Optimization of Conditions for Processing of Lead–Zinc Ores Enrichment Tailings of East Kazakhstan
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
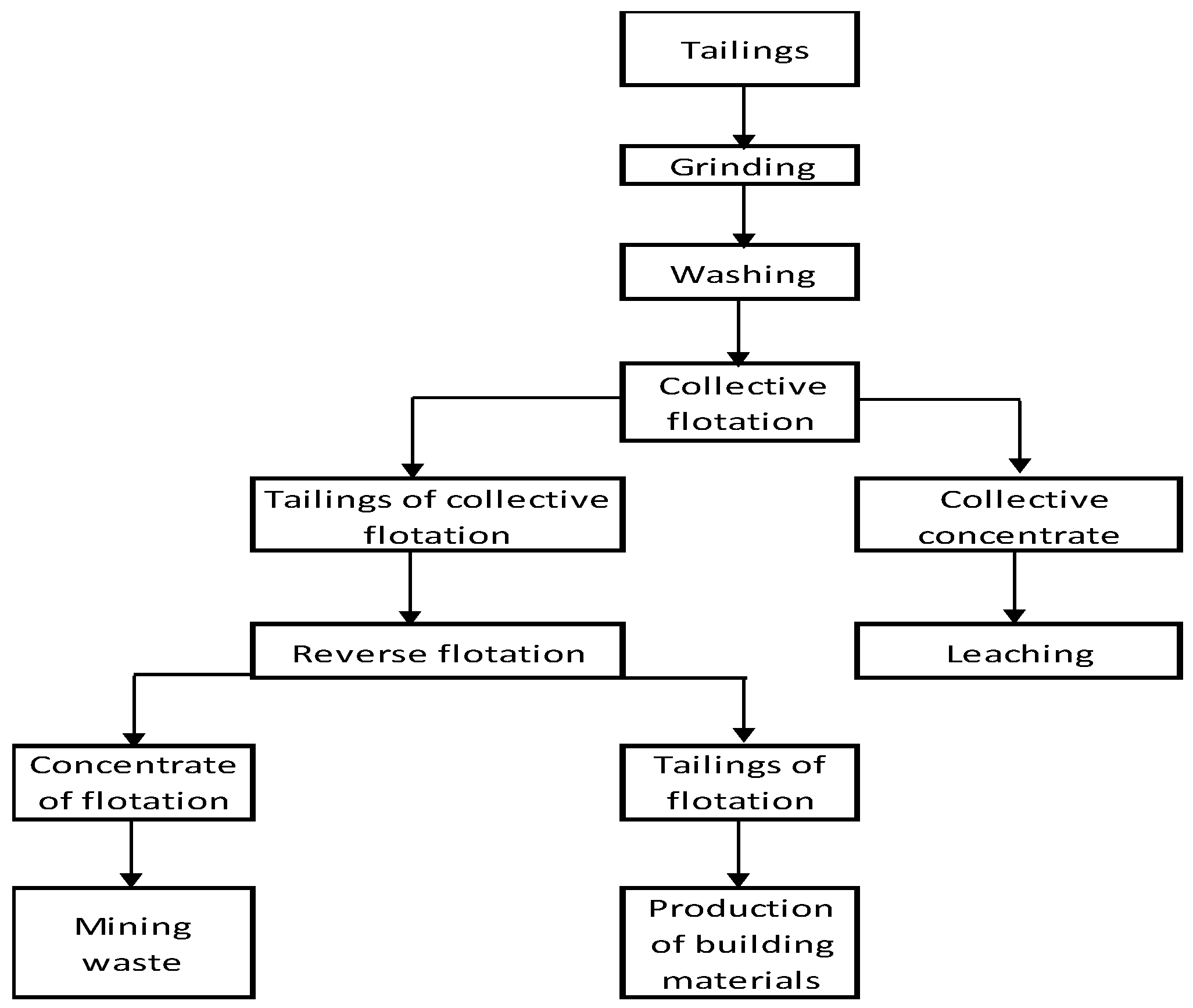
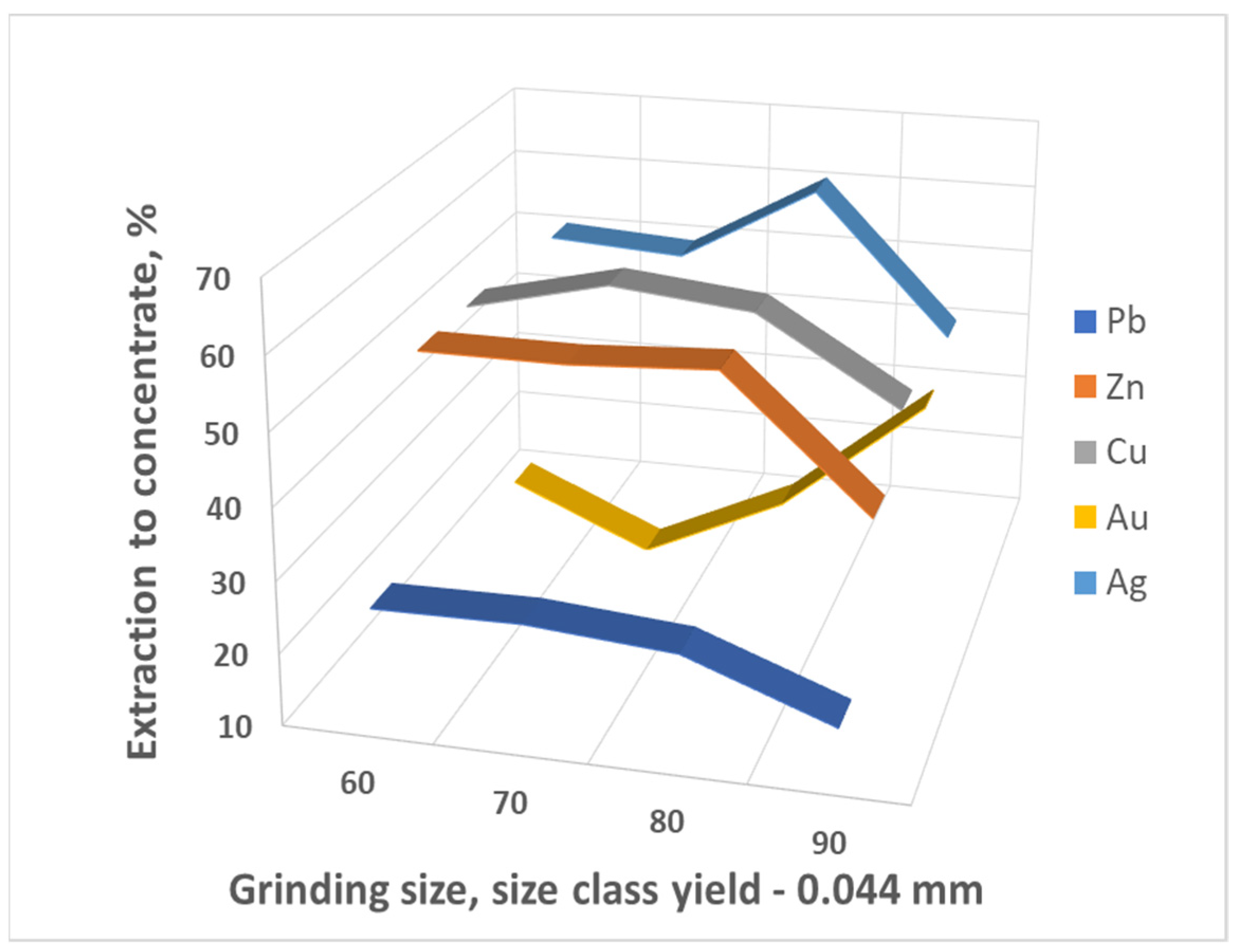
- The need to regrind the material of tailings to a fineness of 73.4% of the class −0.044 mm, which corresponds to the existing experience of processing similar products of technogenic products of enrichment.
