing effects on the figure of merit for NbCoSn, NbFeSb, and ZrNiSn based Half-Heusler thermoelectrics. J. Solid State Chem. 2020, 285, 121203. [Google Scholar] [CrossRef]
- Guo, F.K.; Cui, B.; Liu, Y.; Meng, X.; Cao, J.; Zhang, Y.; He, R.; Liu, W.; Wu, H.; Pennycook, S.J.; et al. Thermoelectric SnTe with Band Convergence, Dense Dislocations, and Interstitials through Sn Self-Compensation and Mn Alloying. Small 2018, 14, 1802615. [Google Scholar] [CrossRef]
- Kim, S., II; Lee, K.H.; Mun, H.A.; Kim, H.S.; Hwang, S.W.; Roh, J.W.; Yang, D.J.; Shin, W.H.; Li, X.S.; Lee, Y.H.; et al. Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 2015, 348, 109–144. [Google Scholar] [CrossRef] [Green Version]
- Su, T.J.; Wang, F.C.; Li, S.K.; Wang, L. Regression relationships established for thermal conductivity of steels as function of chemical composition at various temperatures. Ordnance Mater. Sci. Eng. 2004, 27, 14–16. [Google Scholar]
- Ramachandran, B.; Lin, Y.H.; Kuo, Y.K.; Kuo, C.N.; Gippius, A.A.; Lue, C.S. Thermoelectric properties of Heusler-type Ru2VAl1−xGax alloys. Intermetallics 2018, 92, 36–41. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Li, J.; Wang, Y.X.; Ren, W. High thermoelectric properties in full-Heusler X2YZ alloys (X = Ca, Sr, and Ba; Y = Au and Hg; Z = Sn, Pb, As, Sb, and Bi). J. Phys. D Appl. Phys. 2019, 52, 495303. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Song, S.; Ren, Y.; Guo, J.; Yan, L.; Lu, Z.; Shen, J. Study on Enhancing the Thermoelectric Properties of Ti2CrSn Alloys. Metals 2021, 11, 1503. https://doi.org/10.3390/met11101503
Yu G, Song S, Ren Y, Guo J, Yan L, Lu Z, Shen J. Study on Enhancing the Thermoelectric Properties of Ti2CrSn Alloys. Metals. 2021; 11(10):1503. https://doi.org/10.3390/met11101503
Chicago/Turabian StyleYu, Guangfa, Shihao Song, Yanwei Ren, Jia Guo, Liqin Yan, Zunming Lu, and Jun Shen. 2021. "Study on Enhancing the Thermoelectric Properties of Ti2CrSn Alloys" Metals 11, no. 10: 1503. https://doi.org/10.3390/met11101503




