Influence of Cold Rolling Process and Chemical Composition on the Mechanical Properties and Corrosion Behavior of Zr-Based Metallic Glasses
Abstract
1. Introduction
2. Materials and Methods
3. Results
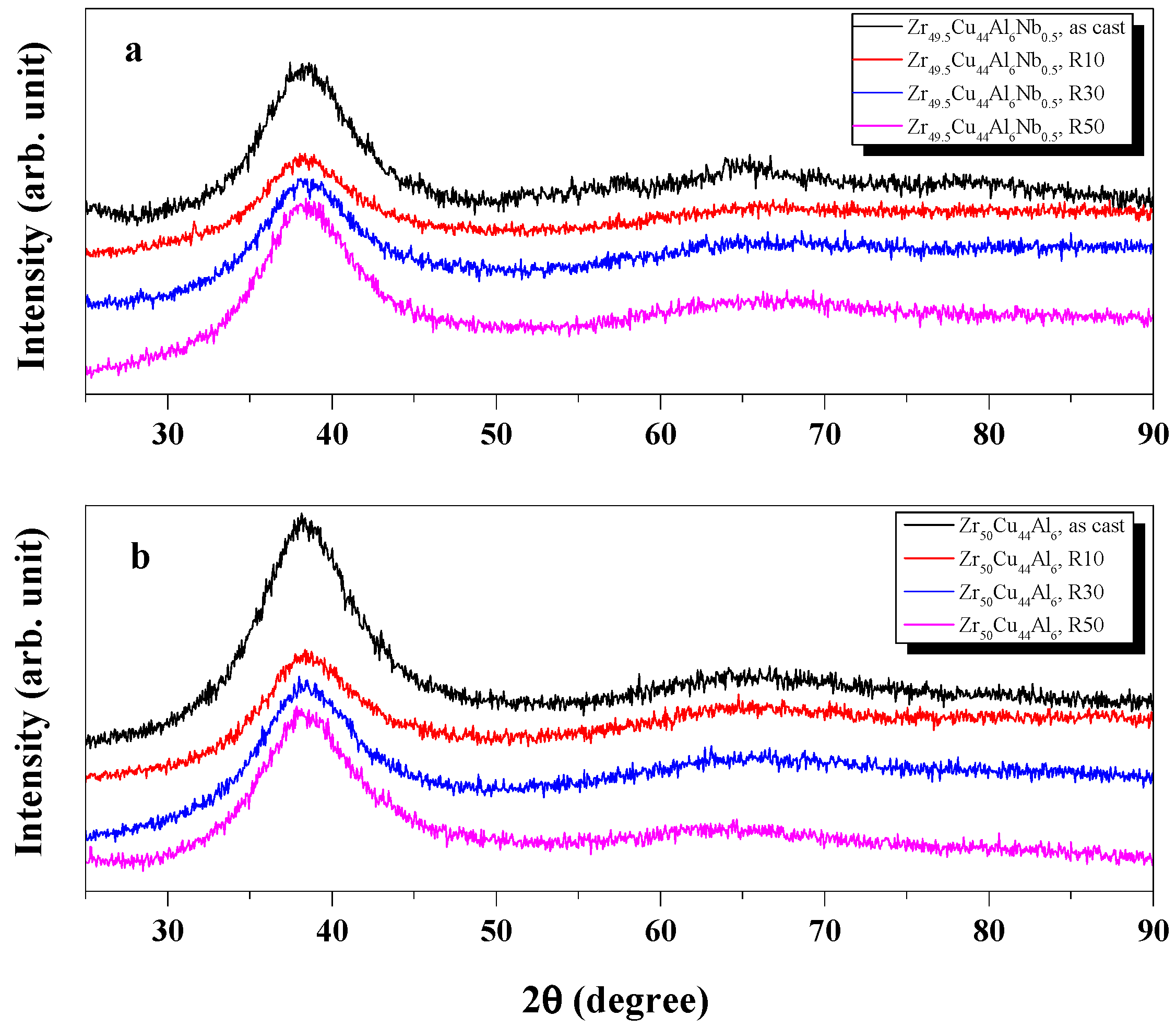
3.1. Structural Evolution by Cold Rolling
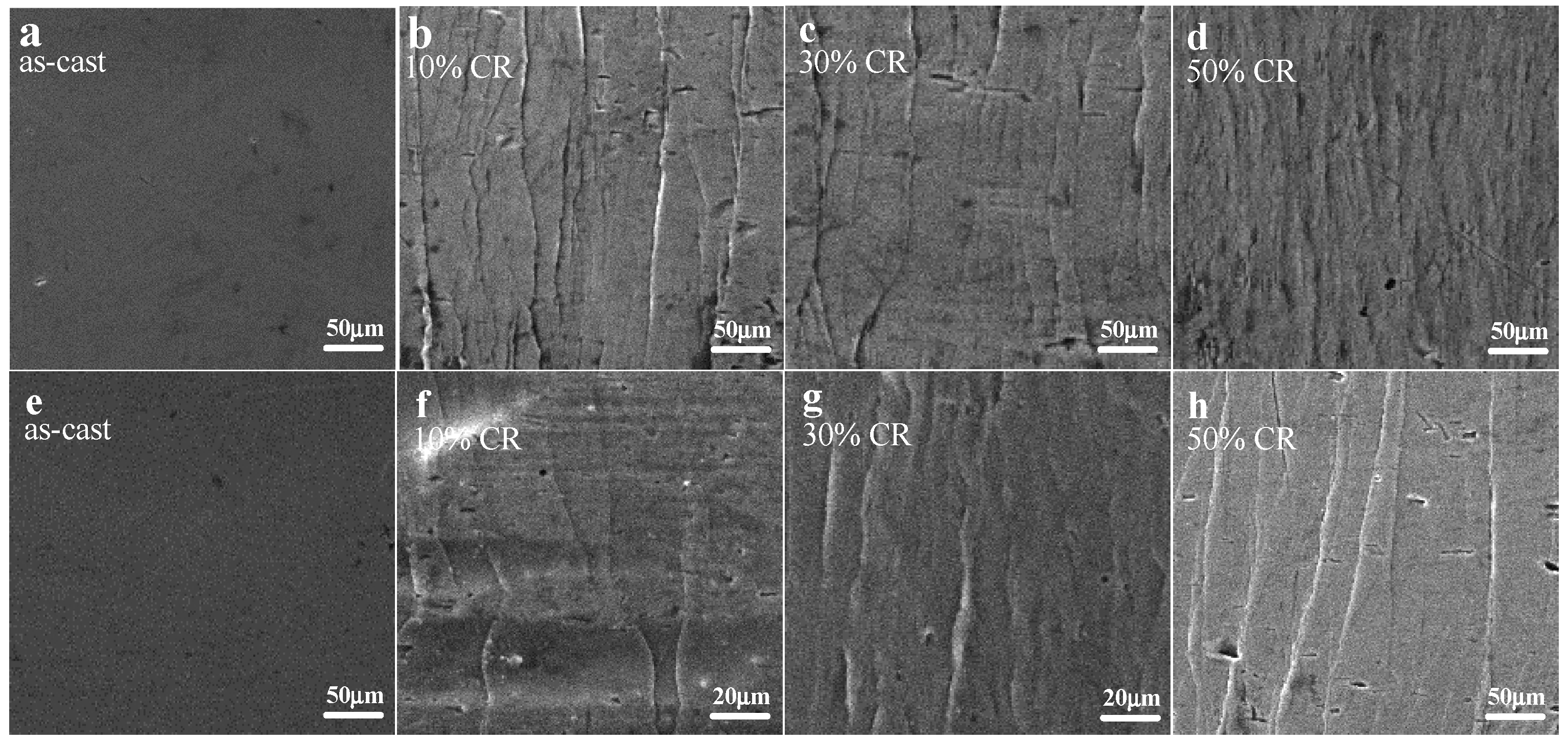
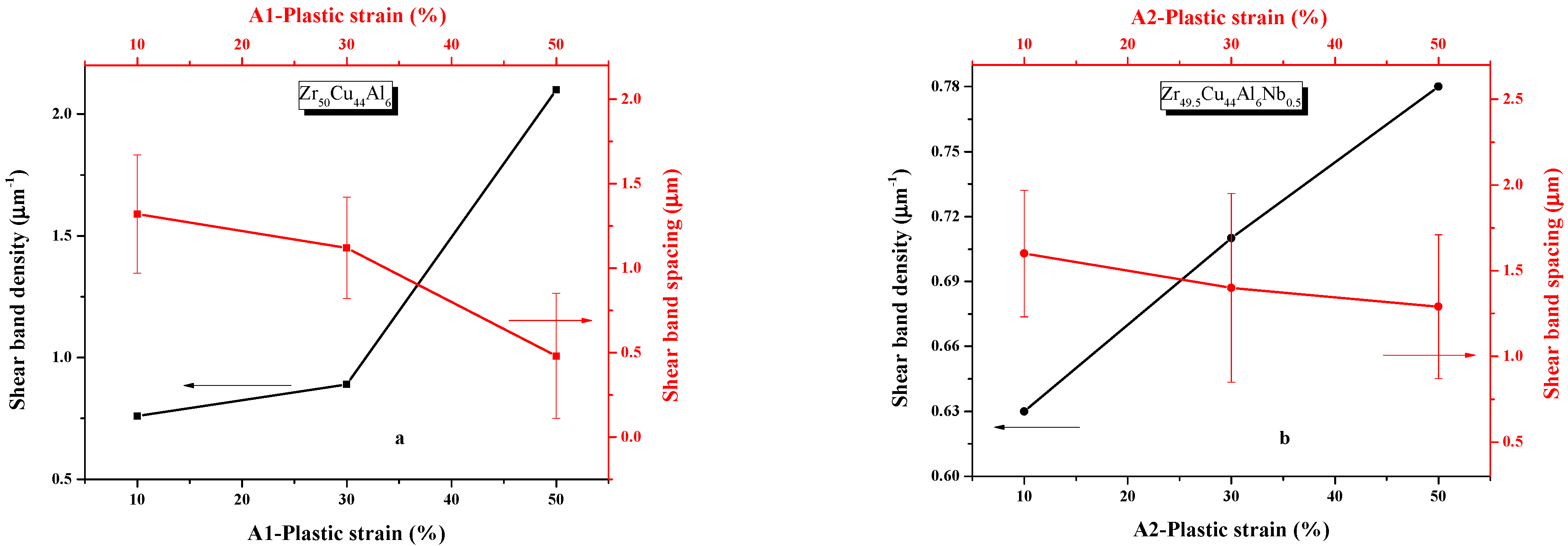
3.2. Shear Band Formation
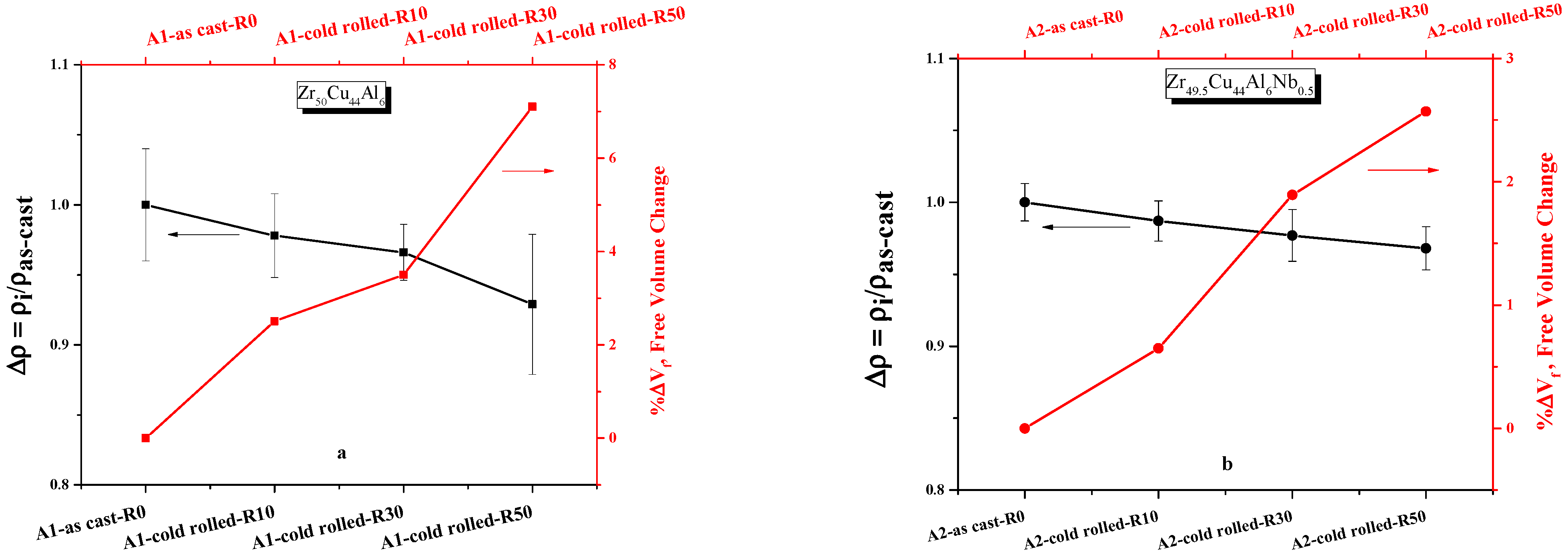
3.3. Characterization of the FV by Density Measurements
3.4. Change in Hardness Due to Cold Rolling
3.5. Corrosion Measurements
4. Discussion
4.1. Shear Band Evolution
4.2. Free Volume Changes
4.3. Changes in Hardness Due to Plastic Strain
4.4. Corrosion Investigation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vishwanadh, B.; Abraham, G.J.; Neogy, S.; Dutta, R.S.; Dey, G.K. Effect of structural defects, surface irregularities, and quenched-in defects on corrosion of Zr-based metallic glasses. Metall. Mater. Trans. A 2009, 40, 1131–1141. [Google Scholar] [CrossRef]
- Stolpe, M.; Kruzic, J.J.; Busch, R. Evolution of shear bands, free volume and hardness during cold rolling of a Zr-based bulk metallic glass. Acta Mater. 2014, 64, 231–240. [Google Scholar] [CrossRef]
- Liu, J.W.; Cao, Q.P.; Chen, L.Y.; Wang, X.D.; Jiang, J.Z. Shear band evolution and hardness change in cold-rolled bulk metallic glasses. Acta Mater. 2010, 58, 4827–4840. [Google Scholar] [CrossRef]
