Effect of Titanium Addition on the Structure, Microstructure, and Selected Mechanical Properties of As-Cast Zr-25Ta-xTi Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehjabeen, A.; Song, T.; Xu, W.; Tang, H.P.; Qian, M. Zirconium Alloys for Orthopaedic and Dental Applications. Adv. Eng. Mater. 2018, 20, 1800207. [Google Scholar] [CrossRef]
- Han, M.-K.; Hwang, M.-J.; Yang, M.-S.; Yang, H.-S.; Song, H.-J.; Park, Y.-J. Effect of zirconium content on the microstructure, physical properties and corrosion behavior of Ti alloys. Mater. Sci. Eng. A 2014, 616, 268–274. [Google Scholar] [CrossRef]
- Mello, D.d.C.R.; de Oliveira, J.R.; Cairo, C.A.A.; Ramos, L.S.d.B.; Vegian, M.R.d.C.; de Vasconcellos, L.G.O.; de Oliveira, F.E.; de Oliveira, L.D.; de Vasconcellos, L.M.R. Titanium alloys: In vitro biological analyzes on biofilm formation, biocompatibility, cell differentiation to induce bone formation, and immunological response. J. Mater. Sci. Mater. Med. 2019, 30, 108. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.; Mareci, D.; Bolat, G.; Santana, J.J.; Rodríguez, J.J.S.; Fernández-Mérida, L.C.; Burtan, L.; Trincă, L.C.; Souto, R.M. Improvement of the Corrosion Resistance of Biomedical Zr-Ti Alloys Using a Thermal Oxidation Treatment. Metals 2020, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.C.; Aristone, F.; Rossi, J.L.; Martinez, L.G.; Cione, F.C.; Tsakiropoulos, P.; Grandini, C.R.; da Silva, W.F. Microstructural characterization of Zr1Nb alloy after hot rolling. Int. J. Refract. Met. Hard Mater. 2019, 80, 216–224. [Google Scholar] [CrossRef]
- Okabe, F.; Kim, H.Y.; Miyazaki, S. Stress induced martensitic transformation and shape memory effect in Zr-Nb-Sn alloys. Scr. Mater. 2018, 162, 412–415. [Google Scholar] [CrossRef]