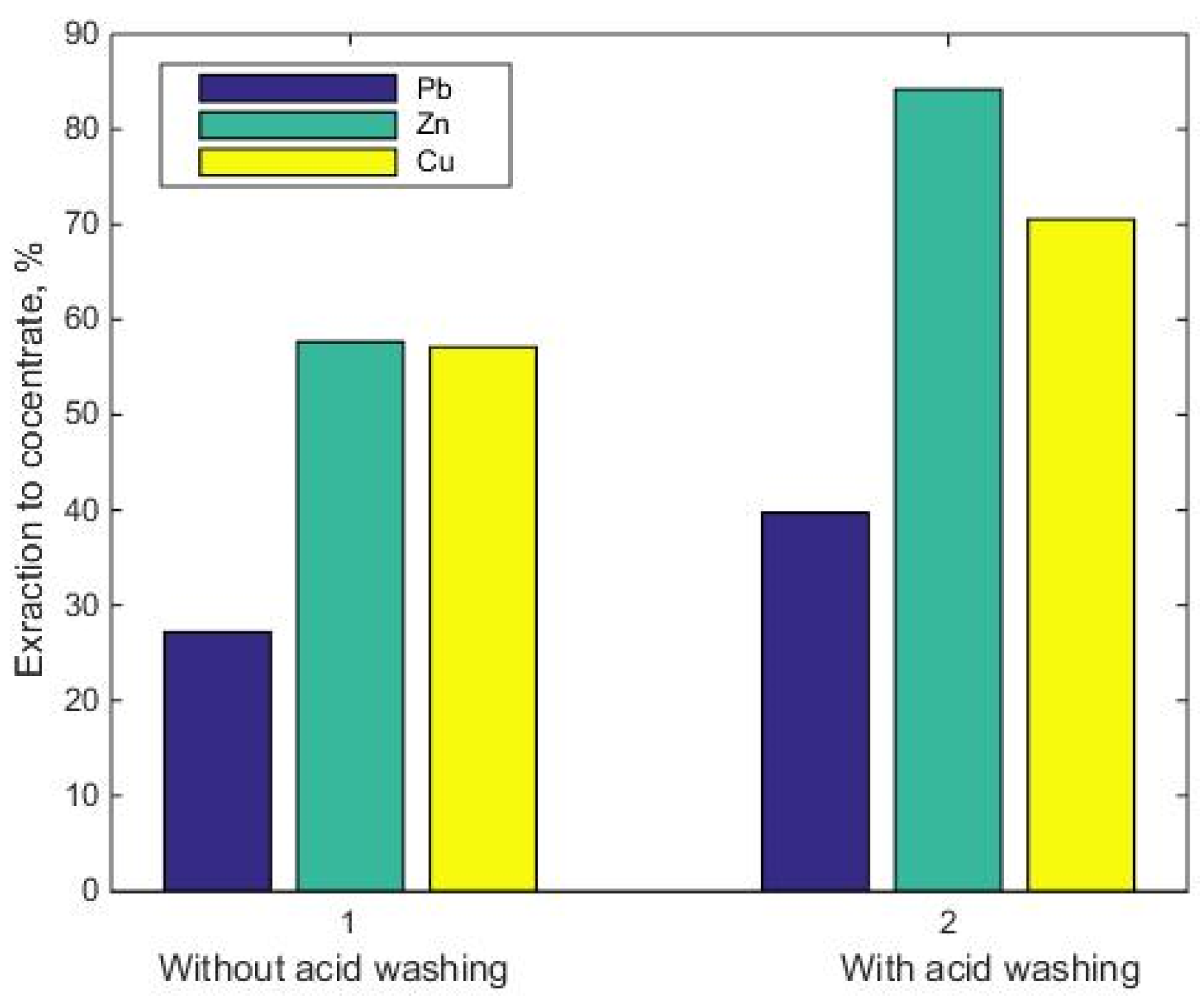
- Pre-washing of the source tailings in a slightly acidic medium increases the recovery of copper, lead and zinc by 42.3%, with minimal losses of these metals with washing drains.