- Hubek, R.; Hilke, S.; Davani, F.A.; Golkia, M.; Shrivastav, G.P.; Divinski, S.V.; Rösner, H.; Horbach, J.; Wilde, G. Shear bands in monolithic metallic glasses: Experiment, theory, and modeling. Front. Mater. 2010, 7, 1–13. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, Y.; Sun, B.A.; Stoica, M.; Li, C.J.; Song, K.K.; Kühn, U.; Pan, F.S.; Eckert, J. Correlation between internal states and plasticity in bulk metallic glass. Appl. Phys. Lett. 2011, 98, 151906. [Google Scholar] [CrossRef]
- Spaepen, F. A microscopic mechanism for steady state inhomogeneous flow in metallic glasses. Acta Metall. 1977, 25, 407–415. [Google Scholar] [CrossRef]
- Argon, A.S. Plastic deformation in metallic glasses. Acta Metall. 1978, 27, 47–58. [Google Scholar] [CrossRef]
- Shi, B.; Xu, Y.; Li, C.; Jia, W.; Li, Z.; Li, J. Evolution of free volume and shear band intersections and its effect on hardness of deformed Zr64.13Cu15.75Ni10.12Al10 bulk metallic glass. J. Alloys Compd. 2016, 669, 167–176. [Google Scholar] [CrossRef]
- Brechtl, J.; Xie, X.; Wang, Z.; Qiao, J.; Liaw, P.K. Complexity analysis of serrated flows in a bulk metallic glass under constrained and unconstrained conditions. Mater. Sci. Eng. A 2020, 771, 138585. [Google Scholar] [CrossRef]
- Song, K.K.; Pauly, S.; Zhang, Y.; Scudino, S.; Gargarella, P.; Surreddi, K.B.; Kühn, U.; Eckert, J. Significant tensile ductility induced by cold rolling in Cu 47.5Zr47.5Al5 bulk metallic glass. Intermetallics 2011, 19, 1394–1398. [Google Scholar] [CrossRef]
- Nie, X.P.; Xu, X.M.; Jiang, Q.K.; Chen, L.Y.; Xu, Y.; Fang, Y.Z.; Xie, G.Q.; Luo, M.F.; Wu, F.M.; Wang, X.D.; et al. Effect of microalloying of Nb on corrosion resistance and thermal stability of ZrCu-based bulk metallic glasses. J. Non Cryst. Solids 2009, 355, 203–207. [Google Scholar] [CrossRef]
