Transfer of Gold, Platinum and Non-Ferrous Metals from Matte to Slag by Flotation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
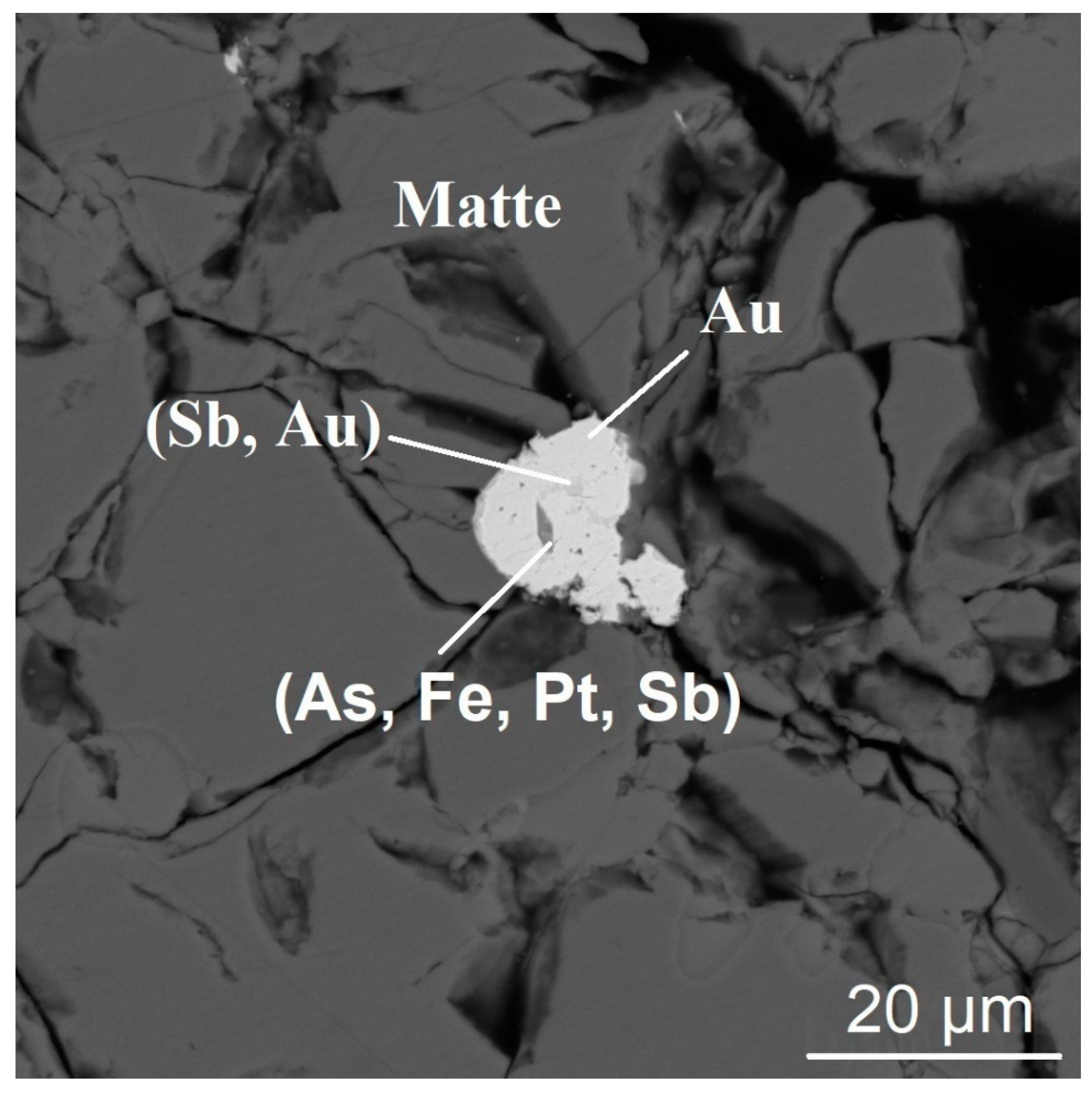
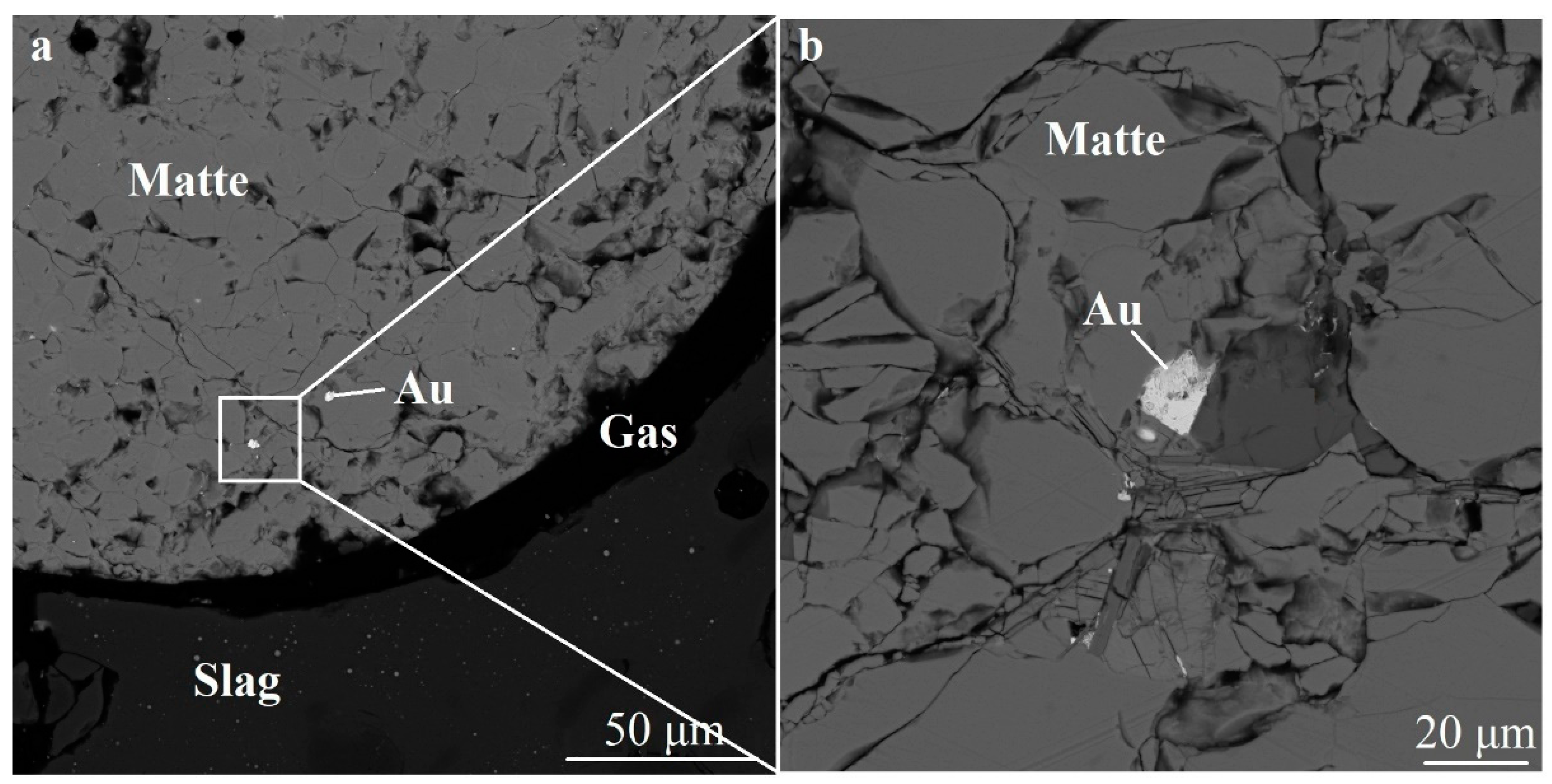
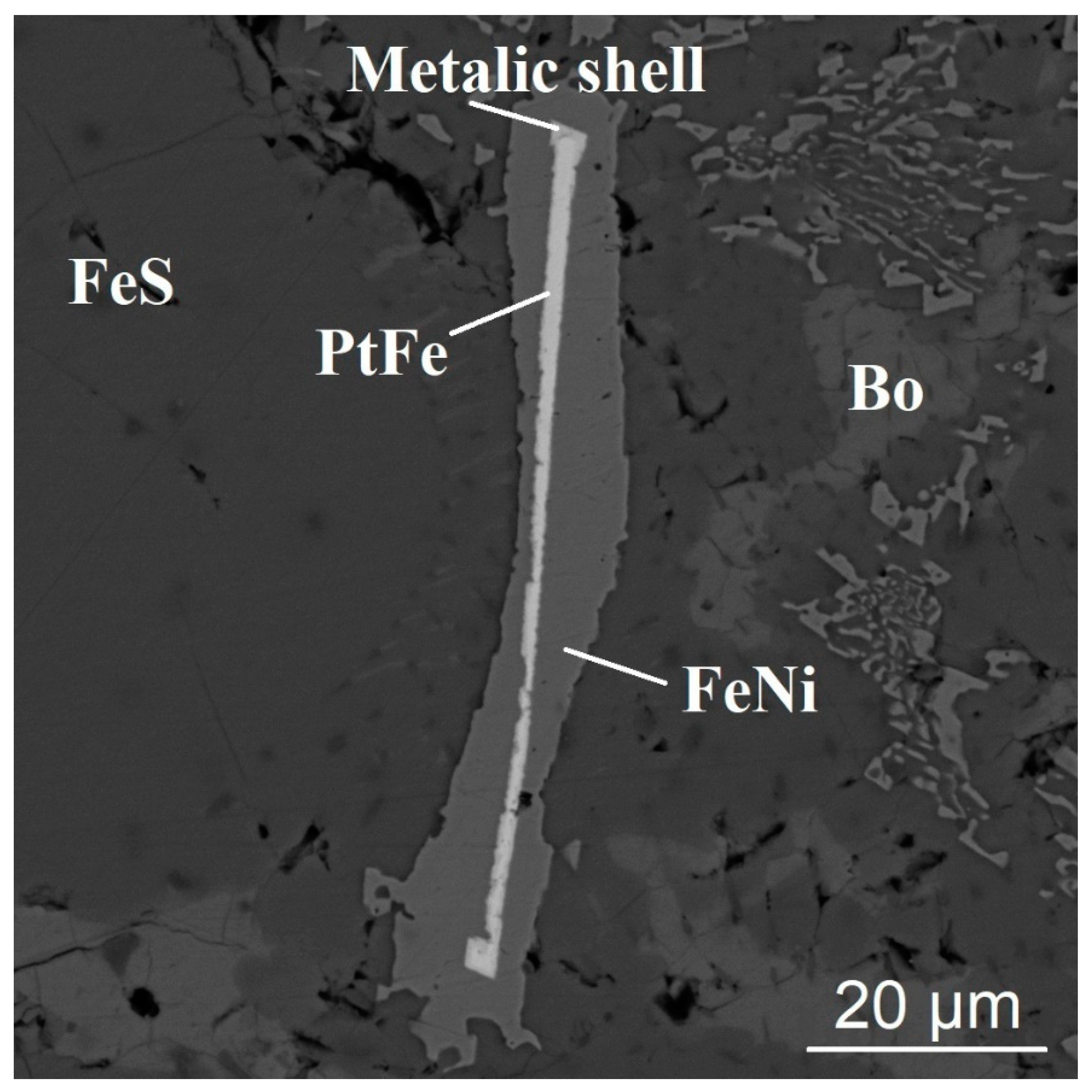
3.1. The Form of Finding of Gold and Platinum in the Melting Products of Sulfide Materials
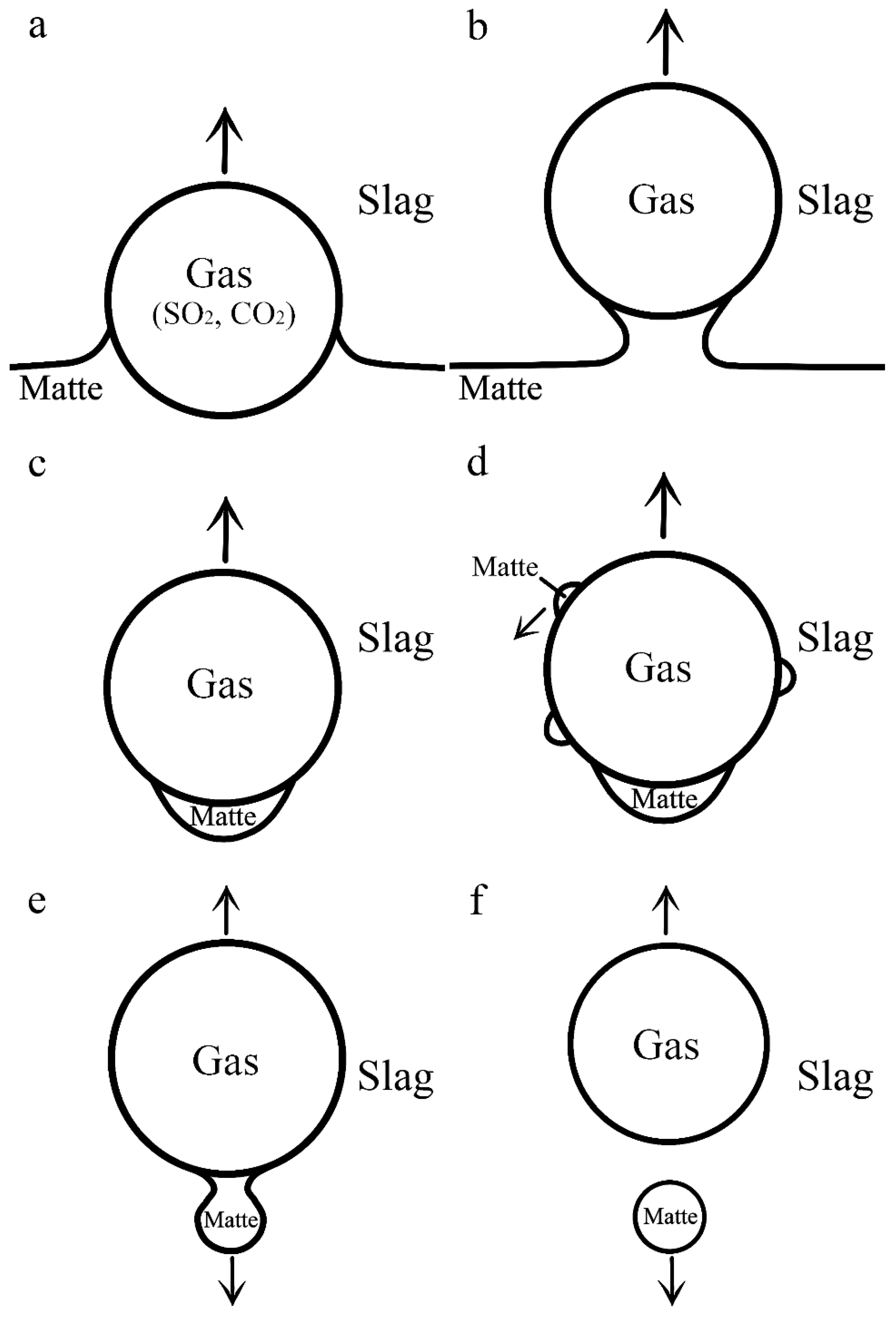
3.2. Flotation of Matte Droplets in an Oxide Melt
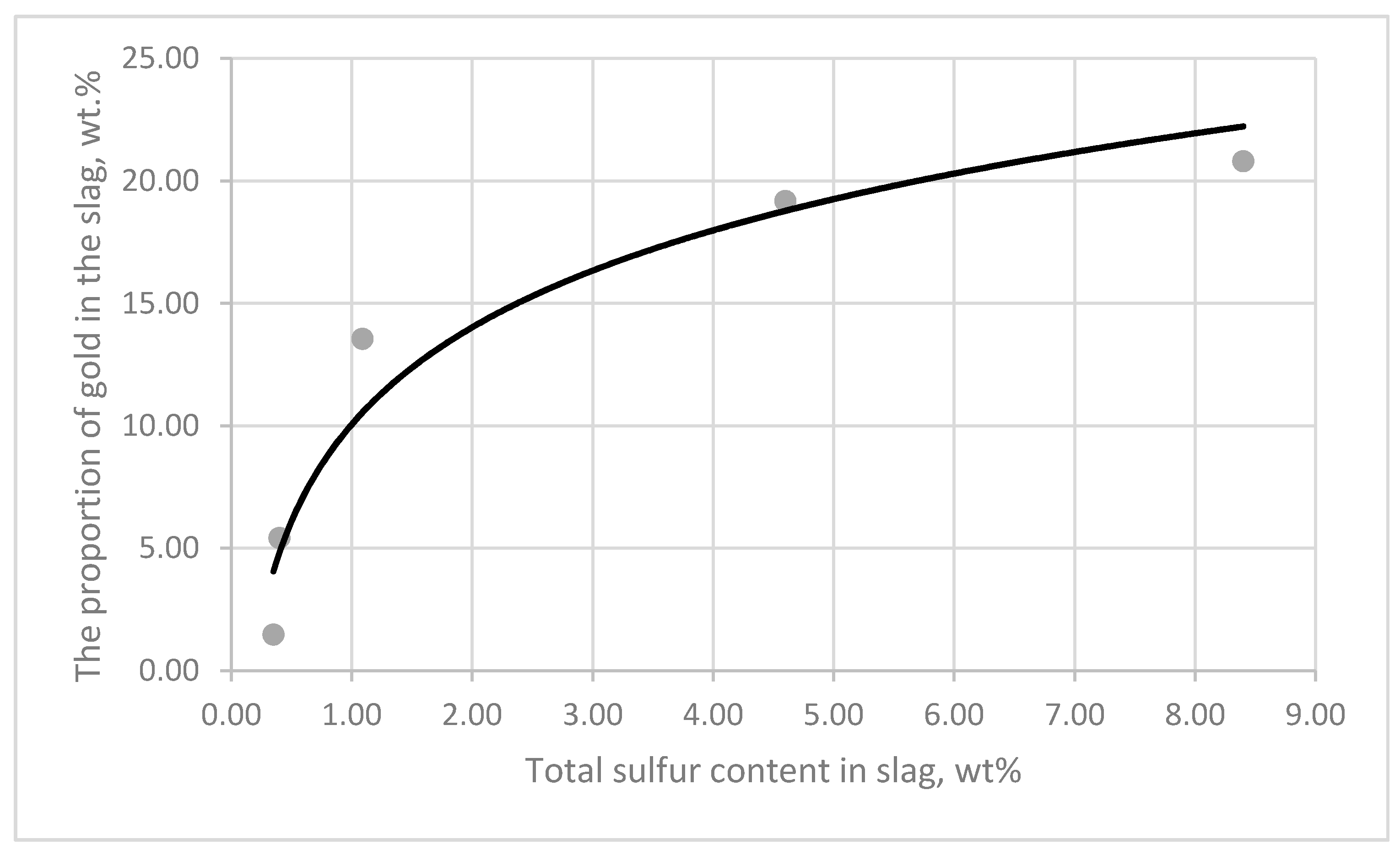
