Leaching of Zinc for Subsequent Recovery by Hydrometallurgical Techniques from Electric Arc Furnace Dusts and Utilisation of the Leaching Process Residues for Ceramic Materials for Construction Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Electric Arc Furnace Dust
2.1.2. Sulfuric Acid and Water
2.1.3. Clay
2.2. Methodology
2.2.1. Chemical Characterization of Electric Arc Furnace Dusts
2.2.2. Leaching of Electric Arc Furnace Dusts in Sulfuric Acid Solutions at Ambient Temperature and Atmospheric Pressure
2.2.3. Chemical Characterization of the Leaching Residue
2.2.4. Conformed of Ceramic Materials for Bricks with the Leaching Residue of Electric Arc Furnace Dusts
3. Results and Discussions
3.1. Chemical Characterization of Electric Arc Furnace Dusts
3.2. Leaching of Electric Arc Furnace Dusts in Sulfuric Acid Solutions at Ambient Temperature and Atmospheric Pressure
3.3. Chemical Characterization of the Leaching Residue
3.4. Conformed of Ceramic Materials for Bricks with the Leaching Residue of Electric Arc Furnace Dusts
4. Conclusions
- Electric arc furnace dusts contain a significant percentage of zinc, around 18%. This zinc seems to exist in the form of oxides, due to the formation process of this waste. There are also other elements such as iron (in high proportion), calcium, silicon, chlorine, sodium, magnesium, potassium, and manganese;
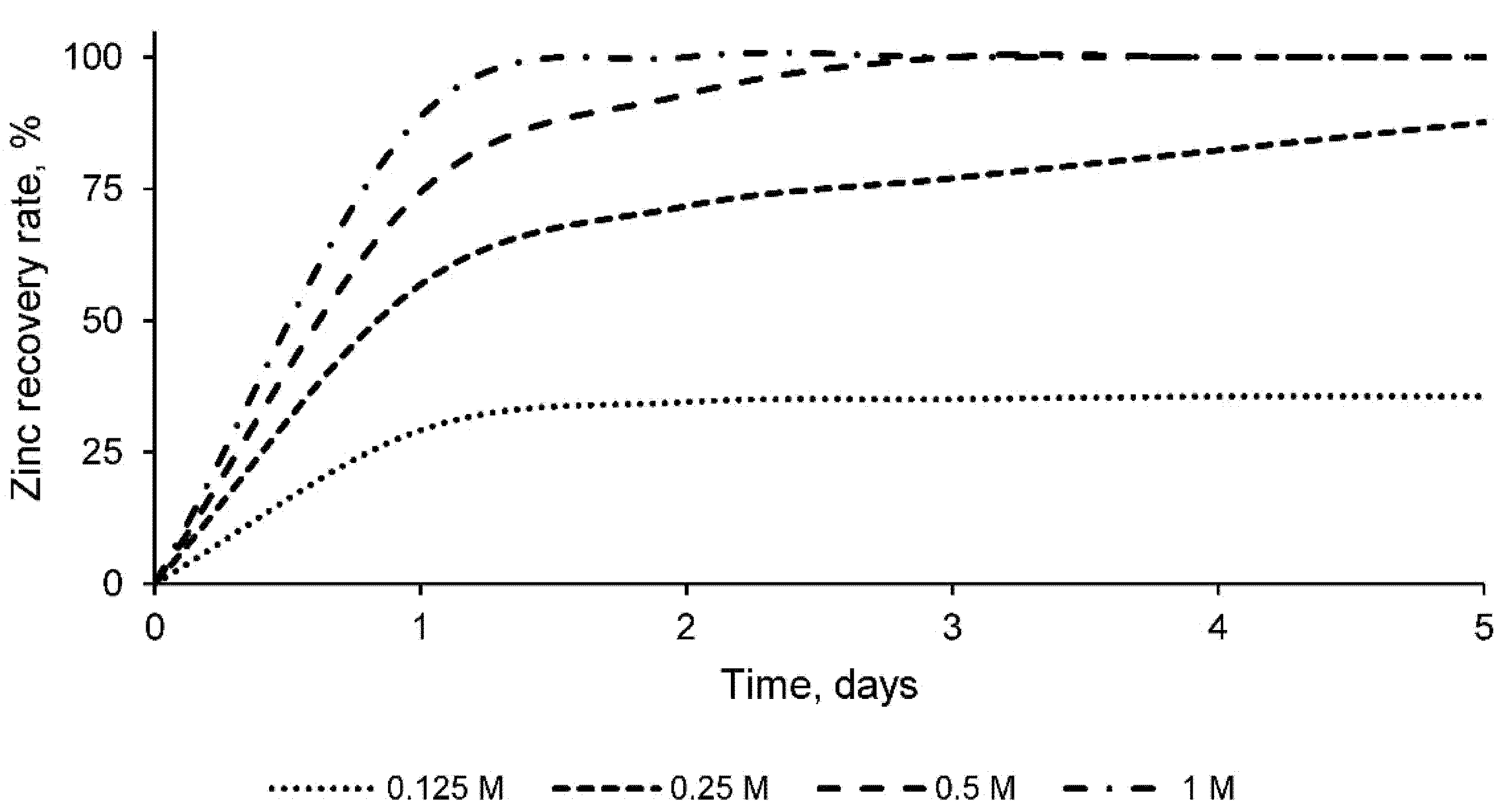
- The 0.5 and 1 molar sulfuric acid solutions leach the total percentage of zinc existing in the sample as determined by X-ray fluorescence analysis (188.3 mg/g). This leaching process was carried out with a solid/liquid ratio of 1:20, constant agitation, ambient temperature, and atmospheric pressure;
- The 1 molar solution of sulfuric acid leaches the zinc from the electric arc furnace dusts in its entirety in 36 h, according to the detailed procedure. Therefore, this solution was selected as optimal and a leaching time of 36 h was set;