- Nie, X.P.; Cao, Q.P.; Wu, Z.F.; Ma, Y.; Wang, X.D.; Ding, S.Q.; Jiang, J.Z. The pitting corrosion behavior of shear bands in a Zr-based bulk metallic glass. Scr. Mater. 2012, 67, 376–379. [Google Scholar] [CrossRef]
- Jiang, W.H.; Jiang, F.; Green, B.A.; Liu, F.X.; Liaw, P.K.; Choo, H.; Qiu, K.Q. Electrochemical corrosion behavior of a Zr-based bulk-metallic glass. Appl. Phys. Lett. 2007, 91, 041904. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, C.L.; Zou, H.; Chan, K.C. The effect of the microalloying of Hf on the corrosion behavior of ZrCuNiAl bulk metallic glass. J. Alloys Compd. 2005, 399, 144–148. [Google Scholar] [CrossRef]
- Raju, V.R.; Kühn, U.; Wolff, U.; Schneider, F.; Eckert, J.; Reiche, R.; Gebert, A. Corrosion behaviour of Zr-based bulk glass-forming alloys containing Nb or Ti. Mater. Lett. 2002, 57, 173–177. [Google Scholar] [CrossRef]
- Gebert, A.; Gostin, P.F.; Uhlemann, M.; Eckert, J.; Schultz, L. Interactions between mechanically generated defects and corrosion phenomena of Zr-based bulk metallic glasses. Acta Mater. 2012, 60, 2300–2309. [Google Scholar] [CrossRef]
- Gebert, A.; Concustell, A.; Greer, A.L.; Schultz, L.; Eckert, J. Effect of shot-peening on the corrosion resistance of a Zr-based bulk metallic glass. Scr. Mater. 2010, 62, 635–638. [Google Scholar] [CrossRef]
- Qin, C.; Asami, K.; Zhang, T.; Zhang, W.; Inoue, A. Corrosion behavior of Cu-Zr-Ti-Nb bulk glassy alloys. Mater. Trans. 2003, 44, 749–753. [Google Scholar] [CrossRef]