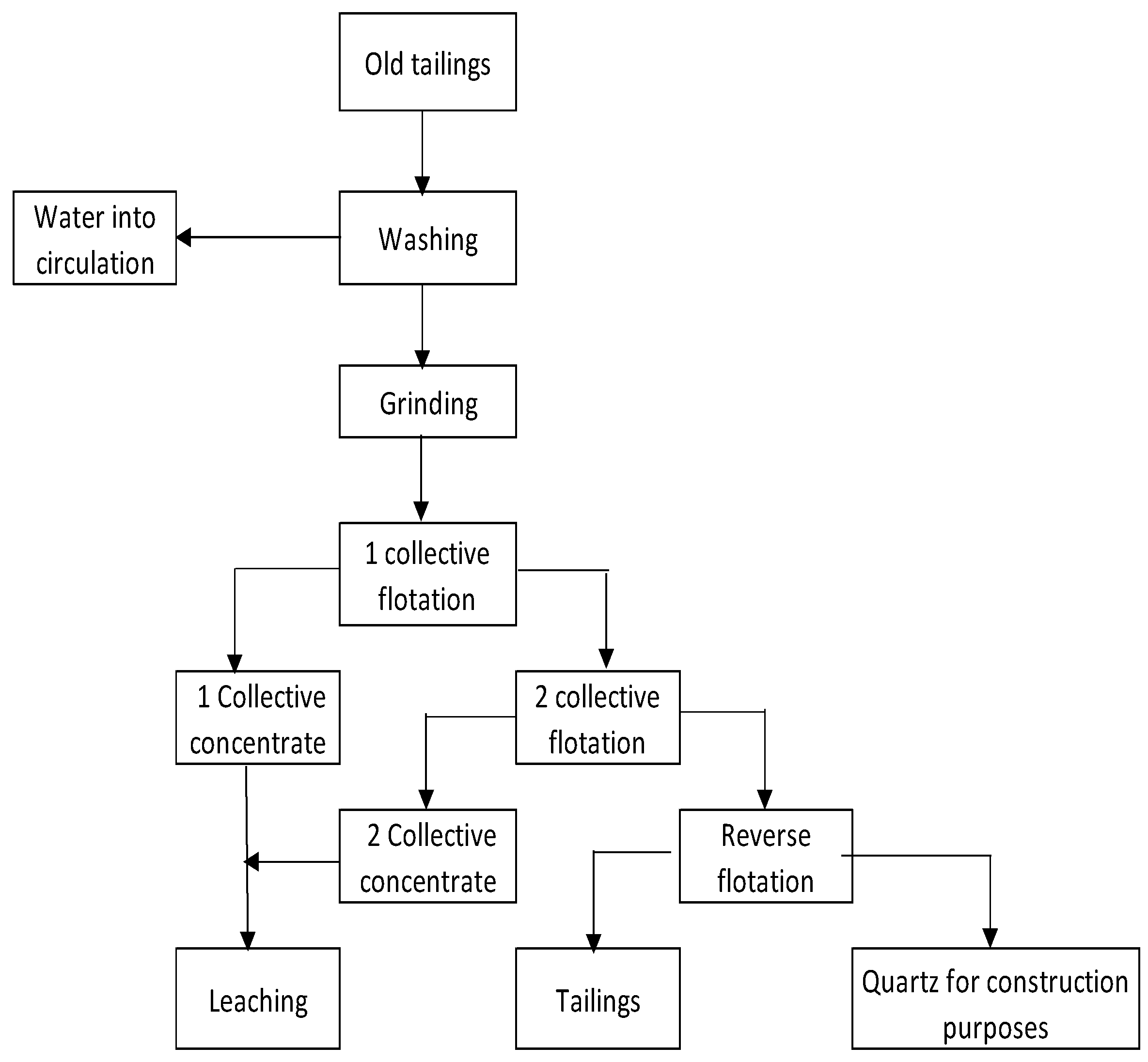
- The feasibility of a multi-stage collective flotation in order to maximize the recovery of valuable components in collective concentrates.
- During the multi-stage collective flotation, it is necessary to consider the physical and chemical properties of the main valuable minerals (sulfide and oxidized forms) and the waste rock minerals, which are included in old tailings.
- The need for simultaneous use of several collectors based on a combination of existing and new types of selective reagents.
- Deep enrichment of the old tailings is feasible, with a maximum recovery of valuable components and a minimum yield (13.10%) of waste products.