- The waste produced in the leaching process with the selected dilution and time was chemically characterized, presenting a higher percentage of sulfur and hydrogen than the electric arc furnace dust. At the same time, the leaching residue contains other chemical elements such as iron (in high proportion), calcium, silicon, lead, aluminum, magnesium, and manganese. In addition, the percentage of zinc in the leaching residue was very low, corroborating the effectiveness of the leaching process;
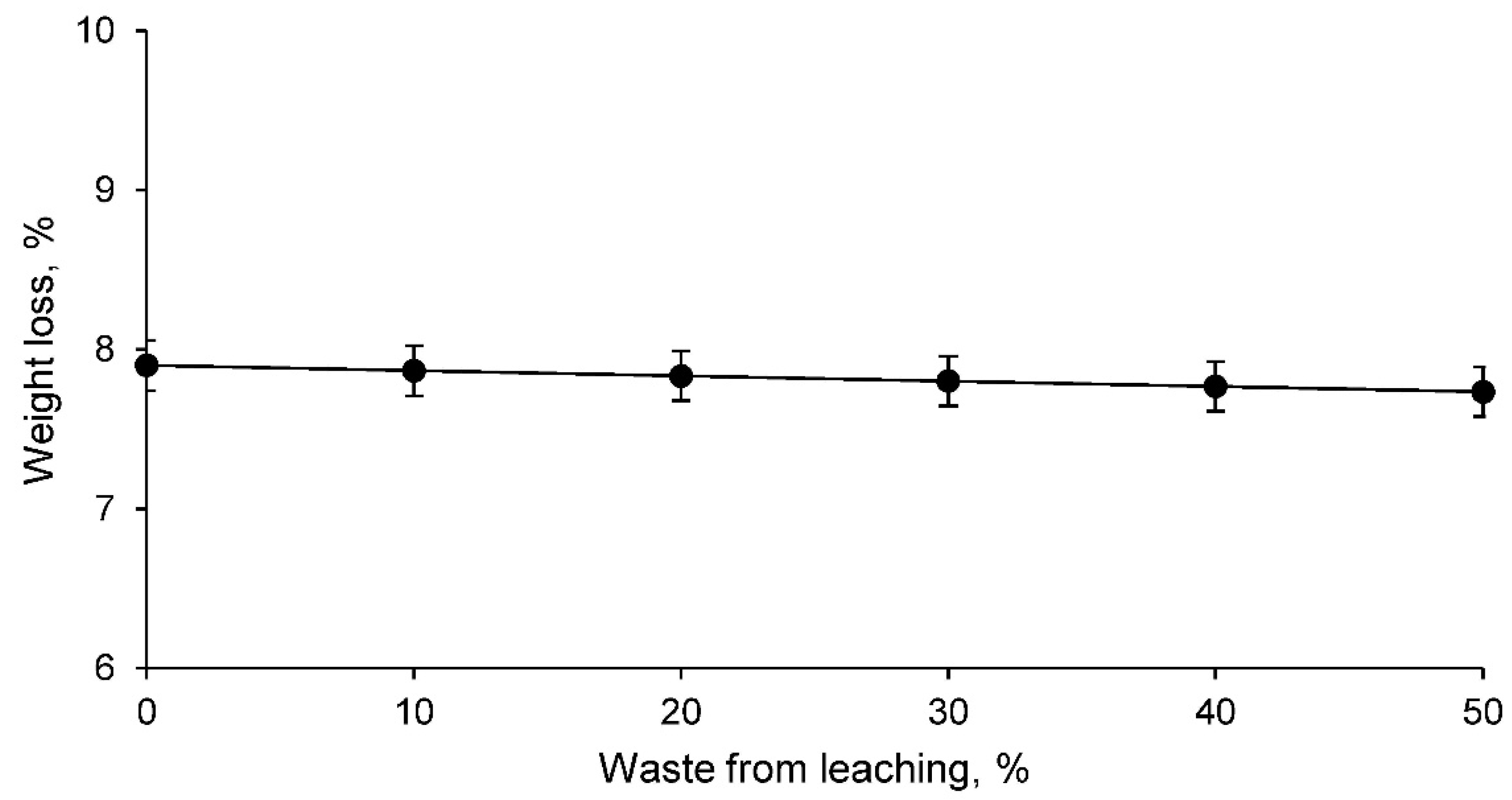
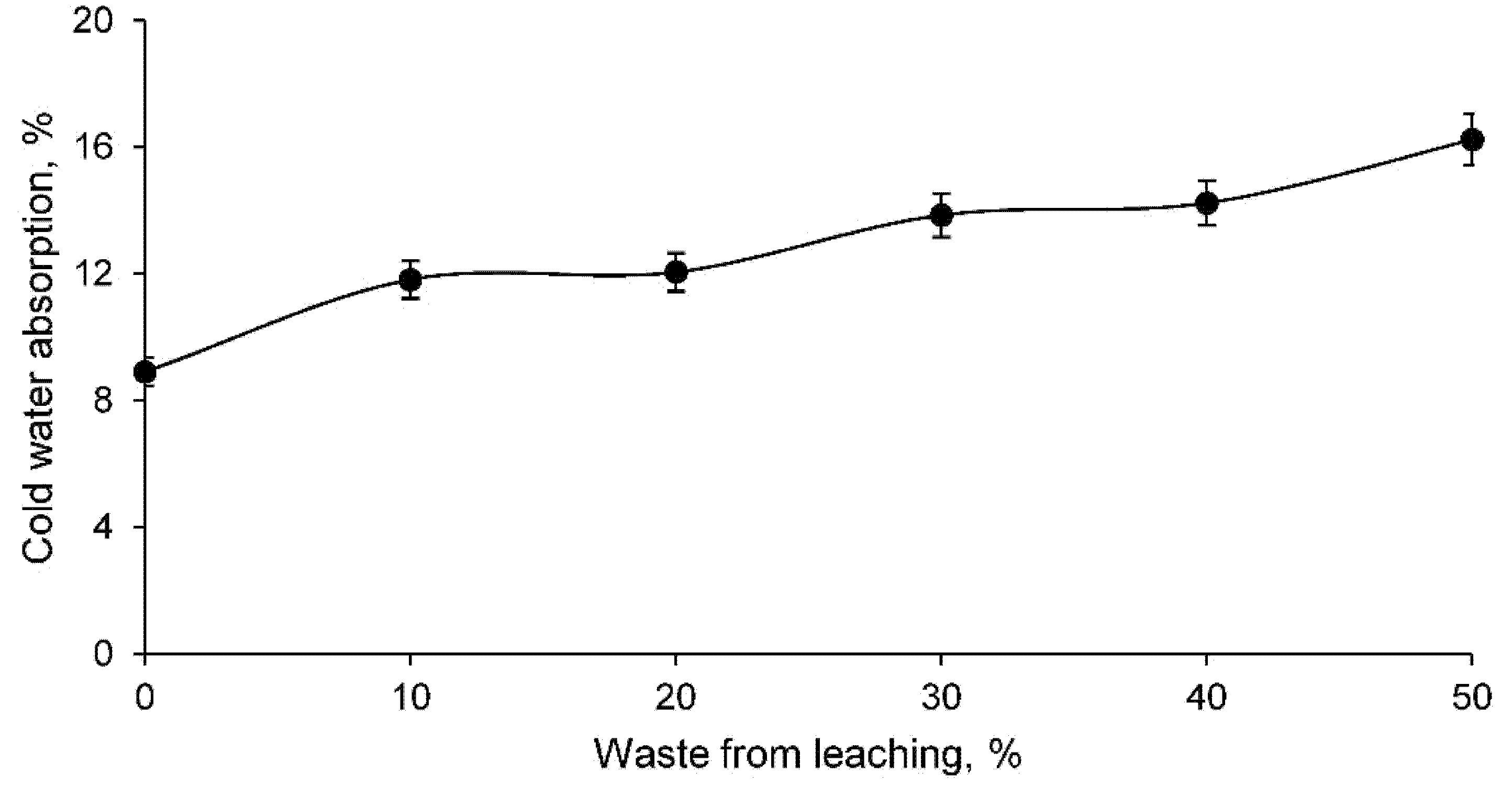
- Ceramics conformed with clay and leaching residue showed a more open structure the higher the percentage of waste. Consequently, ceramics with leaching residue show lower linear shrinkage, higher capillary water absorption, higher cold water absorption, and higher porosity compared to that of a traditional ceramic consisting only of clay. However, the bulk density of ceramics conformed with the leaching residue increases as the percentage of waste increases, mainly due to the existence of heavy metals;
- The strength of ceramics conformed with clay and leaching residue from electric arc furnace dusts is lower the higher the percentage of waste, with unacceptable strength values for samples conformed with 50% clay and 50% leaching residue. Consequently, and according to the physical and mechanical tests carried out, the families of ceramics conformed with the leaching residue that are suitable for use are those incorporating 10%, 20%, 30%, and 40% of waste.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Reck, B.K. On the materials basis of modern society. Proc. Natl. Acad. Sci. USA 2015, 112, 6295–6300. [Google Scholar] [CrossRef] [Green Version]
- Hagelüken, C.; Lee-Shin, J.U.; Carpentier, A.; Heron, C. The EU Circular Economy and Its Relevance to Metal Recycling. Recycling 2016, 1, 242–253. [Google Scholar] [CrossRef]
