Abstract
Non-availability of rich primary resources of rare earth metals (REMs) and the generation of huge amounts of discarded magnets containing REMs, compelled the researchers to explore the possibilities for the recovery of REMs from discarded magnets. Therefore, the present paper reports the recovery of REMs (Nd, Pr, and Dy) from discarded Nd-Fe-B magnets. The process consists of demagnetization, pre-treatment, and hydrometallurgical processing to recover REMs as salt. Leaching studies indicate that 95.5% Nd, 99.9% Pr, and 99.9% Dy were found to be dissolved at the optimized experimental condition i.e., acid concentration 2 M H2SO4, temperature 75 °C, pulp density 100 g/L, and mixing time 60 min. Solvent extraction technique was tried for the selective extraction/separation of REMs and Fe. The result indicates that 99.1% (24.42 g/L) of Nd along with 90% (1.08 g/L) of Pr and total Fe were co-extracted using 35% Cyanex 272 at organic to aqueous (O/A) ratio 1/1, eq. pH 3.5 in 10 min of mixing time. It requires multistage separation and therefore, not feasible in view of economics. Thus, direct precipitation of REMs salt and iron oxide as pigment was studied using two stages of precipitation at different pH. The obtained precipitate of REMs and Fe hydroxides were dried separately to remove the moisture and further treated at elevated temperature to get pure REMs oxide and red oxide.
1. Introduction
Due to rapid industrialization and technological advancement, old electronic devices are replaced with new ones, resulting in the generation of massive amounts of discarded electronic scraps such as personal computers, mobile phones, televisions, etc. [1]. It has been reported that 53.5 million tons (Mt) of e-waste were generated in 2019 and it has been expected that 74.7 Mt of e-waste will be generated by 2030 [2]. The discarded e-waste contains a variety of metals such as base metals (Cu, Ni, Sn, Pb, etc.), rare earth metals (Nd, Pr, Dy, etc.) including hazardous metals that may cause a serious environmental threat if disposed into the landfilling without treatment, as well as the loss of valuables. Therefore, it is needed to develop a sustainable process for the extraction of metals from e-waste, which may also minimize the dependency on primary natural resources.
Various studies have been made to recover rare earth metals (REMs) using pyro-/hydro-metallurgy and hybrid processes. Onal et al. [3] reported the nitration of Nd-Fe-B magnet at 200 °C to convert the refractory magnet into soluble species of REMs. Further, calcined magnets were leached in water at room temperature to dissolve more than 95% REMs while maintaining a pulp density of 60 g/L. In addition, a magnet was roasted at 800 °C to transform the refractory magnet into soluble species of REMs. The roasted product was pressure leached in 0.6 M hydrochloric acid at 180 °C to leach 99% REMs [4]. The mechano-chemical studies have also been made with the addition of ferric sulfate to convert the refractory magnet into their sulfate salt to make the subsequent leaching step easier. The pre-treated product was further leached in water at elevated temperature and subsequently, REMs were precipitated with oxalic acid at pH 1.9 [5].
In addition, oxidative roasting followed by organic acid leaching has been used to recover the REMs from Nd-Fe-B magnet. The refractory Nd-Fe-B magnet was oxidized into the acid susceptible species by heating at 900 °C for 480 min. The oxidized product was dissolved in a mixture of malic and citric acids at 90 °C to leach more than 90% REMs [6]. Kumari et al. [1] reported the sulfuric acid leaching and precipitation process for recovery of Nd, almost ~99.99% REMs (Nd, Dy and Pr) were leached in 1 M sulfuric acid at room temperature. Further, Nd was precipitated with ~98% purity by using ammonia to get Nd salt at pH 1.65; however, minor co-precipitation of Dy and Pr also occurred. In a subsequent study, electrochemical leaching was conducted in a mixture of 0.1 M H2SO4 and 0.05 M H2C2O4 at the current density of 20 A/dm2 to enhance the leaching efficiency of REMs [7]. The obtained leach liquor was subjected to separation and purification studies to extract the REMs. Pavon et al. [8] extracted 99.9% Nd from the leach liquor of Nd-Fe-B magnet at eq. pH 0.8 to 1.5 with 0.3 M Cyanex 572. Further, 58.62% Nd, 98.46% Dy, and 85.59% Pr were extracted at pH 2 with 1 M D2EHPA and stripped with 2 M nitric acid [9].