- Mudali, U.K.; Baunack, S.; Eckert, J.; Schultz, L.; Gebert, A. Pitting corrosion of bulk glass-forming zirconium-based alloys. J. Alloys Compd. 2004, 377, 290–297. [Google Scholar] [CrossRef]
- Baunack, S.; Mudali, U.K.; Gebert, A. Characterization of oxide layers on amorphous Zr-based alloys by Auger electron spectroscopy with sputter depth profiling. Appl. Surf. Sci. 2005, 252, 162–166. [Google Scholar] [CrossRef]
- Qin, C.L.; Zhang, W.; Zhang, Q.S.; Asami, K.; Inoue, A. Electrochemical properties and surface analysis of Cu-Zr-Ag-Al-Nb bulk metallic glasses. J. Alloys Compd. 2009, 483, 317–320. [Google Scholar] [CrossRef]
- Lubenchenko, A.V.; Batrakov, A.A.; Pavolotsky, A.B.; Lubenchenko, O.I.; Ivanov, D.A. XPS study of multilayer multicomponent films. Appl. Surf. Sci. 2018, 427, 711–721. [Google Scholar] [CrossRef]
- Scudino, S.; Surreddi, K.B.; Eckert, J. Mechanical properties of cold-rolled Zr 60Ti 5Ag 5Cu 12.5Ni 10Al 7.5 metallic glass. Phys. Status Solidi 2010, 207, 1118–1121. [Google Scholar] [CrossRef]
- Song, S.X.; Nieh, T.G. Direct measurements of shear band propagation in metallic glasses—An overview. Intermetallics 2011, 19, 1968–1977. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, B.; Ma, Z.; Li, J. Evolution of shear bands, free volume, and structure in room temperature rolled Pd40Ni40P20 bulk metallic glass. Mater. Sci. Eng. A 2015, 623, 145–152. [Google Scholar] [CrossRef]