- Guo, S.; Shang, Y.; Zhang, J.; Zhang, J.; Meng, Q.; Cheng, X.; Zhao, X. A metastable β-type Zr-4Mo-4Sn alloy with low cost, low Young’s modulus and low magnetic susceptibility for biomedical applications. J. Alloys Compd. 2018, 754, 232–237. [Google Scholar] [CrossRef]
- Zhang, J.; Gan, X.; Tang, H.; Zhan, Y. Enhancement of wear and corrosion resistance of low modulus β-type Zr-20Nb-xTi (x = 0, 3) dental alloys through thermal oxidation treatment. Mater. Sci. Eng. C 2017, 76, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Hua, N.; Chen, W.; Zhang, L.; Li, G.; Liao, Z.; Lin, Y. Mechanical properties and bio-tribological behaviors of novel beta-Zr-type Zr-Al-Fe-Nb alloys for biomedical applications. Mater. Sci. Eng. C 2017, 76, 1154–1165. [Google Scholar] [CrossRef]
- Tsuno, A.T.; Tanaka, Y.; Kondo, R.; Nomura, N.; Tsutsumi, Y.; Hanawa, T. Effect of Ta content on the magnetic susceptibility of Zr–Ta binary alloys preventing artefacts for MRI. Adv. Mater. Process. Technol. 2016, 2, 606–614. [Google Scholar] [CrossRef]
- Liang, S.; Yin, L.; Zhou, Y.; Feng, X.; Ma, M.; Liu, R.; Tan, C. Abnormal martensitic transformation of high Zr-containing Ti alloys. J. Alloys Compd. 2014, 615, 804–808. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Wang, B.L.; Qiu, K.J.; Li, L.; Lin, J.P.; Li, H.F.; Zheng, Y.F. Microstructure, mechanical property, corrosion behavior, and in vitro biocompatibility of Zr–Mo alloys. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, R.; Nomura, N.; Tsutsumi, Y.; Doi, H.; Hanawa, T. Microstructure and mechanical properties of as-cast Zr–Nb alloys. Acta Biomater. 2011, 7, 4278–4284. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, P.; Quadros, F.D.F.; Sousa, K.; Donato, T.A.G.; De Araújo, R.O.; Grandini, C.R. Preparation, structural, microstructural, mechanical and cytotoxic characterization of as-cast Ti-25Ta-Zr alloys. J. Mater. Sci. Mater. Med. 2020, 31, 19. [Google Scholar] [CrossRef]
