Study on Adding Ammonium Hydrogen Fluoride to Improve Manganese Leaching Efficiency of Ammonia Leaching Low-Grade Rhodochrosite
Abstract
:1. Introduction
2. Material and Experimental Procedure
2.1. Material and Roasting Treatment
2.2. Experimental Method
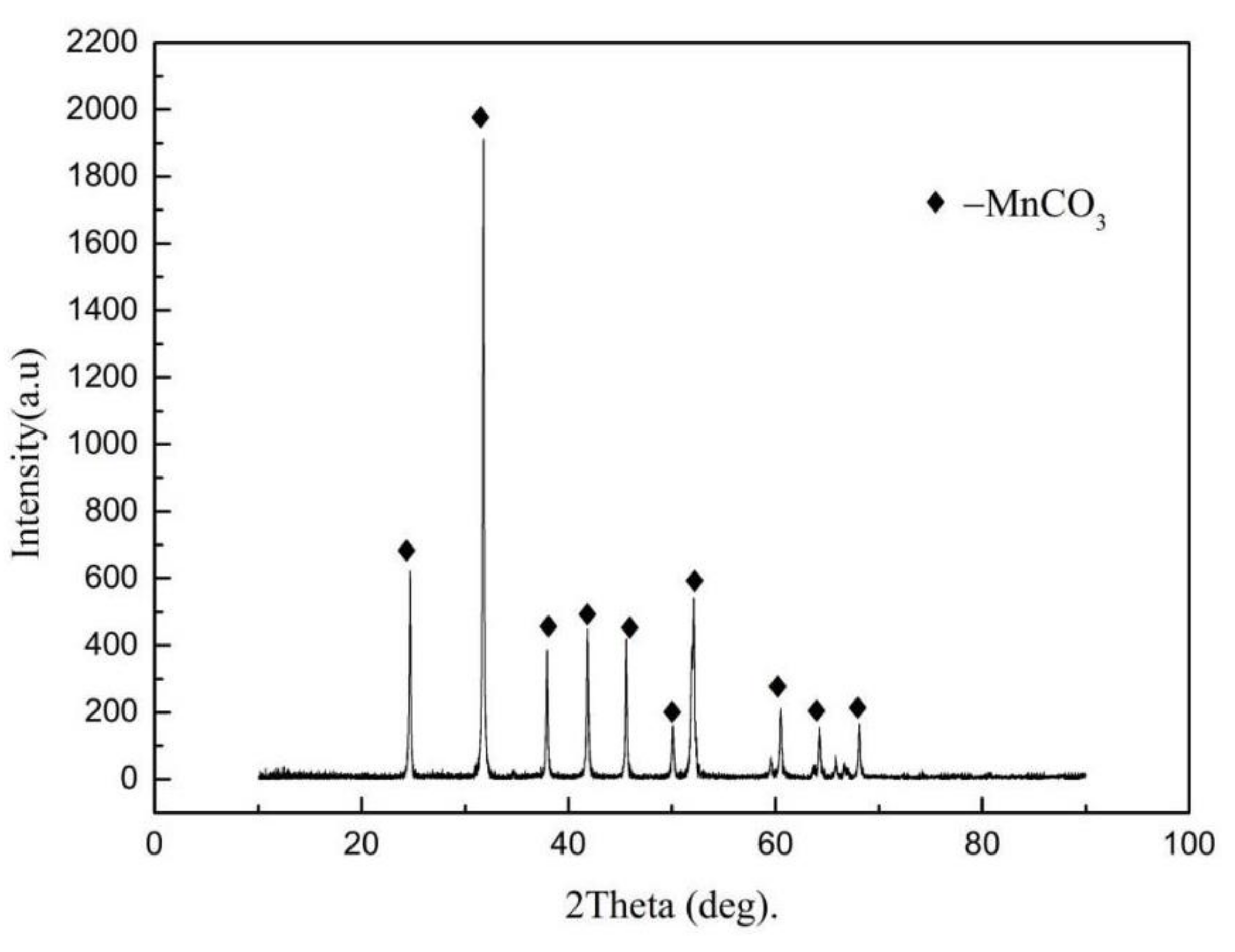
2.3. Preparation of Manganese Carbonate from Ammonia Leaching Solution
3. Results and Discussion
3.1. Effect of Additives on Manganese Leaching Efficiency
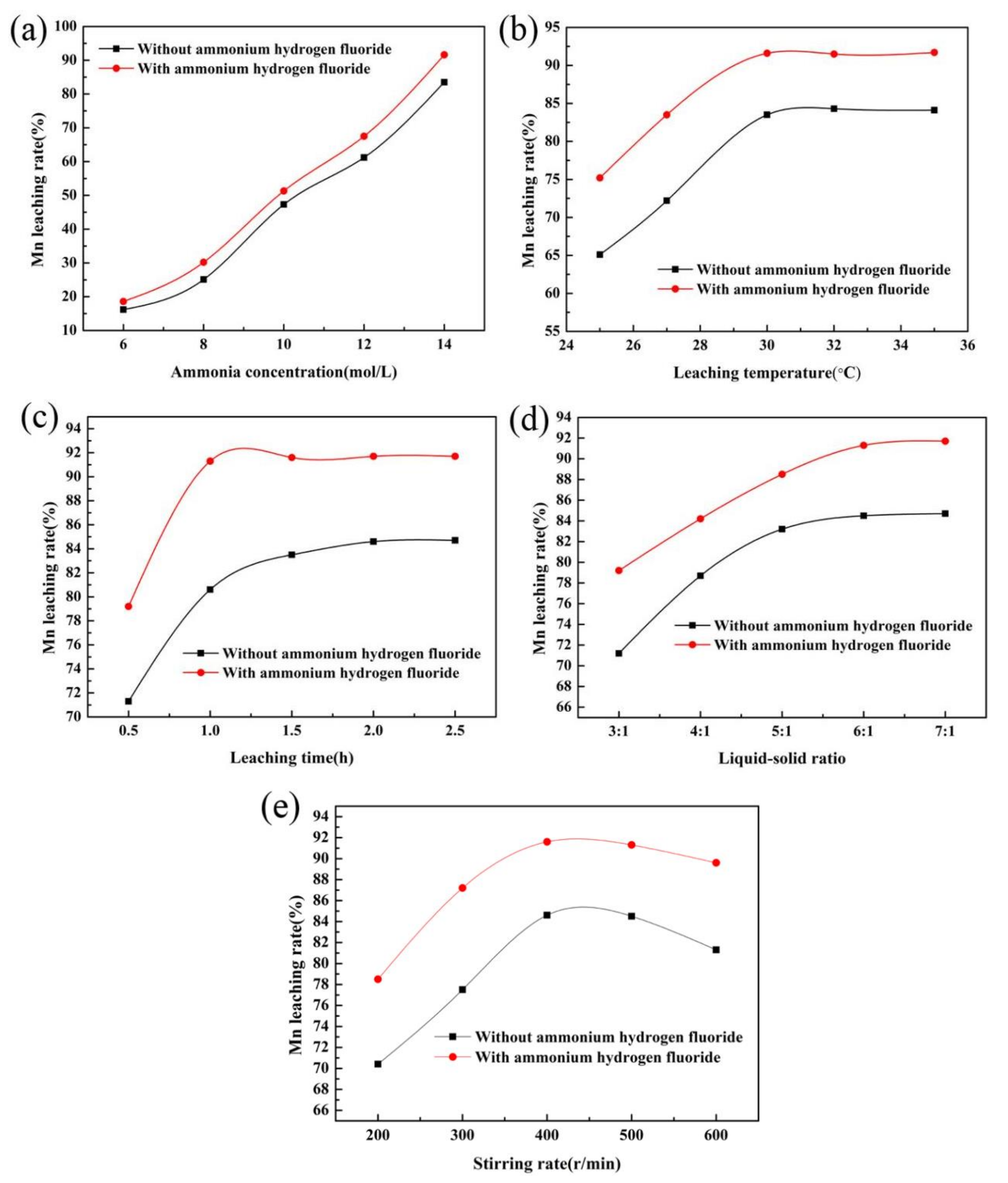
3.2. Effect of Ammonium Hydrogen Fluoride Addition on Mn Leaching Efficiency
3.3. Process Optimization of Ammonia Leaching of Rhodochrosite Calcine with the Addition of Ammonium Hydrogen Fluoride
3.4. Characterization of Ammonia Products
4. Conclusions
- As an additive in the ammonia leaching process, ammonium hydrogen fluoride can effectively improve the leaching efficiency of manganese, which is 7% higher than that without ammonium hydrogen fluoride.