- Slipenyuk, A.; Eckert, J. Correlation between enthalpy change and free volume reduction during structural relaxation of Zr55Cu30Al10Ni 5 metallic glass. Scr. Mater. 2004, 50, 39–44. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses; Taylor and Francis Group: Boca Raton, FL, USA, 2011; pp. 265–273. [Google Scholar]
- Zhang, Y.; Hahn, H. Characterization of the free volume in a Zr45.0Cu 39.3Al7.0Ag8.7 bulk metallic glass by reverse Monte Carlo simulation and density measurements. J. Non Cryst. Solids 2011, 357, 1420–1425. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, J.-H.; Eckert, J. On the structural relaxation of bulk metallic glass under warm deformation. Intermetallics 2009, 17, 222–226. [Google Scholar] [CrossRef]
- Nowosielski, R.; Januszka, A. Structure and density of Fe 36 Co 36 B 19.2 Si 4.8 Nb 4 bulk glassy alloy. J. Achiev. Mater. Manuf. Eng. 2012, 52, 67–74. [Google Scholar]
- Bei, H.; Xie, S.; George, E.P. Softening caused by profuse shear banding in a bulk metallic glass. Phys. Rev. Lett. 2006, 96, 105503. [Google Scholar] [CrossRef]
- Gonzaález, S.; Chen, N.; Zhang, Q.S.; Louzguine-Luzgin, D.V.; Perepezko, J.H.; Inoue, A. Effect of shear bands initiated in the pre-yield region on the deformation behaviour of Zr-based metallic glasses. Scr. Mater. 2011, 64, 713–716. [Google Scholar] [CrossRef]
- Haruyama, O.; Kisara, K.; Yamashita, A.; Kogure, K.; Yokoyama, Y.; Sugiyama, K. Characterization of free volume in cold-rolled Zr55Cu 30Ni5Al10 bulk metallic glasses. Acta Mater. 2013, 61, 3224–3232. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, D.; Zhang, L.K.; Zhao, X.Y.; Zhang, A.L. Effect of ceramic rolling on the mechanical properties of Zr54Cu38Al8 bulk metallic glass. Mater. Sci. Eng. A 2014, 592, 64–69. [Google Scholar] [CrossRef]