- Jawed, S.; Rabadia, C.D.; Liu, Y.; Wang, L.; Qin, P.; Li, Y.; Zhang, X.; Zhang, L. Strengthening mechanism and corrosion resistance of beta-type Ti-Nb-Zr-Mn alloys. Mater. Sci. Eng. C 2020, 110, 110728. [Google Scholar] [CrossRef]
- Torrento, J.; Grandini, C.; Sousa, T.; Rocha, L.; Gonçalves, T.; Sottovia, L.; Rangel, E.; Cruz, N.; Correa, D. Bulk and surface design of MAO-treated Ti-15Zr-15Mo-Ag alloys for potential use as biofunctional implants. Mater. Lett. 2020, 269, 127661. [Google Scholar] [CrossRef]
- Correa, D.; Kuroda, P.; Lourenço, M.; Buzalaf, M.; Grandini, C. Adjustment of the microstructure and selected mechanical properties of biomedical Ti-15Zr-Mo alloys through oxygen doping. J. Alloys Compd. 2018, 775, 158–167. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Zhao, X.; Sheng, Y.; Li, W.; Chang, C.-L.; Zhang, Q.; Yu, Z.; Wang, X. Microstructure, mechanical properties and corrosion behaviors of biomedical Ti-Zr-Mo-xMn alloys for dental application. Corros. Sci. 2019, 161, 108195. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Hori, T.; Nakano, T. Microstructure of equiatomic and non-equiatomic Ti-Nb-Ta-Zr-Mo high-entropy alloys for metallic biomaterials. J. Alloys Compd. 2018, 753, 412–421. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Kuroda, P.; Lourenço, M.L.; Buzalaf, M.; Mendoza, M.; Archanjo, B.; Achete, C.; Rocha, L.; Grandini, C. Microstructure and selected mechanical properties of aged Ti-15Zr-based alloys for biomedical applications. Mater. Sci. Eng. C 2018, 91, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Correa, D.; Kuroda, P.; Lourenço, M.; Fernandes, C.; Buzalaf, M.; Zambuzzi, W.; Grandini, C. Development of Ti-15Zr-Mo alloys for applying as implantable biomedical devices. J. Alloys Compd. 