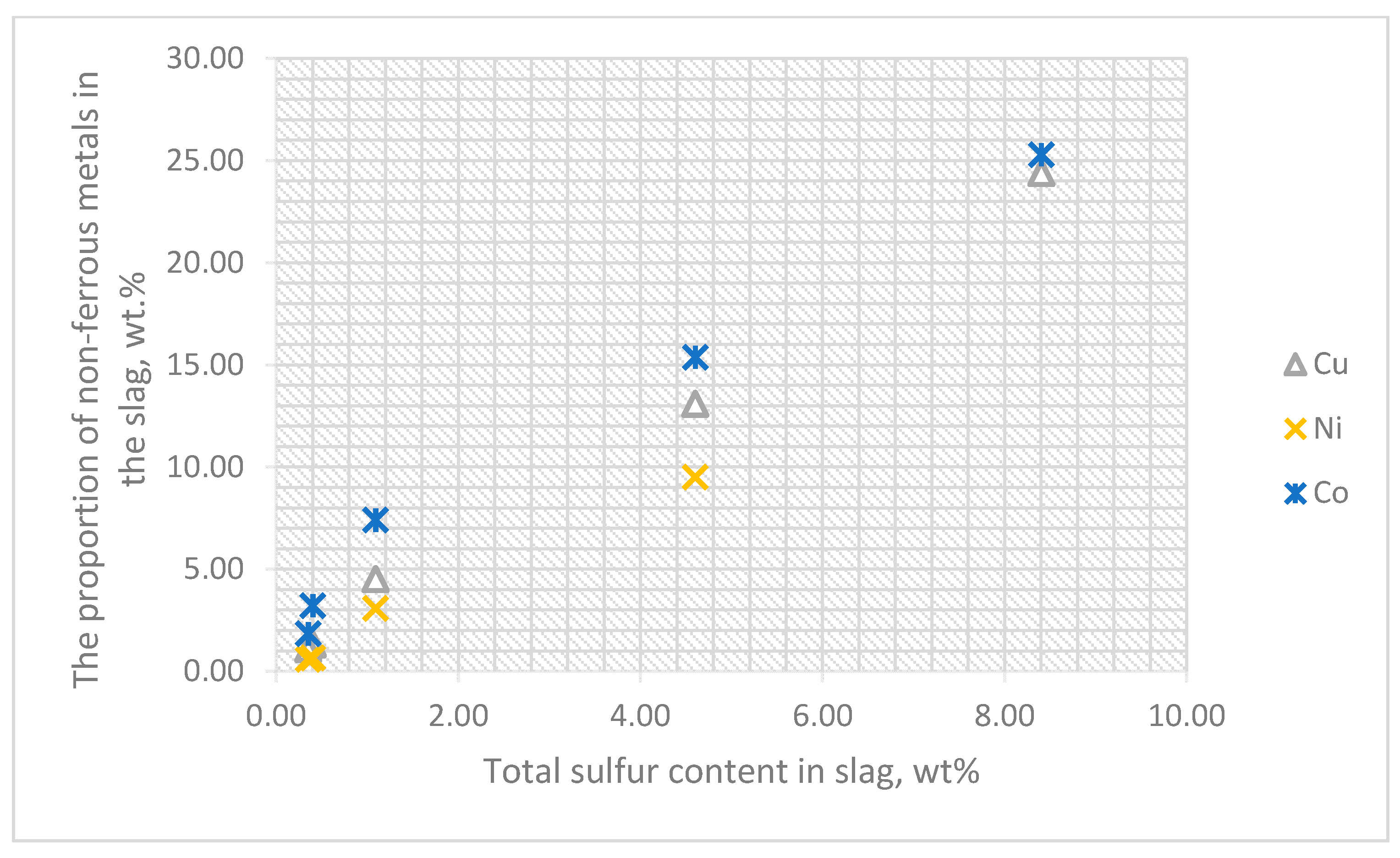
3.3. Influence of CaCO3 and CaF2 Fluxes on the Distribution of Gold, Platinum and Non-Ferrous Metals between Matte and Slag
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrov, G.V.; Baudouin, A.Y.; Mardar, I.I.; Ivanov, B.S.; Boginskaya, A.S. Resources of noble metals in technogenic objects of the mining and metallurgical complex of Russia. Success Mod. Nat. Sci. 2013, 3, 145–148. [Google Scholar]