- Zhang, P.N.; Li, J.F.; Hu, Y.; Zhou, Y.H. Effect of rolling on the microstructure and mechanical property of Zr52.5Cu17.9Ni14.6Al10Ti5 bulk metallic glass. J. Alloys Compd. 2008, 462, 88–93. [Google Scholar] [CrossRef]
- Tang, C.; Li, Y.; Zeng, K. Characterization of mechanical properties of a Zr-based metallic glass by indentation techniques. Mater. Sci. Eng. A 2004, 384, 215–223. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Y.; Si, J.; Liu, Y.; Hui, X. Plasticizing and work hardening in phase separated Cu-Zr-Al-Nb bulk metallic glasses by deformation induced nanocrystallization. Mater. Des. 2018, 142, 74–82. [Google Scholar] [CrossRef]
- Scully, J.R.; Gebert, A.; Payer, J.H. Corrosion and related mechanical properties of bulk metallic glasses. J. Mater. Res. 2007, 22, 302–313. [Google Scholar] [CrossRef]
- Gebert, A.; Gostin, F.; Kühn, U.; Schultz, L. Corrosion of a Zr-based bulk metallic glass with different surface finishing states. ECS Trans. 2009, 16, 1–7. [Google Scholar] [CrossRef]
- Guo, S.F.; Zhang, H.J.; Liu, Z.; Chen, W.; Xie, S.F. Corrosion resistances of amorphous and crystalline Zr-based alloys in simulated seawater. Electrochem. Commun. 2012, 24, 39–42. [Google Scholar] [CrossRef]