- The optimum technological conditions are as follows: ammonia concentration is 14.5 mol/L, ammonia leaching temperature is 31.6 °C, the dosage of ammonium hydrogen fluoride is 7.3%, ammonia leaching time is 60 min, liquid-solid ratio is 6:1, and stirring rate is 400 r/min.
- The addition of ammonium hydrogen fluoride will not affect the quality of ammonia evaporation products, and the recovery of manganese is as high as 97.3%. The manganese grade of the manganese carbonate product is 44.13%.
Author Contributions
Funding
Conflicts of Interest
References
- Toro, N.; Rodríguez, F.; Rojas, A.; Robles, P.; Ghorbani, Y. Leaching manganese nodules with iron-reducing agents—A critical review. Miner. Eng. 2021, 163, 106748. [Google Scholar] [CrossRef]
- Roskill. Manganese: Global Industry Markets & Outlook to 2020; Roskill Information Services: London, UK, 2015. [Google Scholar]
- Singh, V.; Chakraborty, T.; Tripathy, S.K. A Review of low-grade Manganese Ore Upgradation Processes. J. Miner. Process. Extr. Metall. Rev. 2020, 41, 417–438. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purcell, W. Reducing agents in the leaching of manganese ores: A comprehensive review. Hydrometallurgy 2019, 187, 168–186. [Google Scholar] [CrossRef]
- Zhang, Y.; You, Z.; Li, G.; Jiang, T. Manganese extraction by sulfur-based reduction roasting–acid leaching from low-grade manganese oxide ores. Hydrometall 2013, 133, 126–132. [Google Scholar] [CrossRef]
- Alaoui, A.; Kacemi, K.E.; Ass, K.E.; Darmane, Y.; Kitane, S. Kinetic study of the leaching of manganese mine tailings by organic reductant in sulphuric acid solution. Miner. Process. Extr. Met. 2016, 125, 109–116. [Google Scholar] [CrossRef]
- Erik, P.; Eti, P.; Widi, A. Selective-reductive leaching of manganese from low-grade manganese ore using tannic acid as reductant. J. Min. Eng. 2019, 71, 1003–1012. [Google Scholar]
- Lu, Y.; Ma, H.; Huang, R.; Yuan, A.; Huang, Z.; Zhou, Z. Reductive Leaching of Low-Grade Pyrolusite with Formic Acid. Met. Mater. Trans. A 2015, 46, 1709–1715. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, B.; Ge, W.; Yan, C.J.; Yang, X. Magnetic Separation and Magnetic Properties of Low-Grade Manganese Carbonate Ore. JOM 2014, 67, 361–368. [Google Scholar] [CrossRef]