- Bellemans, I.; De Wilde, E.; Moelans, N.; Verbeken, K. Metal losses in pyrometallurgical operations—A review. Adv. Colloid Interface Sci. 2018, 255, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Shi, J.; Taskinen, P.; Lindberg, D.; Jokilaakso, A. Precious Metal Distributions Between Copper Matte and Slag at High PSO2 in WEEE Reprocessing. Met. Mater. Trans. B 2021, 52, 871–882. [Google Scholar] [CrossRef]
- Piskunen, P.; Avarmaa, K.; O’Brien, H.; Klemettinen, L.; Johto, H.; Taskinen, P. Precious Metal Distributions in Direct Nickel Matte Smelting with Low-Cu Mattes. Met. Mater. Trans. B 2017, 49, 98–112. [Google Scholar] [CrossRef]
- Avarmaa, K.; O’Brien, H.; Johto, H.; Taskinen, P. Equilibrium Distribution of Precious Metals Between Slag and Copper Matte at 1250–1350 °C. J. Sustain. Met. 2015, 1, 216–228. [Google Scholar] [CrossRef]
- Ip, S.W.; Toguri, J.M. Entrainment behavior of copper and copper matte in copper smelting operations. Met. Mater. Trans. B 1992, 23, 303–311. [Google Scholar] [CrossRef]
- Ip, S.W.; Toguri, J.M. Surface and interfacial tension of the Ni-Fe-S, Ni-Cu-S, and fayalite slag systems. Met. Mater. Trans. B 1993, 24, 657–668. [Google Scholar] [CrossRef]
- Sakai, T.; Ip, S.W.; Toguri, J.M. Interfacial phenomena in the liquid copper-calcium ferrite slag system. Met. Mater. Trans. B 1997, 28, 401–407. [Google Scholar] [CrossRef]
- Cheng, X.; Cui, Z.; Contreras, L.; Chen, M.; Nguyen, A.; Zhao, B. Matte Entrainment by SO2 Bubbles in Copper Smelting Slag. JOM 2019, 71, 1897–1903. [Google Scholar] [CrossRef]
- Geguzin, Y.I. Bubbles; Nauka: Moscow, Russia, 1985; p. 176. [Google Scholar]
- HSC Chemistry for Windows. Chemical Reaction and Equilibrium Software withis Extensive Thermochemical Database/Outotec Reseach Oy Information Servise, Finland. Available online: www.outotec.com/hsc (accessed on 10 July 2021).