- Cao, G.; Liu, K.; Liu, G.; Zong, H.; Bala, H.; Zhang, B. Improving the glass-forming ability and the plasticity of Zr-Cu-Al bulk metallic glass by addition of Nb. J. Non Cryst. Solids. 2019, 513, 105–110. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Zhou, W.; Hou, J.X.; Sheng, M.Q. Microstructural change and corrosion behavior during rolling of Zr-based bulk metallic glass. Int. J. Electrochem. Sci. 2016, 11, 7163–7172. [Google Scholar] [CrossRef][Green Version]
- Nie, X.P.; Yang, X.H.; Chen, L.Y.; Yeap, K.B.; Zeng, K.Y.; Li, D.; Pan, J.S.; Wang, X.D.; Cao, Q.P.; Ding, S.Q.; et al. The effect of oxidation on the corrosion resistance and mechanical properties of a Zr-based metallic glass. Corros. Sci. 2011, 53, 3557–3565. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Chen, C.L.; Lin, L.Y.; Wang, Z.C.; Ketov, S.V.; Miyama, M.J.; Trifonov, A.S.; Lubenchenko, A.V.; Ikuhara, Y. Bulk metallic glassy surface native oxide: Its atomic structure, growth rate and electrical properties. Acta Mater. 2015, 97, 282–290. [Google Scholar] [CrossRef]



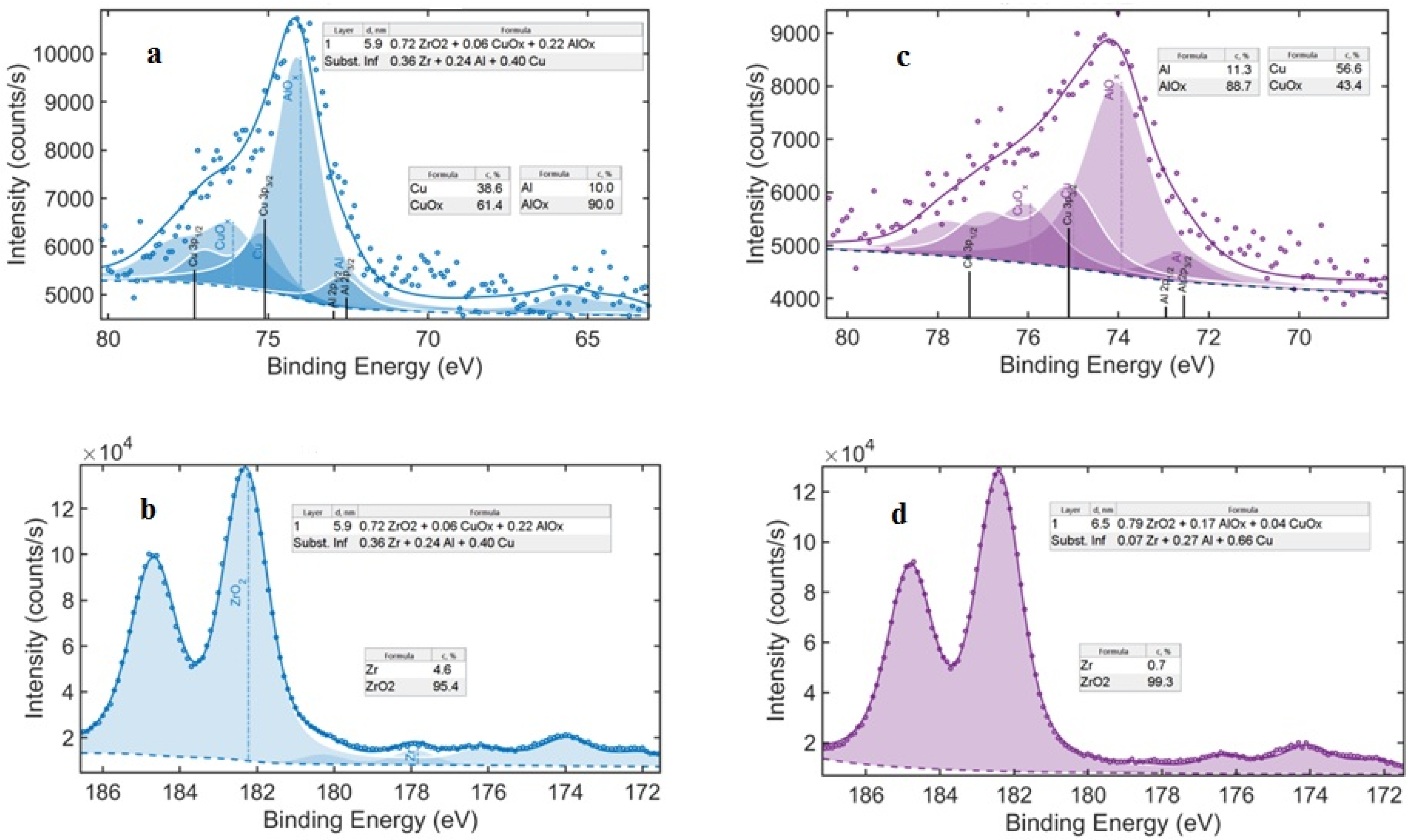
| Alloys | Plastic Strain, (%) | Ecorr, (V) | icorr, (Am−2) |
|---|---|---|---|
| 0.5 M NaCl | |||
| Zr50Cu44Al6 Zr49.5Cu44Al6Nb0.5 Zr50Cu44Al6 Zr49.5Cu44Al6Nb0.5 | 0 0 50 50 | −0.4 −0.375 −0.375 −0.374 | 2.8 × 10−5 2.1 × 10−5 2.0 × 10−3 1.8 × 10−3 |
| 0.5 M Na2SO4 | |||
| Zr50Cu44Al6 Zr49.5Cu44Al6Nb0.5 Zr50Cu44Al6 Zr49.5Cu44Al6Nb0.5 | 0 0 50 50 | −0.397 −0.368 −0.164 −0.152 | 2.6 × 10−5 1.9 × 10−5 5.1 × 10−5 5.0 × 10−5 |
| Zr50Cu44Al6 (As-Cast) | Zr50Cu44Al6 (50% Thickness Reduction) | |||||
|---|---|---|---|---|---|---|
| Ct, % | Oxide Formula 0.72 ZrO2 + 0.06 CuOx + 0.22 AlOx | Cp, % | Ct, % | Oxide Formula 0.79 ZrO2 + 0.04 CuOx + 0.17 AlOx | Cp, % | |