2018, 749, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Lide, D. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 85th ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 2712. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Zirconium and Hafnium. In Metals Handbook Desk Edition, 2nd ed.; Davis, J.R. (Ed.) ASM International: Metals Park, OH, USA, 1998. [Google Scholar]
- Boyer, R.; Welsch, G.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Materials Park, OH, USA, 1994. [Google Scholar]
- Nasser, S. Tantalum. In Materials for Medical Devices; Narayan, R.J., Ed.; ASM International: Materials Park, OH, USA, 2012; Volume 23. [Google Scholar]
- Wu, C.Y.; Xin, Y.H.; Wang, X.F.; Lin, J.G. Effects of Ta content on the phase stability and elastic properties of [beta] Ti-Ta alloys from first-principles calculations. Solid State Sci. 2010, 12, 2120–2124. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2005, 398, 28–36. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Wu, S.-C.; Sung, Y.-C.; Ho, W.-F. The structure and mechanical properties of as-cast Zr–Ti alloys. J. Alloys Compd. 2009, 488, 279–283. [Google Scholar] [CrossRef]
- Chausov, M.; Maruschak, P.; Pylypenko, A.; Markashova, L. Enhancing plasticity of high-strength titanium alloys VT 22 under impact-oscillatory loading. Philos. Mag. 2016, 97, 389–399. [Google Scholar] [CrossRef]