- Crystal Impact Match. Available online: https://crystalimpact.com/match/ (accessed on 2 July 2021).
- Amdur, A.M.; Pavlov, V.V.; Fedorov, S.; Matushkina, A.N. Dispersed ore gold characterisation. Can. Met. Q. 2020, 59, 331–334. [Google Scholar] [CrossRef]
- Cabri, L.J.; Feather, C.E. Platinum-Iroi Alloys: A Nomenclature Based on a Study of Natutral and Synthetic Alloys. Can. Mineral. 1975, 13, 117–126. [Google Scholar]
- Maestro, A.; Santini, E.; Zabiegaj, D.; Llamas, S.; Ravera, F.; Liggieri, L.; Ortega, F.; Rubio, R.G.; Guzman, E. Particle and Particle-Surfactant Mixtures at Fluid Interfaces: Assembly, Morphology, and Rheological Description. Adv. Condens. Matter Phys. 2015, 2015, 917516. [Google Scholar] [CrossRef] [Green Version]
- Vakilov, A.N.; Mamonova, M.V.; Prudnikov, V.V. Adhesion of metals and semiconductors in the framework of the dielectric formalism. Solid State Phys. 1997, 39, 964–967. [Google Scholar] [CrossRef]
- Kaitmazov, N.G. (Ed.) Metal Production beyond the Arctic Circle; Antey Limited: Norilsk, Russia, 2008; p. 296. [Google Scholar]
- Tsemekhman, L.S.; Fomichev, V.B.; Ertseva, L.N.; Kaitmazov, N.G.; Kozyrev, S.M.; Maksimov, V.I.; Shneerson, Y.M.; Dyachenko, V.T. Atlas of Mineral Raw Materials, Technological Industrial Products and Commercial Products of the Polar Division of OJSC MMC Norilsk Nickel; Ore and Metals: Moscow, Russia, 2010; p. 336. [Google Scholar]
- Makarov, A.B.; Talalay, A.G.; Khasanova, G.G. Geological and industrial types of technogenic deposits. In Bulletin of the IG Komi Scientific Center of the Ural Branch of the Russian Academy of Sciences; Komi Scientific Center of the Ural Branch of the Russian Academy of Sciences: Syktyvkar, Russia, 2018; Volume 8, pp. 80–85. [Google Scholar] [CrossRef]
- Makarov, V.A.; Mikheev, V.G.; Samorodskiy, P.N. Mineralogy of slag dumps at Norilsk Nickel plant. Gorn. Zhurnal 2016, 3, 50–55. [Google Scholar] [CrossRef]
- Zherebtsov, S.N.; Chernyshov, E.A. Features of the physicochemical properties of fluxes used in electroslag remelting technologies. In Proceedings of the Nizhny Novgorod State Technical University Name R.E. Alekseeva; Nizhny Novgorod State Technical University named after R.E. Alekseeva: Nizhny Novgorod, Russia, 2016; Volume 1, pp. 228–235. [Google Scholar]

| Component | Content, wt.% | ||
|---|---|---|---|
| Copper–Nickel Sulfide Ore | Gold-Bearing Pyrite Ore | Copper–Nickel Matte | |
| SiO2 | 5.60 | 10.2 | - |
| Al2O3 | 2.40 | 0.37 | - |
| CaO | 0.30 | 1.12 | - |
| MgO | 0.40 | 1.32 | - |
| Fe | 54.6 | 37.7 | 57.9 |
| MnO | 0.20 | - | - |