- In order to maximize the recovery of valuable components from collective concentrates, it is advisable to process them hydrometallurgically with subsequent enrichment of leaching cakes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shadrunova, I.V.; Gorlova, O.E.; Zhilina, V.A. The new paradigm of an environmentally-driven resource-saving technologies for processing of mining. In IOP Conference Series Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 687, p. 066048. [Google Scholar] [CrossRef]
- Ceng, C.; Wang, H.; Hu, W.; Li, L.; Shi, C. Recovery of iron and copper from copper tailings by coal-based direct reduction and magnetic separation. J. Iron Steel Res. Int. 2017, 24, 991–997. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Kostov, A.; Tasić, V.; Milosević, N. Influence of pyrometallurgical copper production on the environment. J. Hazard. Mater. 2009, 164, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Palden, T.; Regadio, M.; Onghena, B.; Binnemans, K. Selective metal recovery from jarosite residue by leaching with acid-equilibrated ionic liquids and precipitation-stripping. ACS Sustain. Chem. Eng. 2019, 7, 4239–4246. [Google Scholar] [CrossRef]
- Asadi, T.; Azizi, A.; Lee, J.; Jahani, M. Leaching of zinc from a lead-zinc flotation tailing sample using ferric sulphate and sulfuric acid media. J. Environ. Chem. Eng. 2017, 5, 4769–4775. [Google Scholar] [CrossRef]
- Hussaini, S.; Kursunoglu, S.; Top, S.; Ichlas, Z.T.; Kaya, M. Testing of 17-different leaching agents for the recovery of zinc from a carbonate-type Pb-Zn ore flotation tailing. Min. Eng. 2021, 168, 106935. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Méndez, A.; Rodríguez-Pacheco, R.; Paz-Ferreiro, J.; Gascó, G. Recovery of Zinc and Copper from Mine Tailings by Acid Leaching Solutions Combined with Carbon-Based Materials. Appl. Sci. 2021, 11, 5166. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Kursunoglu, N.; Hussaini, S.; Kaya, M. Selection of an appropriate acid type for the recovery of zinc from a flotation tailing by the analytic hierarchy process. J. Clean. Prod. 2020, 283, 124659. [Google Scholar] [CrossRef]
- Sayilgan, E.; Karacan, G. Characterization and evalution of removal conditions of lead-zinc-copper flotation plant waste. J. Eng. Sci. Des. 2019, 7, 175–181. [Google Scholar]
- Gao, X.; Jiang, L.; Mao, Y.; Yao, B.; Jiang, P. Progress, Challenges, and Perspectives of Bioleaching for Recovering Heavy Metals from Mine Tailings. Adsorp. Sci. Technol. 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Zhong, Y. Removal of metals from lead-zinc mine tailings using bioleaching and followed by sulfide precipitation. Chemosphere. 2017, 185, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Sycheva, E.A.; Akylbekov, A.; Ushakov, N.N.; Kushakova, L.B. Combined Method for Processing Tailings of Polymetallic Ores. East Mining and Metallurgical Research Institute of Non-ferrous Metals. KZ Patent No. 5305, 15 October 1993. p. 3. [Google Scholar]
- Bagheri, B.; Vazifeh Mehrabani, J.; Farrokhpay, S. Recovery of sphalerite from a high zinc grade tailing. J. Hazard. Mater. 2019, 381, 120946. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Tang, C.; Hao, Y.; Wang, Z.; Yang, G.; Wang, Y.; Mu, Y. Solidification/Stabilization of heavy metals and its efficiency in lead-zinc tailings using different chemical agents. Environ. Technol. 2020, 41, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dryuchkova, O.A.; Bykov, R.A.; Mamyachenkov, S.V. The Ways of Development of Combined Hydrometallurgical Technology for Processing of Complex Ores’ Beneficiation Tailings. Mater. Sci. Forum. 2019, 946, 564–568. [Google Scholar] [CrossRef]
- Mantsevich, M.I.; Malinsky, R.A.; Lapshina, G.A.; Khersonsky, M.I. Development of technologies for processing non-ferrous metal ores aimed at improving the environmental safety of mining and metallurgical industries. Min. Inf. Anal. Bull. 2013, 11, 74–81. [Google Scholar]
- Mitrofanov, S.I. The study of minerals for enrichment. In Textbook; Gosgortehizdat: Moscow, Russia, 1962; p. 729. [Google Scholar]
- GOST 8736-2014 Sand for Construction Works; Specifications: Moscow, Russia, 2015.