- Broadbent, C. Steel’s recyclability: Demonstrating the benefits of recycling steel to achieve a circular economy. Int. J. Life Cycle Assess. 2016, 21, 1658–1665. [Google Scholar] [CrossRef] [Green Version]
- Madias, J. Electric Furnace Steelmaking. Treatise Process Metall. 2014, 3, 271–300. [Google Scholar]
- Naito, M.; Takeda, K.; Matsui, Y. Ironmaking Technology for the Last 100 Years: Deployment to Advanced Technologies from Introduction of Technological Know-how, and Evolution to Next-generation Process. ISIJ Int. 2015, 55, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Jaimes, W.; Maroufi, S. Sustainability in steelmaking. Curr. Opin. Green Sustain. Chem. 2020, 24, 42–47. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- De Buzin, P.J.W.K.; Heck, N.C.; Vilela, A.C.F. EAF dust: An overview on the influences of physical, chemical and mineral features in its recycling and waste incorporation routes. J. Mater. Res. Technol. 2017, 6, 194–202. [Google Scholar] [CrossRef]
- Guézennec, A.G.; Huber, J.C.; Patisson, F.; Sessiecq, P.; Birat, J.P.; Ablitzer, D. Dust formation in Electric Arc Furnace: Birth of the particles. Powder Technol. 2005, 157, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; French, D.; Sakurovs, R.; Grigore, M.; Sun, H.; Cham, T.; Hilding, T.; Hallin, M.; Lindblom, B.; Sahajwalla, V. Minerals and iron-making reactions in blast furnaces. Prog. Energy Combust. Sci. 2008, 34, 155–197. [Google Scholar] [CrossRef]
- Agency, E.P. European Waste Catalogue and Hazardous Waste List; Environmental Protection Agency Ireland: Wexford, Ireland, 2002; ISBN 1840950838. [Google Scholar]
- Xanthopoulos, P.; Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.E. Zinc recovery from purified electric arc furnace dust leach liquors by chemical precipitation. J. Environ. Chem. Eng. 2017, 5, 3550–3559. [Google Scholar] [CrossRef]