- Lin, Q.-Q.; Gu, G.-H.; Wang, H.; Zhu, R.-F.; Liu, Y.-C.; Fu, J.-G. Preparation of manganese sulfate from low-grade manganese carbonate ores by sulfuric acid leaching. Int. J. Miner. Met. Mater. 2016, 23, 491–500. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, M.; Zhang, M. Extraction of molybdenum and vanadium from the spent diesel exhaust catalyst by ammonia leaching method. J. Hazard. Mater. 2015, 286, 402–409. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yin, Z.L.; Xiong, S.F.; Chen, Y.G.; Chen, Q.Y. Leaching and Kinetic Modeling of Calcareous Bornite in Ammonia Ammonium Sulfate Solution with Sodium Persulfate. Hydrometallurgy 2014, 144–145, 86–90. [Google Scholar] [CrossRef]
- Hosseini, T.; Han, B.; Selomulya, C.; Haque, N.; Zhang, L. Chemical and morphological changes of weathered Victorian brown coal fly ash and its leaching characteristic upon the leaching in ammonia chloride and hydrochloric acid. Hydrometallurgy 2015, 157, 22–32. [Google Scholar] [CrossRef]
- Chen, S.-L.; Guo, X.-Y.; Shi, W.-T.; Li, D. Extraction of valuable metals from low-grade nickeliferous laterite ore by reduction roasting-ammonia leaching method. J. Cent. South Univ. Technol. 2010, 17, 765–769. [Google Scholar] [CrossRef]
- Li, S.W.; Li, H.Y.; Chen, W.H.; Peng, J.H.; Yin, S.H.; Zhang, L.B.; Yang, K. Ammonia Leaching of Zinc from Low-grade Oxide Zinc Ores Using the Enhancement of the Microwave Irradiation. Int. J. Chem. React. Eng. 2017, 16, 107–115. [Google Scholar] [CrossRef]
- Ehsan, B.; Valeh, A. Investigation of Copper Ammonia Leaching from Smelter Slags: Characterization, Leaching and Kinetics. Metall. Mater. Trans. B 2015, 46, 2305–2314. [Google Scholar]
- Radmehr, V.; Koleini, S.M.J.; Khalesi, M.R.; Mohammadi, M.R.T. Ammonia Leaching: A New Approach of Copper Industry in Hydrometallurgical Processes. J. Inst. Eng. India Ser. D 2013, 94, 95–104. [Google Scholar] [CrossRef]
- Chen, J.B. Reduction roasting and ammonia leaching of high-iron manganese ore from Zunyi. China’s Manganese Ind. 1995, 13, 38–43. [Google Scholar]