- Quadros, F.D.F.; Kuroda, P.A.B.; Sousa, K.D.S.J.; Donato, T.A.G.; Grandini, C.R. Preparation, structural and microstructural characterization of Ti-25Ta-10Zr alloy for biomedical applications. J. Mater. Res. Technol. 2019, 8, 4108–4114. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Buzalaf, M.A.R.; Grandini, C.R. Effect of molybdenum on structure, microstructure and mechanical properties of biomedical Ti-20Zr-Mo alloys. Mater. Sci. Eng. C 2016, 67, 511–515. [Google Scholar] [CrossRef] [Green Version]
- ASTM. Standard Test Method for Analysis of Titanium and Titanium Alloys by Atomic Emission Plasma Spectrometry; ASTM E2371-13; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM. Standard Test Method for Determination of Oxygen and Nitrogen in Titanium and Titanium Alloys by the Inert Gas Fusion Technique; ASTM E1409-13; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM. Test Methods for Vickers Hardness and Knoop Hardness of Metallic Materials; ASTM E92-16; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM. Standard Test Method for Dynamic Young’s Modulus, Shear Modulus, and Poisson’s Ratio by Impulse Excitation of Vibration; ASTM E1876-15; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM. Standard Specification for Zirconium and Zirconium Alloy Strip, Sheet, and Plate; ASTM B551/B551M-12; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Collings, E.W. The Physical Metallurgy of Titanium Alloys; ASM International: Metals Park, OH, USA, 1989. [Google Scholar]
- Zhou, Y.-L.; Niinomi, M. Ti–25Ta alloy with the best mechanical compatibility in Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. C 2009, 29, 1061–1065. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Decomposition of martensite α″ during aging treatments and resulting mechanical properties of Ti−Ta alloys. Mater. Sci. Eng. A 2004, 384, 92–101. [Google Scholar] [CrossRef]