- Martins, F.M.; Neto, J.M.; da Cunha, C.J. Mineral phases of weathered and recent electric arc furnace dust. J. Hazard. Mater. 2008, 154, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part I: Characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef]
- Guo, X.; Zhong, J.; Song, Y.; Tian, Q. Substance flow analysis of zinc in China. Resour. Conserv. Recycl. 2010, 54, 171–177. [Google Scholar] [CrossRef]
- Yu, G.; Peng, N.; Zhou, L.; Liang, Y.J.; Zhou, X.Y.; Peng, B.; Chai, L.Y.; Yang, Z.H. Selective reduction process of zinc ferrite and its application in treatment of zinc leaching residues. Trans. Nonferrous Met. Soc. China 2015, 25, 2744–2752. [Google Scholar] [CrossRef]
- Su, Y.M.; Huang, W.C.; Liu, Y.C.; Chang, C.K.; Kuo, Y.L. Utilization of electric arc furnace dust as regenerable sorbents for the removal of hydrogen sulfide. Ceram. Int. 2017, 43, S694–S699. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Yan, J.; Li, Z.; Hwang, J.Y.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Clean. Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Youcai, Z.; Stanforth, R. Extraction of zinc from zinc ferrites by fusion with caustic soda. Miner. Eng. 2000, 13, 1417–1421. [Google Scholar] [CrossRef]
- Langová, Š.; Leško, J.; Matýsek, D. Selective leaching of zinc from zinc ferrite with hydrochloric acid. Hydrometallurgy 2009, 95, 179–182. [Google Scholar] [CrossRef]
- Havlík, T.; Souza, B.V.E.; Bernardes, A.M.; Schneider, I.A.H.; Miškufová, A. Hydrometallurgical processing of carbon steel EAF dust. J. Hazard. Mater. 2006, 135, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, B.; Du, J.; Liu, C.; Wang, S. CO2 emission linkage analysis in global construction sectors: Alarming trends from 1995 to 2009 and possible repercussions. J. Clean. Prod. 2019, 221, 863–877. [Google Scholar] [CrossRef]
- Almeida, M.I.; Dias, A.C.; Demertzi, M.; Arroja, L. Contribution to the development of product category rules for ceramic bricks. J. Clean. Prod. 2015, 92, 206–215. [Google Scholar] [CrossRef]