| K2O | 0.30 | 0.24 | - |
| Na2O | 0.30 | 0.80 | 0.38 |
| S | 31.3 | 45.1 | 29.8 |
| Cu | 4.67 | 1.83 | 7.02 |
| Ni | 4.28 | - | 4.98 |
| Co | 0.30 | - | 0.15 |
| Au | 0.4 × 10−4 | 1.2 × 10−4 | 13.1 × 10−4 |
| Pt | 1.1 × 10−4 ÷ 3.1 × 10−4 | - | - |
| Mineral. | Content, wt.% | ||
|---|---|---|---|
| Copper–Nickel Sulfide Ore | Gold-Bearing Pyrite Ore | Copper–Nickel Matte | |
| Qurtz SiO2 | 8.0 | - | - |
| Clinochlor Mg6Si4O10(OH)8 | 3.0 | - | - |
| Sericite KAl2(AlSi3O10)(OH)2 | 2.0 | - | - |
| Olivine (Fe, Mg)2SiO4 | - | 0.7 | - |
| Enstatite Mg2Si2O6 | - | 0.7 | - |
| Dolomite CaMgCO3 | - | 1.3 | - |
| Plagioclase (Na, Ca)AlSi3O8 | - | 3.3 | - |
| Magnetite Fe2+Fe3+2O4 | - | 12.0 | 4.9 |
| Pyrite FeS2 | 79.6 | - | - |
| Troilite FeS | - | - | 72.4 |
| Pyrrhotite Fe7S8 | - | 42.8 | - |
| Chalcopyrite CuFeS2 | 5.1 | 20.0 | - |
| Pentlandite Ni4.5Fe4.5S8 | - | 15.0 | - |
| Sphalerite ZnS | 2.0 | - | - |
| Bornite Cu5FeS4 | 0.1 | - | 10.1 |
| Tetratenite FeNi | - | 7.0 | |
| σAu, N/m | σPt, N/m | σm1, N/m | σm2, N/m | σs1, N/m |
| 1.02 | 1.95 | 0.36 | 0.35 | 0.41 |
| σs2, N/m | σm-s1, N/m | σm-s2, N/m | θAu | θPt |
| 0.45 | 0.11–0.14 | 0.05–0.11 | 19.5° | 24–37° |
| Component | Content of Components in wt.% | |||
|---|---|---|---|---|
| Without CaCO3 | CaCO3 in the Amount of 10% | |||
| Matte | Slag | Matte | Slag | |
| Cu | 4.66 | 0.38 | 4.68 | 0.15 |
| Ni | 4.28 | 0.45 | 4.39 | 0.14 |
| Co | 0.11 | 0.02 | 0.11 | 0.008 |
| Pt | 4.1 × 10−4 | 2.15 × 10−4 | 3.8 × 10−4 | 2.06 × 10−4 |
| Au | 1.77 × 10−4 | 0.45 × 10−4 | 1.83 × 10−4 | 0.29 × 10−4 |
| S | 30.5 | 4.60 | 28.7 | 1.13 |
| Component | Content of Components in wt.% | |||
|---|---|---|---|---|
| Without CaF2 | CaF2 in the Amount of 10% | |||
| Matte | Slag | Matte | Slag | |
| Cu | 7.02 | 0.1 | 7.25 | 0.05 |
| Ni | 4.98 | 0.03 | 5.12 | 0.01 |
| Co | 0.15 | 0.005 | 0.16 | 0.002 |
| Au | 13.1 × 10−4 | 0.75 × 10−4 | 13.5 × 10−4 | Not detected |
| S | 29.8 | 0.41 | 30.9 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amdur, A.M.; Fedorov, S.A.; Yurak, V.V. Transfer of Gold, Platinum and Non-Ferrous Metals from Matte to Slag by Flotation. Metals 2021, 11, 1602. https://doi.org/10.3390/met11101602
Amdur AM, Fedorov SA, Yurak VV. Transfer of Gold, Platinum and Non-Ferrous Metals from Matte to Slag by Flotation. Metals. 2021; 11(10):1602. https://doi.org/10.3390/met11101602
Chicago/Turabian StyleAmdur, Alexey M., Sergei A. Fedorov, and Vera V. Yurak. 2021. "Transfer of Gold, Platinum and Non-Ferrous Metals from Matte to Slag by Flotation" Metals 11, no. 10: 1602. https://doi.org/10.3390/met11101602