- Chen, Y.M.; Yi, B.B. The theory and practice of ammonia leaching in the treatment of low-grade manganese. Wuhan Univ. Technol. 2004, 13, 100–105. [Google Scholar]
- Heindl, R.; Ruppert, J.; Skow, M.; Conley, J. Manganese from Steel-Plant Slags by a Lime-Clinkering and Carbonate-Leaching Process: Part I, Laboratory Development (in Two Parts). BuMines Rep. Inv. 1955, 5124, 23–61. [Google Scholar]
- Mcintosh, S.N.; Baglin, E.G. Recovery of Manganese from Steel Plant Slag by Carbamate Leaching; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1992. [Google Scholar]
- Tu, Z.B.; Liang, X.P.; Yang, X.G.; Ren, S.L.; Wu, C.B.; Wang, Y. Recovery of Manganese by Roasting-Ammonia Leaching from Low-Grade Manganese Carbonate Ores. In Rare Metal Technology, The Minerals, Metals & Materials Series; Azimi, G., Kim, H., Alam, S., Ouchi, T., Neelameggham, N., Baba, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Sun, W.; Liu, J.; Wang, L.; Yi, Y. Aluminum-Enhanced Coal Pyrite Leaching during SO2 Removal with Coal Slurry. Water Air Soil Pollut. 2016, 227, 1–8. [Google Scholar] [CrossRef]
- Zhou, J.F.; Liu, C.C.; Ma, J.Y.; Qin, Y.H.; Wu, Z.K.; Yang, L.; Wang, T.L.; Wang, W.G.; Wang, C.W. Intensification of potassium leaching from phosphorus-potassium associated ore with lauryl alcohol. Sep. Purif. Technol. 2018, 191, 1–7. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.-M.; Liu, T.; Huang, J.; Zhao, J.; Zhang, G.; Liu, J. Comparison of direct acid leaching process and blank roasting acid leaching process in extracting vanadium from stone coal. Int. J. Miner. Process. 2014, 128, 40–47. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, K.; Tian, X.; Qin, W. Vanadium leaching from carbonaceous shale using fluosilicic acid. Int. J. Miner. Process. 2011, 100, 184–187. [Google Scholar] [CrossRef]
- Patakamuri, G.; Eduard, G.; Yeonuk, C.; Ye, Z.B. Pressure oxidation leaching of an enargite concentrate in the presence of polytetrafluoroethylene beads. Hydrometallurgy 2015, 157, 340–347. [Google Scholar]