- Suárez-Macías, J.; Terrones-Saeta, J.M.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Retention of Contaminants Elements from Tailings from Lead Mine Washing Plants in Ceramics for Bricks. Minerals 2020, 10, 576. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Study of the incorporation of biomass bottom ashes in ceramic materials for the manufacture of bricks and evaluation of their leachates. Materials 2020, 13, 2099. [Google Scholar] [CrossRef] [PubMed]







| Ceramic Family | % of Clay | % of Leaching Residue |
|---|---|---|
| RL0 | 100 | 0 |
| RL1 | 90 | 10 |
| RL2 | 80 | 20 |
| RL3 | 70 | 30 |
| RL4 | 60 | 40 |
| RL5 | 50 | 50 |
| Element | Wt % | Est. Error |
|---|---|---|
| Fe | 23.12 | 0.16 |
| Zn | 18.83 | 0.17 |
| Ca | 4.78 | 0.09 |
| Si | 2.06 | 0.05 |
| Cl | 3.93 | 0.10 |
| Na | 2.52 | 0.15 |
| Mg | 1.66 | 0.05 |
| K | 1.42 | 0.05 |
| Mn | 1.18 | 0.05 |
| Al | 0.752 | 0.03 |
| Pb | 0.805 | 0.040 |
| Sx | 0.329 | 0.016 |
| Cr | 0.186 | 0.0093 |
| Cu | 0.194 | 0.0097 |
| Px | 0.0766 | 0.0038 |
| Ti | 0.0656 | 0.0033 |
| Cd | 0.0646 | 0.0032 |
| Sn | 0.0512 | 0.0061 |
| Ni | 0.0267 | 0.0014 |
| W | 0.0259 | 0.0078 |
| Pt | 0.0204 | 0.0059 |
| Pd | 0.0181 | 0.0026 |
| Co | 0.0143 | 0.0021 |
| Zr | 0.0135 | 0.0023 |
| Ru | 0.0100 | 0.0014 |
| Ag | 0.0104 | 0.0029 |
| Chemical Element | Concentration in Leachate, mg/g |
|---|---|
| Fe | 96.4 ± 1.1 |
| Zn | 189.7 ± 3.0 |
| Ca | 13.3 ± 0.2 |
| Al | 3.7 ± 0.0 |
| Mn | 7.9 ± 0.1 |
| Mg | 13.1 ± 0.2 |
| Sample | Nitrogen, % | Carbon, % | Hydrogen, % | Sulphur, % |
|---|---|---|---|---|
| Leaching residue | 0.024 ± 0.001 | 0.053 ± 0.001 | 5.451 ± 0.177 | 3.745 ± 0.088 |
| Element | Wt % | Est. Error |
|---|---|---|
| Fe | 28.85 | 0.17 |
| Ca | 8.26 | 0.11 |
| Sx | 4.53 | 0.06 |
| Si | 2.12 | 0.05 |
| Pb | 2.01 | 0.07 |
| Al | 1.01 | 0.04 |
| Mg | 1.12 | 0.04 |