| Size Class, mm | +1 | −1 + 0.63 | −0.63 + 0.5 | −0.5 + 0.315 | −0.315 + 0.071 | 0.071 + 0.044 | −0.044 + 0 | Total |
| Yield, % | 0.09 | 0.85 | 0.38 | 0.89 | 42.13 | 13.71 | 41.94 | 100 |
| Element | Pb | Zn | Cu | Fe | Au * | Ag * | Stotal | Ba | Al2O3 | MgO | SiO2 | Albite | Muscovite | Kaolinite |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content, %(g/t) | 0.07 | 0.2 | 0.07 | 6.94 | 0.32 | 3.18 | 5.01 | 0.62 | 13.9 | 4.34 | 28.9 | 3.6 | 27.64 | 8.71 |
| Pb | Zn | Cu | ||||||
|---|---|---|---|---|---|---|---|---|
| Minerals | Content, % | Elements | Content, % | Elements | Content, % | |||
| Absolute | Relative | Absolute | Relative | Absolute | Relative | |||
| Galena | 0 | 0 | Sphalerite | 1,23 | 100 | Chalcopyrite | 0.18 | 23.68 |
| Carakolite | 0.38 | 100 | Smithsonite | 0 | 0 | Azurite | 0.26 | 34.21 |
| malachite | 0.3 | 39.47 | ||||||
| Oxidized | 0.38 | 100 | Oxidized | 0 | 0 | Oxidized | 0.56 | 73.68 |
| Sulphide | 0 | 0 | Sulphide | 1.23 | 100 | Sulphide | 0.18 | 23.68 |
| Total | 0.38 | 100 | Total | 1.23 | 100 | Total | 0.76 | 100 |
| The List of the Main Components | Mineral Composition of the Main Valuable Components, % | Distribution of Mineral Grains in the Main Components of Tailings, % | ||
|---|---|---|---|---|
| Total | Including | |||
| Aggregates | Free Grains | |||
| Galena (Ga) | - | - | 100 | - |
| Sphalerite (Sl) | 10 | 48 | 52 | 45% Py; 33% Py–Cp; 11% Py |
| Chalcopyrite (Cp) | 4 | 86 | 14 | 50% Sl–Py; Py |
| Pyrite (Py) | 86 | 6 | 94 | 34% Sl; 25% Sl–Cp; Cp; 8% Sl |
| Total | 100 | |||
| Product Name | Yield, % | Content, % | Recovery, % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Pb | Au * | Fe | S | SiO2 | Cu | Zn | Pb | Au | Fe | S | SiO2 | ||
| Collective flotation concentrate 1 | 22.82 | 0.28 | 0.66 | 0.2 | 0.7 | 18.02 | 17.56 | 6.44 | 64.96 | 74.83 | 46.02 | 53 | 59.6 | 77.08 | 3.35 |
| Final collective flotation concentrate 1 | 2.76 | 0.08 | 0.16 | 0.16 | 1.12 | 15.38 | 9.99 | 7.32 | 2.25 | 2.22 | 4.33 | 10.27 | 6.15 | 5.3 | 0.46 |
| Collective flotation concentrate 2 | 22.49 | 0.07 | 0.11 | 0.11 | 0.09 | 2.71 | 1.29 | 25 | 16.46 | 12.04 | 25.19 | 6.89 | 8.84 | 5.56 | 12.81 |
| Final collective flotation concentrate 2 | 1.83 | 0.09 | 0.17 | 0.07 | 4.17 | 22.55 | 8.78 | 11.8 | 1.57 | 1.54 | 1.3 | 25.44 | 5.98 | 3.09 | 0.49 |
| Total collective product | 49.9 | 0.17 | 0.36 | 0.15 | 0.57 | 11.14 | 9.49 | 15.1 | 85.24 | 90.63 | 76.84 | 95.6 | 80.57 | 91.03 | 17.11 |
| Collective flotation tailings | 50.1 | 0.03 | 0.04 | 0.05 | 0.03 | 2.68 | 0.93 | 72.6 | 14.76 | 9.37 | 23.16 | 4.4 | 19.43 | 8.97 | 82.89 |
| tailings (feed) | 100 | 0.1 | 0.2 | 0.1 | 0.3 | 6.9 | 5.2 | 43.9 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Product Name | Yield, % | Content, % | Recovery, % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Pb | Au * | Fe | S | SiO2 | Cu | Zn | Pb | Au | Fe | S | SiO2 | ||
| Total reverse flotation concentrate | 67.25 | 0.02 | 0.02 | 0.01 | 0.01 | 2.35 | 1.04 | 20 | 40.44 | 34.84 | 17.78 | 33.04 | 46.76 | 75.36 | 19.05 |