| Zr | 36 | ZrO2 Zr | 95.4 4.6 | 7 | ZrO2 Zr | 99.3 0.7 |
| Cu | 40 | CuOx Cu | 61.4 38.6 | 66 | CuOx Cu | 43.4 56.6 |
| Al | 24 | AlOx Al | 90.0 10.0 | 27 | AlOx Al | 88.7 11.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbarpour, A.; Milkova, D.A.; Zanaeva, E.N.; Parkhomenko, M.S.; Cheverikin, V.V.; Lubenchenko, A.; Bazlov, A.I. Influence of Cold Rolling Process and Chemical Composition on the Mechanical Properties and Corrosion Behavior of Zr-Based Metallic Glasses. Metals 2021, 11, 1514. https://doi.org/10.3390/met11101514
Akbarpour A, Milkova DA, Zanaeva EN, Parkhomenko MS, Cheverikin VV, Lubenchenko A, Bazlov AI. Influence of Cold Rolling Process and Chemical Composition on the Mechanical Properties and Corrosion Behavior of Zr-Based Metallic Glasses. Metals. 2021; 11(10):1514. https://doi.org/10.3390/met11101514
Chicago/Turabian StyleAkbarpour, Ali, Daria A. Milkova, Erzhena N. Zanaeva, Mark S. Parkhomenko, Vladimir V. Cheverikin, Alexander Lubenchenko, and Andrey I. Bazlov. 2021. "Influence of Cold Rolling Process and Chemical Composition on the Mechanical Properties and Corrosion Behavior of Zr-Based Metallic Glasses" Metals 11, no. 10: 1514. https://doi.org/10.3390/met11101514
APA StyleAkbarpour, A., Milkova, D. A., Zanaeva, E. N., Parkhomenko, M. S., Cheverikin, V. V., Lubenchenko, A., & Bazlov, A. I. (2021). Influence of Cold Rolling Process and Chemical Composition on the Mechanical Properties and Corrosion Behavior of Zr-Based Metallic Glasses. Metals, 11(10), 1514. https://doi.org/10.3390/met11101514