| Mn | 1.31 | 0.05 |
| Zn | 0.84 | 0.02 |
| Cr | 0.267 | 0.013 |
| Cu | 0.148 | 0.0074 |
| K | 0.122 | 0.0061 |
| Ba | 0.119 | 0.040 |
| Ti | 0.0759 | 0.0038 |
| Cl | 0.123 | 0.0062 |
| Sn | 0.0795 | 0.0067 |
| Ni | 0.0367 | 0.0018 |
| Px | 0.0195 | 0.0025 |
| Zr | 0.0262 | 0.0031 |
| Co | 0.0206 | 0.0025 |
| Sr | 0.0189 | 0.0024 |
| Ru | 0.0091 | 0.0019 |
| Bi | 0.0107 | 0.0040 |
| Mo | 0.0068 | 0.0015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrones-Saeta, J.M.; Suárez-Macías, J.; Moreno-López, E.R.; Corpas-Iglesias, F.A. Leaching of Zinc for Subsequent Recovery by Hydrometallurgical Techniques from Electric Arc Furnace Dusts and Utilisation of the Leaching Process Residues for Ceramic Materials for Construction Purposes. Metals 2021, 11, 1603. https://doi.org/10.3390/met11101603
Terrones-Saeta JM, Suárez-Macías J, Moreno-López ER, Corpas-Iglesias FA. Leaching of Zinc for Subsequent Recovery by Hydrometallurgical Techniques from Electric Arc Furnace Dusts and Utilisation of the Leaching Process Residues for Ceramic Materials for Construction Purposes. Metals. 2021; 11(10):1603. https://doi.org/10.3390/met11101603
Chicago/Turabian StyleTerrones-Saeta, Juan María, Jorge Suárez-Macías, Evaristo Rafael Moreno-López, and Francisco Antonio Corpas-Iglesias. 2021. "Leaching of Zinc for Subsequent Recovery by Hydrometallurgical Techniques from Electric Arc Furnace Dusts and Utilisation of the Leaching Process Residues for Ceramic Materials for Construction Purposes" Metals 11, no. 10: 1603. https://doi.org/10.3390/met11101603
APA StyleTerrones-Saeta, J. M., Suárez-Macías, J., Moreno-López, E. R., & Corpas-Iglesias, F. A. (2021). Leaching of Zinc for Subsequent Recovery by Hydrometallurgical Techniques from Electric Arc Furnace Dusts and Utilisation of the Leaching Process Residues for Ceramic Materials for Construction Purposes. Metals, 11(10), 1603. https://doi.org/10.3390/met11101603