- Gray, G.T.I.; Morris, C.E.; Lawson, A.C. Omega phase formation in titanium and titanium alloys. In Proceedings of the Conference: 7. World Conference and Exhibition on Titanium, San Diego, CA, USA, 28 June–2 July 1992. [Google Scholar]
- Manda, P.; Chakkingal, U.; Singh, A. Hardness characteristic and shear band formation in metastable β-titanium alloys. Mater. Charact. 2014, 96, 151–157. [Google Scholar] [CrossRef]
- Lütjering, G.; Williams, J.C.; Gysler, A. Microstructure and mechanical properties of titanium alloys. Microstruct. Prop. Mater. 2000, 1–77. [Google Scholar] [CrossRef]
- He, G.; Eckert, J.; Löser, W.; Hagiwara, M. Processing dependence of Young’s modulus of Ti-base nanostructured alloys. Solid State Commun. 2004, 129, 711–715. [Google Scholar] [CrossRef]
- Allıoğlu, Ş.; Acar, P. Design of β-Titanium microstructures for implant materials. Mater. Sci. Eng. C 2020, 110, 110715. [Google Scholar] [CrossRef] [PubMed]
- Liang, S. Review of the Design of Titanium Alloys with Low Elastic Modulus as Implant Materials. Adv. Eng. Mater. 2020, 22, 2000555. [Google Scholar] [CrossRef]
- Manda, P.; Chakkingal, U.; Singh, A.K. Effect of Alloying Elements in Hot-Rolled Metastable β-Titanium Alloys. Part II: Mechanical Properties. Metall. Mater. Trans. A 2016, 47, 3447–3463. [Google Scholar] [CrossRef]
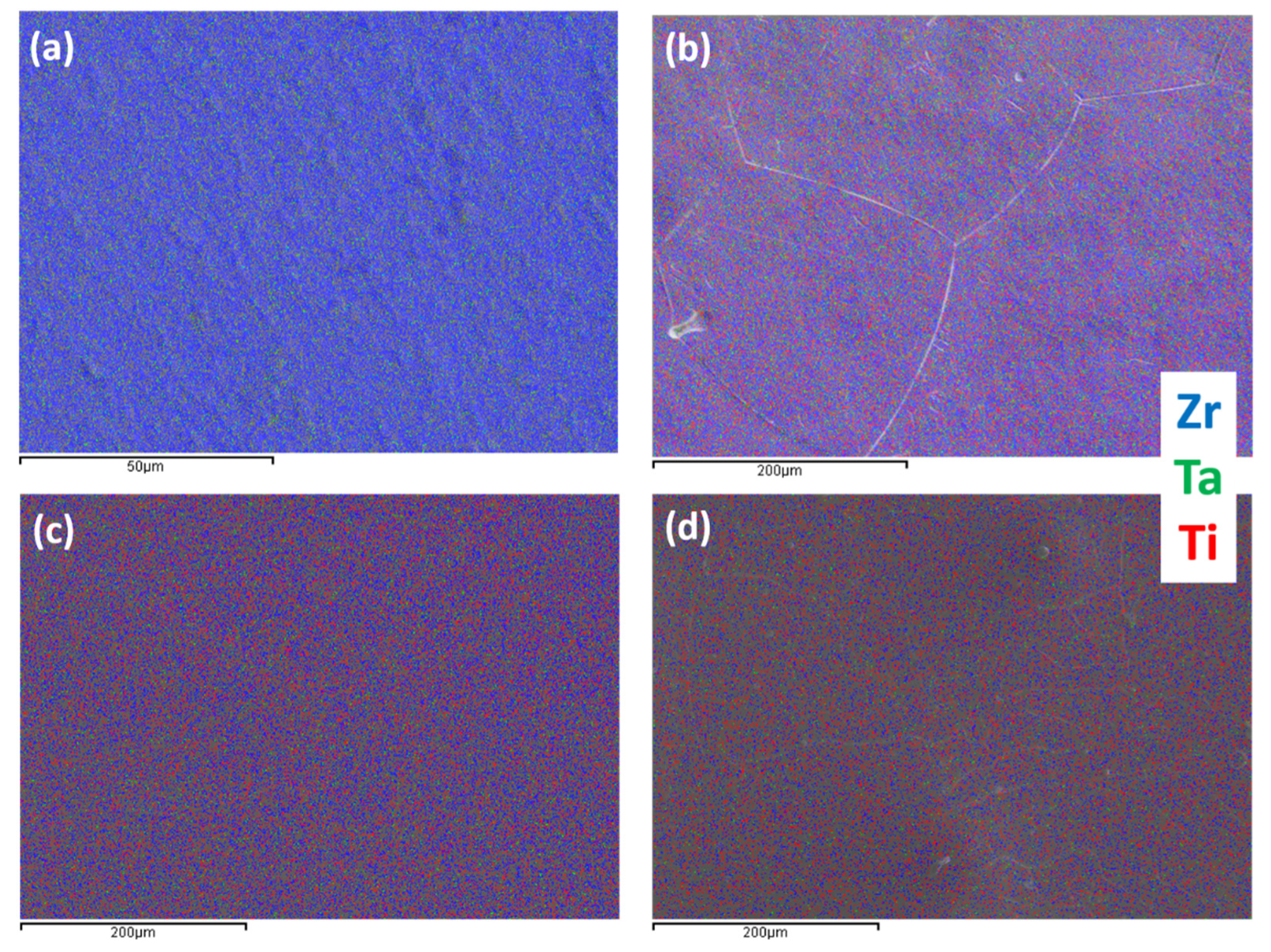
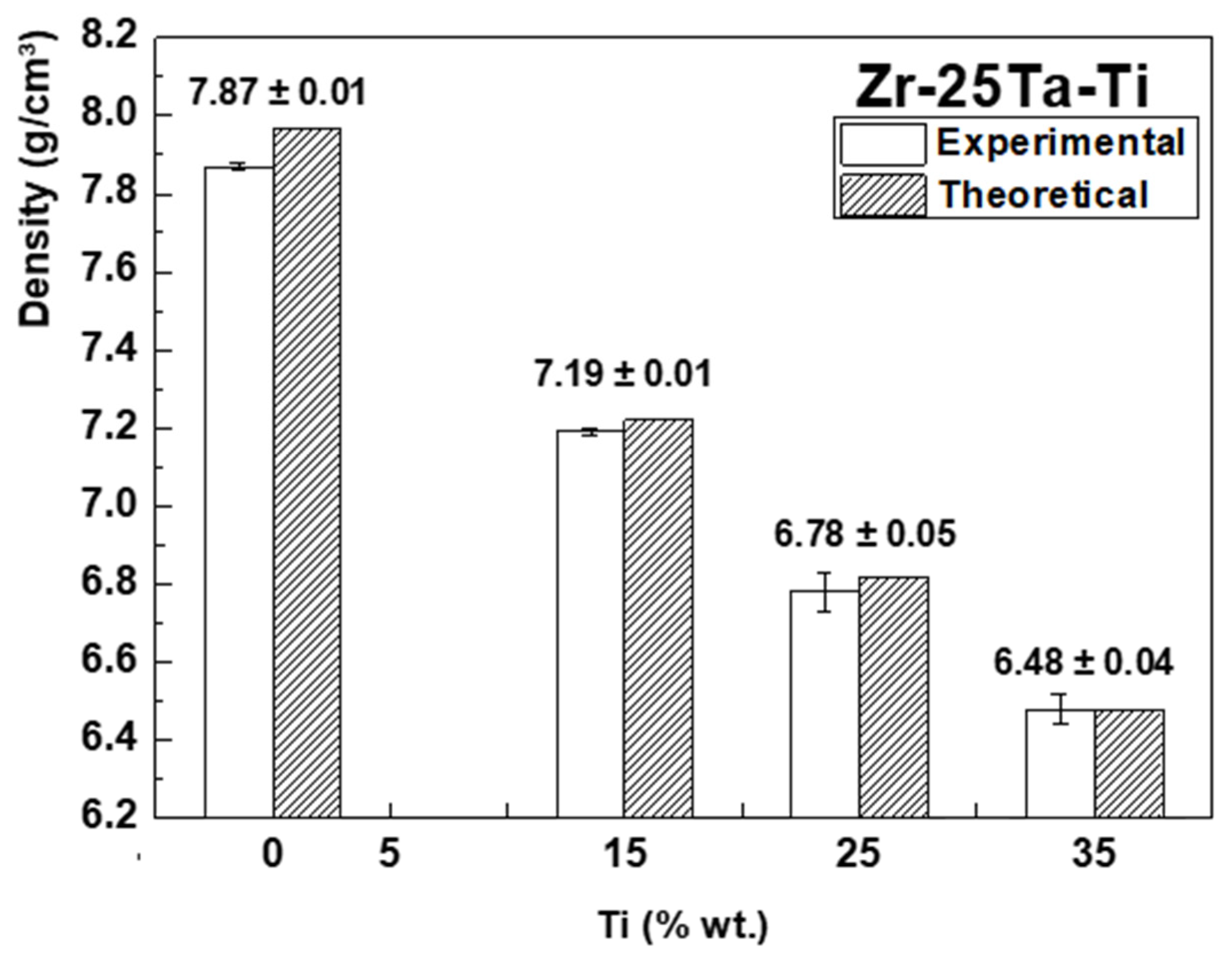
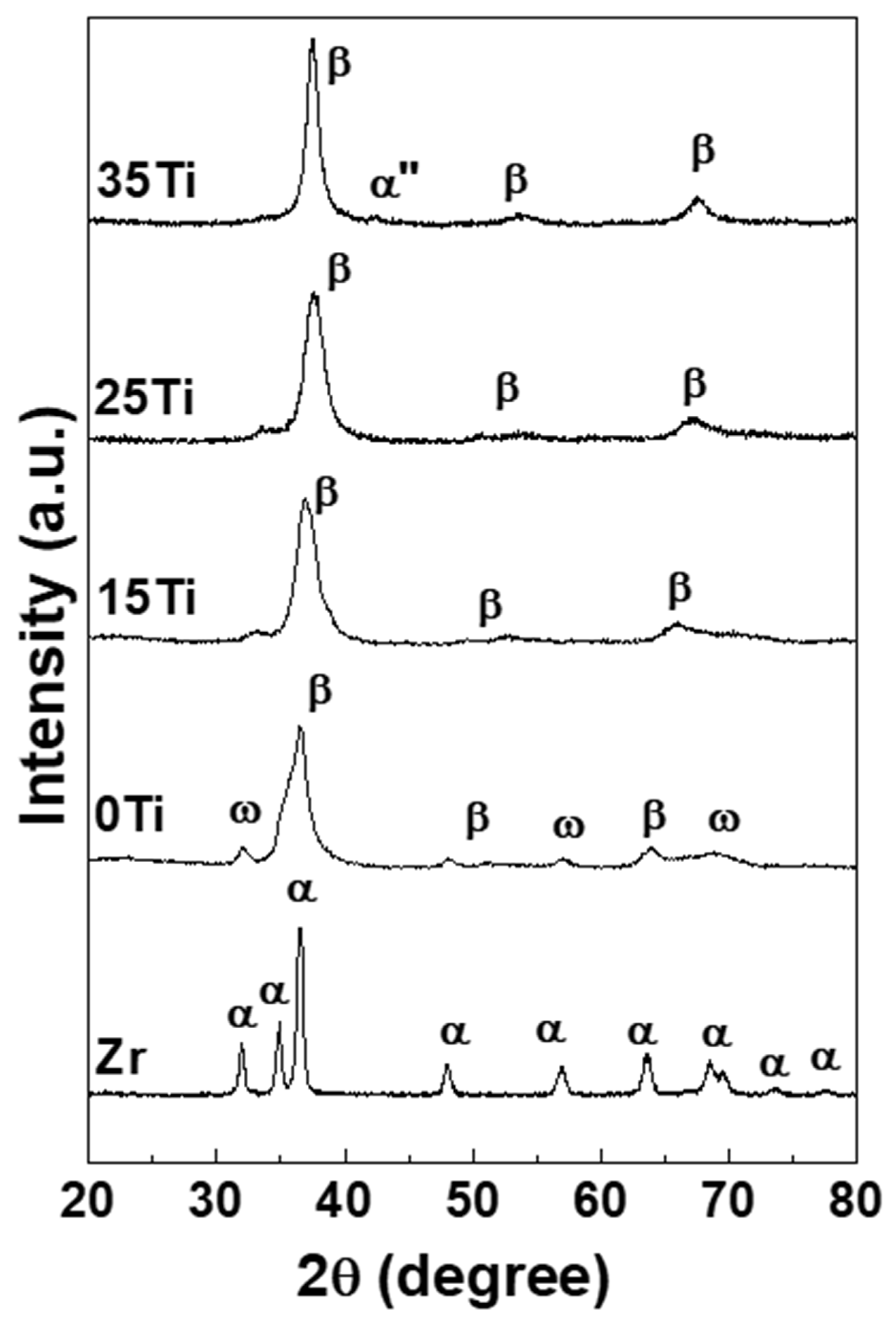



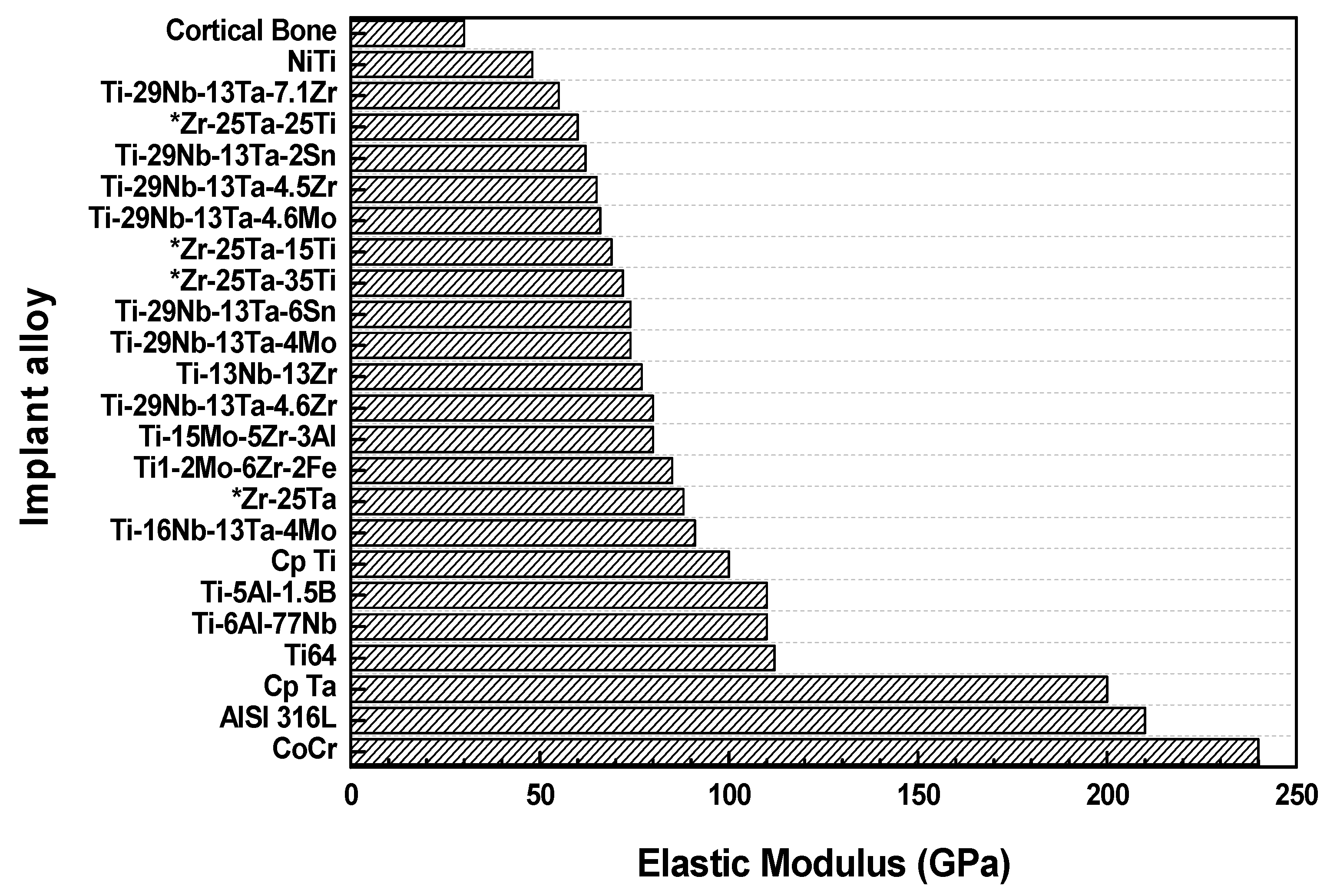
| Element (wt %) | Zr-25Ta | Zr-25Ta-15Ti | Zr-25Ta-25Ti | Zr-25Ta-35Ti [14] |
|---|---|---|---|---|
| Al | 0.34 | 0.27 | 0.01 | 0.16 |
| Cr | 0.01 | 0.01 | 0.01 | 0.01 |
| Cu | 0.01 | 0.01 | 0.01 | 0.66 |
| Fe | 0.41 | 0.05 | 0.09 | 0.05 |
| Hf | 1.00 | 0.77 | 1.10 | 0.20 |
| Mo | 0.01 | 0.11 | 0.09 | 0.01 |