| Reverse flotation tailings | 32.75 | 0.03 | 0.04 | 0.05 | 0.03 | 2.68 | 0.34 | 85 | 59.56 | 65.16 | 82.22 | 66.96 | 53.24 | 24.64 | 80.95 |
| Tailings of two-stage collective flotation | 100 | 0.03 | 0.04 | 0.04 | 0.03 | 3.38 | 0.93 | 70.6 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Operation Name | OperationTime, min | pH | Reagent Consumption, g/t | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2SO4 | CaO | NaHCO₃ | Liquid Glass | Na2S | CuSO4 | Collector Aero 7249 | Sodium Butyl Xanthate | Sodium Oleate | Butyl Aeroflot | Frothing Agent T92 | |||
| Washing | 240 | - | 50 | - | - | - | - | - | - | - | - | - | - |
| Tailings Regrinding | 13 | - | - | - | 1000 | 400 | - | - | - | 40 | - | - | - |
| 1 Collective Flotation | 10 | 8–9 | - | - | 3000 | - | - | - | - | 60 | - | 50 | 50 |
| 1 Final flotation | 2 | - | - | - | - | - | - | - | - | - | - | 20 | 20 |
| 2 Collective Flotation | 8 | 12 | - | 5000 | - | 500 | 200 | - | 150 | 350 | - | - | 50 |
| 2 Final Flotation | 2 | - | - | - | - | - | - | - | - | - | - | 20 | 20 |
| Main Reverse Flotation | 10 | 7–8 | - | - | - | 300 | 500 | 50 | - | - | 400 | - | - |
| Final Reverse flotation | 2 | - | - | - | - | - | - | - | - | - | 40 | - | - |
| Total Reagent Consumption | 34 | - | 50 | 5000 | 4000 | 1200 | 700 | 50 | 150 | 450 | 440 | 90 | 140 |
| Product Name | Yield, % | Content, % | Recovery, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Pb | Au * | Ag * | Fe | Cu | Zn | Pb | Au | Ag | Fe | ||
| Total collective concentrate | 48.70 | 0.19 | 0.38 | 0.16 | 0.56 | 5.39 | 10.14 | 86.78 | 91.69 | 80.81 | 95.90 | 82.5 | 78.78 |
| Total reverse flotation concentrate | 13.10 | 0.02 | 0.02 | 0.01 | 0.01 | 0.67 | 2.35 | 2.50 | 1.29 | 1.33 | 0.59 | 2.76 | 4.91 |
| Reverse flotation tailings | 38.20 | 0.03 | 0.04 | 0.05 | 0.03 | 1.22 | 2.68 | 10.72 | 7.02 | 17.87 | 3.51 | 14.74 | 16.31 |
| Tailings (feed) | 100 | 0.10 | 0.20 | 0.10 | 0.29 | 3.18 | 6.27 | 100 | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seksenova, N.; Bykov, R.; Mamyachenkov, S.; Daumova, G.; Kozhakanova, M. Optimization of Conditions for Processing of Lead–Zinc Ores Enrichment Tailings of East Kazakhstan. Metals 2021, 11, 1802. https://doi.org/10.3390/met11111802
Seksenova N, Bykov R, Mamyachenkov S, Daumova G, Kozhakanova M. Optimization of Conditions for Processing of Lead–Zinc Ores Enrichment Tailings of East Kazakhstan. Metals. 2021; 11(11):1802. https://doi.org/10.3390/met11111802
Chicago/Turabian StyleSeksenova, Nazym, Rudolf Bykov, Sergey Mamyachenkov, Gulzhan Daumova, and Malika Kozhakanova. 2021. "Optimization of Conditions for Processing of Lead–Zinc Ores Enrichment Tailings of East Kazakhstan" Metals 11, no. 11: 1802. https://doi.org/10.3390/met11111802
APA StyleSeksenova, N., Bykov, R., Mamyachenkov, S., Daumova, G., & Kozhakanova, M. (2021). Optimization of Conditions for Processing of Lead–Zinc Ores Enrichment Tailings of East Kazakhstan. Metals, 11(11), 1802. https://doi.org/10.3390/met11111802