- Tu, Z.B.; Liang, X.P.; Wang, Y.; Wu, C.B. Removal of Phosphorus from High-Phosphorus Manganese Ores by Ammonia-Ammonium Carbonate Leaching Method. Metals 2019, 9, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Li, C.; Li, J.; Liu, H.; Wu, S. Leaching of vanadium from carbonaceous shale. Hydrometallurgy 2009, 99, 97–99. [Google Scholar] [CrossRef]
- Li, M.; Wei, C.; Fan, G.; Wu, H.; Li, C.; Li, X. Acid leaching of black shale for the extraction of vanadium. Int. J. Miner. Process. 2010, 95, 62–67. [Google Scholar] [CrossRef]
- Yang, X.; Fang, Q.; Ouyang, H. Density Functional Theory Study of Leaching Performance of Different Acids on Pyrochlore (100) Surface. JOM 2018, 70, 1000–1004. [Google Scholar] [CrossRef]
- He, D.; Feng, Q.; Zhang, G.; Luo, W.; Ou, L. Study on leaching vanadium from roasted residue of stone coal. Min. Met. Explor. 2008, 25, 181–184. [Google Scholar] [CrossRef]
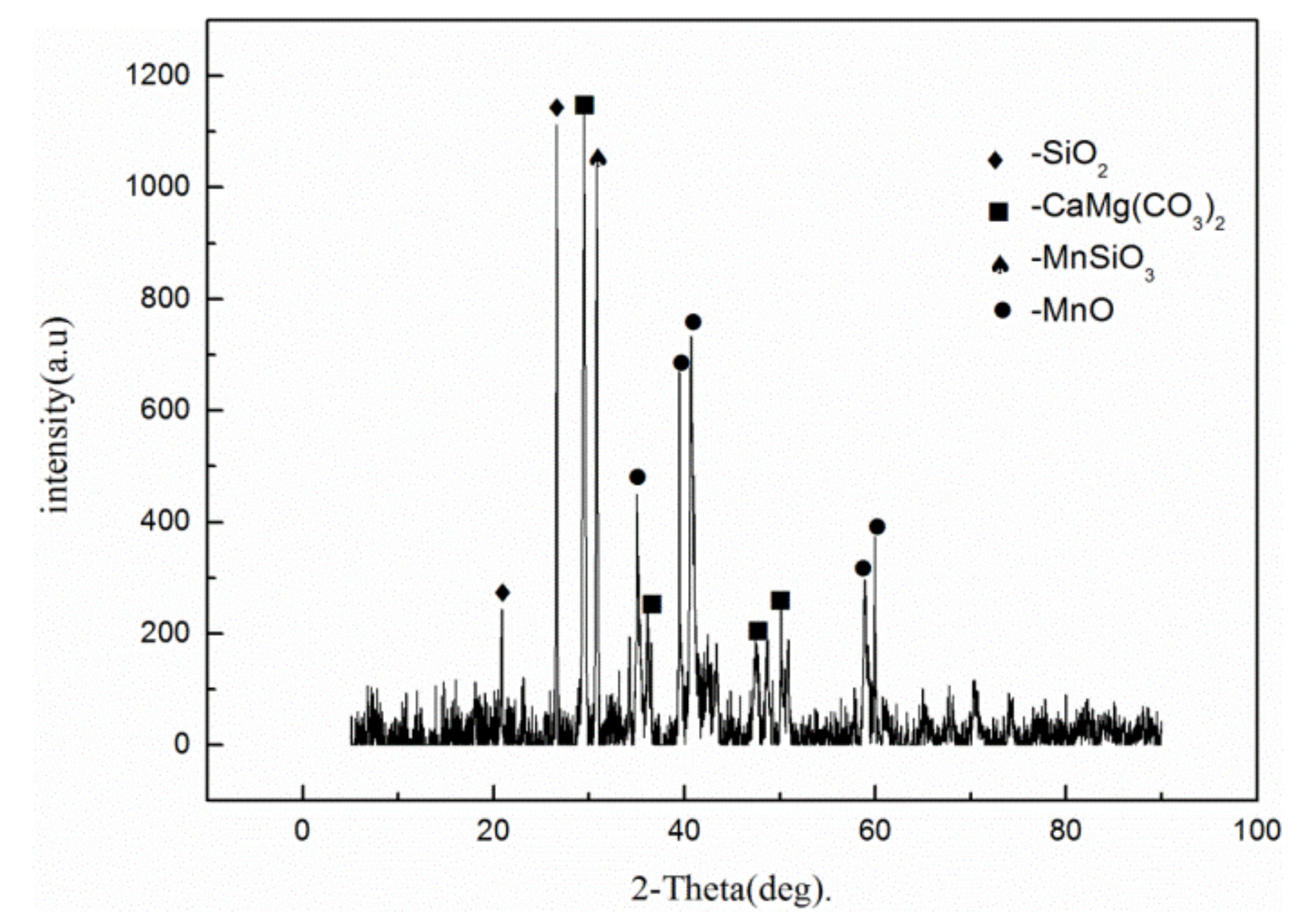


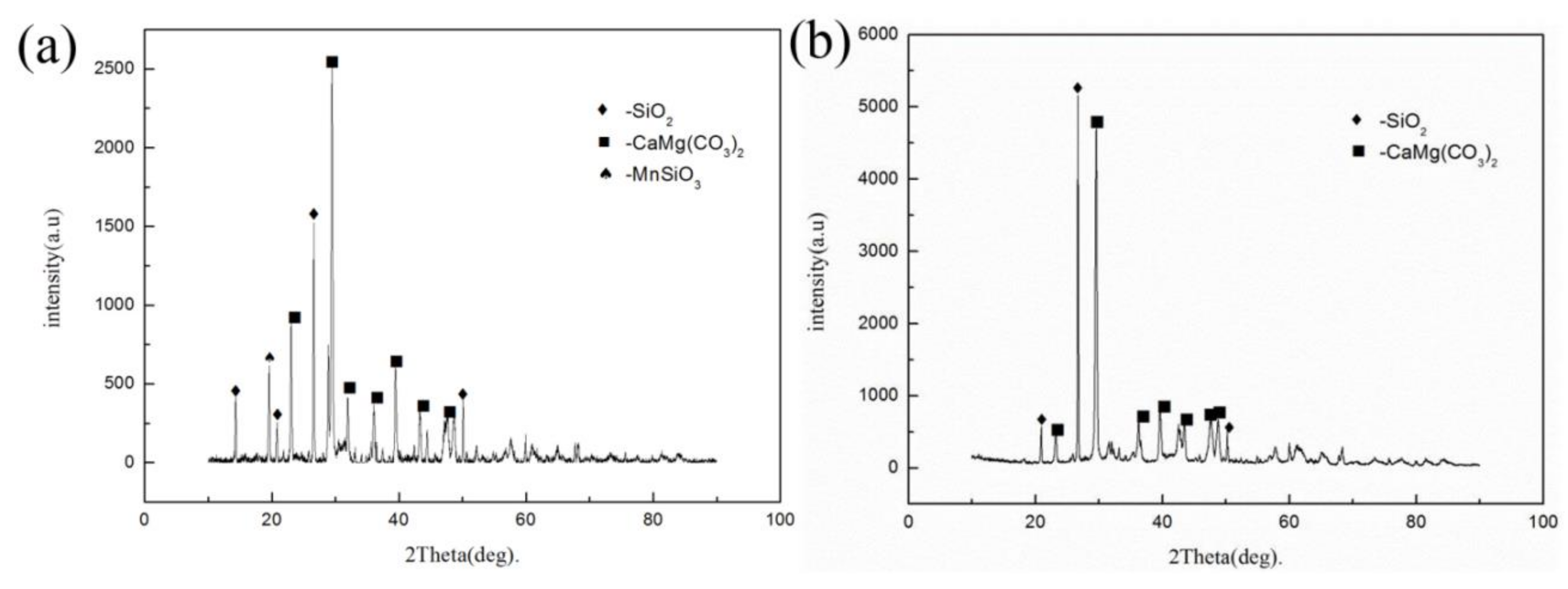
| Element | Mn | Ca | Si | Al | Mg | Fe | P | S |
|---|---|---|---|---|---|---|---|---|
| Content (w/%) | 23.19 | 9.41 | 4.15 | 1.30 | 2.73 | 1.24 | 0.51 | 0.51 |
| Investigate Factors | Symbol | Level | ||||
|---|---|---|---|---|---|---|
| α = −1.682 | −1 | 0 | 1 | α = +1.682 | ||
| Ammonia leaching temperature | °C | 26.64 | 28 | 30 | 32 | 33.36 |
| Ammonia concentration | mol/L | 6.64 | 8 | 10 | 12 | 13.36 |
| Ammonium hydrogen fluoride addition | w/% | 2.64 | 4 | 6 | 8 | 9.36 |
| Number | Ammonia Concentration (mol/L) | Ammonia Leaching Temperature (°C) | Ammonium Hydrogen Fluoride Addition (w/%) | Leaching Efficiency (%) |
|---|---|---|---|---|
| 1 | 10.00 | 33.36 | 6.00 | 52.4 |
| 2 | 10.00 | 30.00 | 2.64 | 49.3 |
| 3 | 13.36 | 30.00 | 6.00 | 90.6 |
| 4 | 10.00 | 30.00 | 6.00 | 51.3 |
| 5 | 12.00 | 32.00 | 4.00 | 64.9 |
| 6 | 10.00 | 30.00 | 6.00 | 51.3 |
| 7 | 12.00 | 28.00 | 8.00 | 66.8 |
| 8 | 6.64 | 30.00 | 6.00 | 23.3 |
| 9 | 8.00 | 32.00 | 8.00 | 31.2 |
| 10 | 8.00 | 28.00 | 8.00 | 30.7 |
| 11 | 8.00 | 32.00 | 4.00 | 27.1 |
| 12 | 10.00 | 30.00 | 6.00 | 51.3 |
| 13 | 10.00 | 30.00 | 6.00 | 51.3 |
| 14 | 8.00 | 28.00 | 4.00 | 26.3 |
| 15 | 10.00 | 30.00 | 6.00 | 51.3 |
| 16 | 10.00 | 26.64 | 6.00 | 48.9 |
| 17 | 12.00 | 32.00 | 8.00 | 68.4 |
| 18 | 10.00 | 30.00 | 9.36 | 54.2 |
| 19 | 12.00 | 28.00 | 4.00 | 63.8 |