| Ni | 0.18 | 0.18 | 0.01 | 0.08 |
| Si | 0.01 | 0.01 | 0.01 | 0.07 |
| Ta | 21.7 | 21.64 | 23.5 | 23.5 |
| Zr | 72.2 | 59.3 | 45.1 | 37.2 |
| Ti | Balance | Balance | Balance | Balance |
| Alloy | O (wt %) | N (wt %) |
|---|---|---|
| Zr-25Ta | 0.132 ± 0.004 | 0.004 ± 0.001 |
| Zr-25Ta-15Ti | 0.131 ± 0.005 | 0.003 ± 0.001 |
| Zr-25Ta-25Ti | 0.178 ± 0.039 | 0.010 ± 0.001 |
| Zr-25Ta-35Ti [14] | 0.144 ± 0.003 | 0.012 ± 0.001 |
| Alloy | Ta (wt %) | Ti (wt %) |
|---|---|---|
| Zr-25Ta | 28.6 | – |
| Zr-25Ta-15Ti | 27.6 | 14.7 |
| Zr-25Ta-25Ti | 25.8 | 24.5 |
| Zr-25Ta-35Ti | 26.9 | 35.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroda, P.A.B.; Pedroso, B.L.T.; Pontes, F.M.L.; Grandini, C.R. Effect of Titanium Addition on the Structure, Microstructure, and Selected Mechanical Properties of As-Cast Zr-25Ta-xTi Alloys. Metals 2021, 11, 1507. https://doi.org/10.3390/met11101507
Kuroda PAB, Pedroso BLT, Pontes FML, Grandini CR. Effect of Titanium Addition on the Structure, Microstructure, and Selected Mechanical Properties of As-Cast Zr-25Ta-xTi Alloys. Metals. 2021; 11(10):1507. https://doi.org/10.3390/met11101507
Chicago/Turabian StyleKuroda, Pedro Akira Bazaglia, Barbara Letícia Tomaz Pedroso, Fenelon Martinho Lima Pontes, and Carlos Roberto Grandini. 2021. "Effect of Titanium Addition on the Structure, Microstructure, and Selected Mechanical Properties of As-Cast Zr-25Ta-xTi Alloys" Metals 11, no. 10: 1507. https://doi.org/10.3390/met11101507
APA StyleKuroda, P. A. B., Pedroso, B. L. T., Pontes, F. M. L., & Grandini, C. R. (2021). Effect of Titanium Addition on the Structure, Microstructure, and Selected Mechanical Properties of As-Cast Zr-25Ta-xTi Alloys. Metals, 11(10), 1507. https://doi.org/10.3390/met11101507