| 20 | 10.00 | 30.00 | 6.00 | 51.3 |
| Sources of Variance | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 5754.23 | 9 | 639.36 | 43.07 | <0.0001 | significant |
| A | 5672.43 | 1 | 5672.43 | 382.13 | <0.0001 | |
| B | 3.16 | 1 | 3.16 | 3.21 | 0.0345 | |
| C | 10.91 | 1 | 10.91 | 10.74 | 0.0013 | |
| AB | 0.011 | 1 | 0.011 | 0.012 | 0.9786 | |
| AC | 0.78 | 1 | 0.78 | 0.053 | 0.8232 | |
| BC | 0.031 | 1 | 0.031 | 0.021 | 0.9643 | |
| A2 | 1.379 × 10–4 | 1 | 1.379 × 10–4 | 1.025 | 0.0006 | |
| B2 | 31.64 | 1 | 31.64 | 2.13 | 0.1750 | |
| C2 | 40.49 | 1 | 40.49 | 2.73 | 0.1296 | |
| Residual | 148.44 | 10 | 14.84 | |||
| Lack of Fit | 148.25 | 5 | 29.65 | 787.19 | <0.0001 | significant |
| Pure Error | 0.19 | 5 | 0.038 | |||
| Cor Total | 5902.67 | 19 |
| Ammonia Concentration (mol/L) | Ammonia Leaching Temperature (°C) | Ammonium Hydrogen Fluoride Addition (w/%) | Leaching Efficiency Predicted Value (%) | Leaching Efficiency Experiment Value (%) |
|---|---|---|---|---|
| 14.5 | 31.6 | 7.3 | 93.2 | 92.0 |
| Main Components | Mn | Ca | Mg |
|---|---|---|---|
| Content/% | 44.13 | 1.31 | 1.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Liang, X.; Wu, C.; Cui, T.; Wang, Y. Study on Adding Ammonium Hydrogen Fluoride to Improve Manganese Leaching Efficiency of Ammonia Leaching Low-Grade Rhodochrosite. Metals 2021, 11, 1496. https://doi.org/10.3390/met11091496
Yang P, Liang X, Wu C, Cui T, Wang Y. Study on Adding Ammonium Hydrogen Fluoride to Improve Manganese Leaching Efficiency of Ammonia Leaching Low-Grade Rhodochrosite. Metals. 2021; 11(9):1496. https://doi.org/10.3390/met11091496
Chicago/Turabian StyleYang, Peng, Xiaoping Liang, Chengbo Wu, Tengfei Cui, and Yu Wang. 2021. "Study on Adding Ammonium Hydrogen Fluoride to Improve Manganese Leaching Efficiency of Ammonia Leaching Low-Grade Rhodochrosite" Metals 11, no. 9: 1496. https://doi.org/10.3390/met11091496
APA StyleYang, P., Liang, X., Wu, C., Cui, T., & Wang, Y. (2021). Study on Adding Ammonium Hydrogen Fluoride to Improve Manganese Leaching Efficiency of Ammonia Leaching Low-Grade Rhodochrosite. Metals, 11(9), 1496. https://doi.org/10.3390/met11091496