Besides the solvent extraction, precipitation studies have also been investigated for the extraction of REMs from leach liquor of Nd-Fe-B magnet. Lee et al. [10] used sodium hydroxide to precipitate Nd as Nd(OH)3 at pH 0.6. Rabatho et al. [11] leached 98% of Nd and 81% of Dy in 1 M HNO3 at 25 °C in presence of 0.3 M H2O2, but Fe remained in the residue. Further, 81.8% Dy and 91.5% Nd were recovered by precipitation using oxalic acid (H2C2O4) in a range of pH 8 at room temperature [11]. A lot of research on this topic is reported, but due to various factors it is still not applied for commercial exploitation in industries. Another aspect of this research is recycling, which will play an important role in the circular economy as well as in the conservation of natural resources. The important findings in the developed process are closed-loop, which means all the materials will be recycled in the production flow with no waste, i.e., zero waste generation.
Keeping in view of the above, the present paper reports the novel process for selective recovery of REMs oxides (Nd, Pr, and Dy) from discarded magnets of the hard disk considering the cost of the process. The magnets were separated by being dismantled, demagnetized by heat treatment, crushed to reduce the particle size, leached to dissolve the REMs, and selective extraction of REMs using solvent extraction/precipitation to produce REMs oxides. Compared to previous studies, the developed process reports less energy for demagnetization of discarded Nd-Fe-B magnets as well as a smaller number of stages to get REMs like rare earth oxides.
2. Materials and Methods
2.1. Pre-Treatment of Nd-Fe-B Magnets
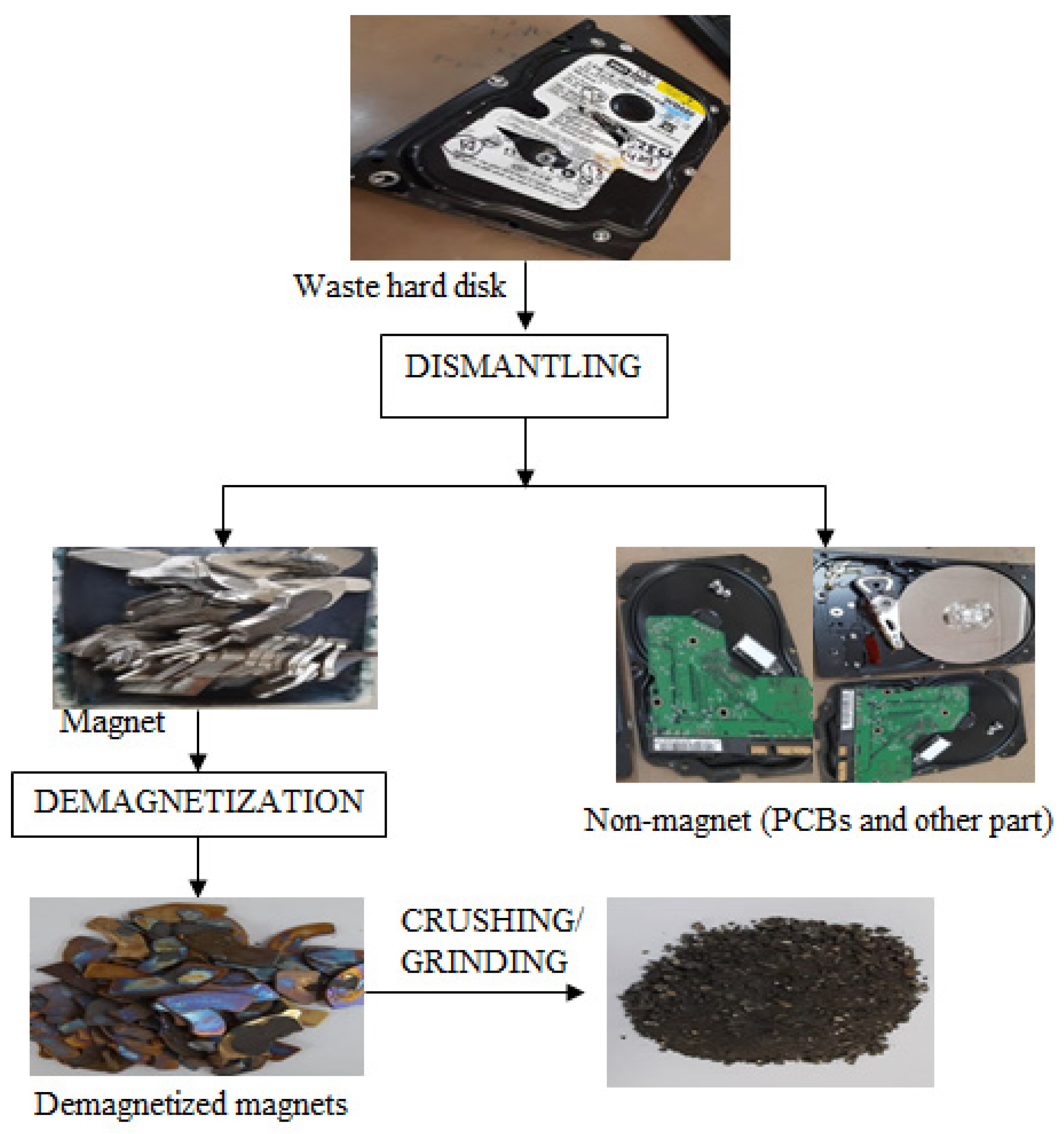
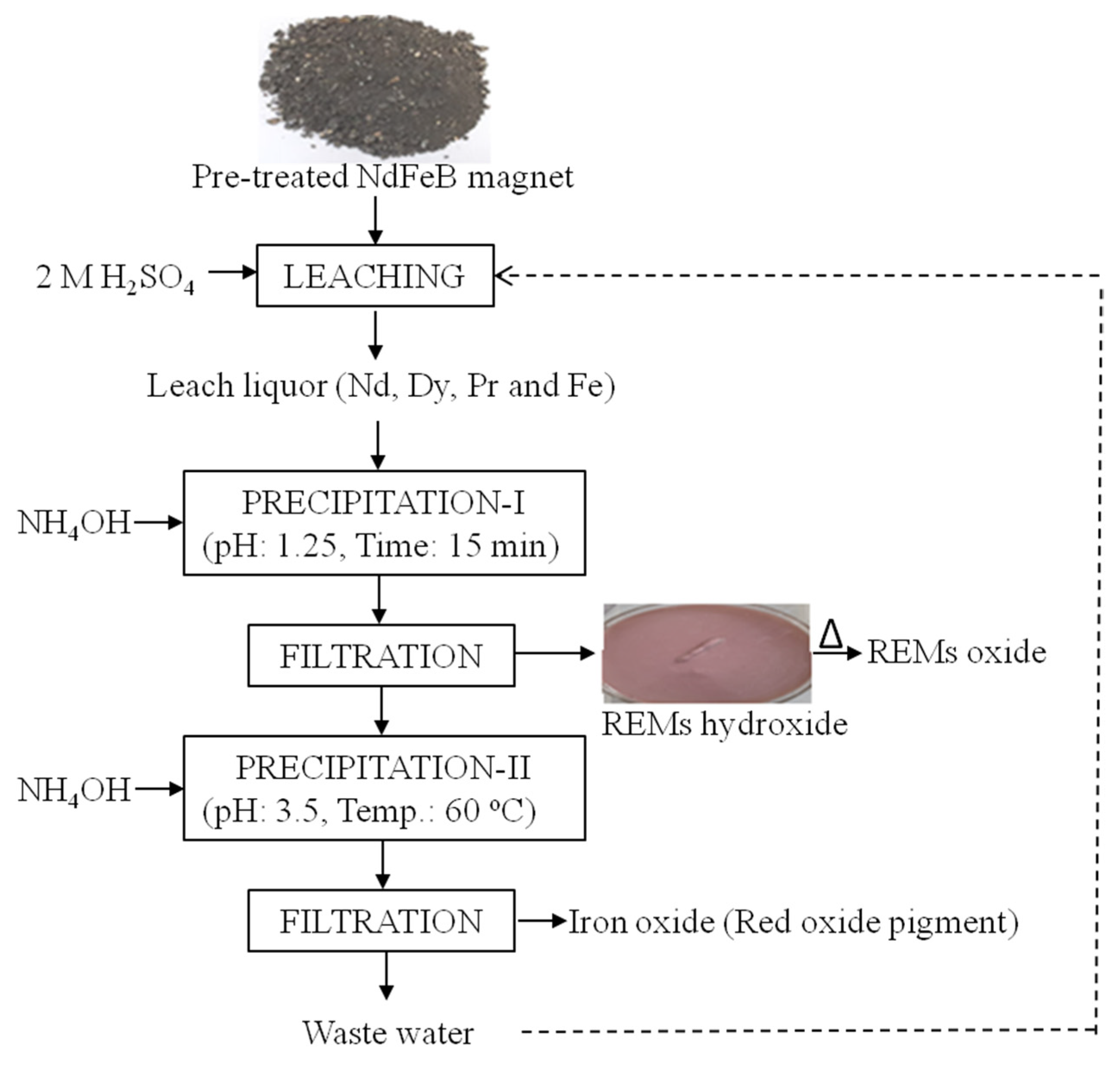
At first, a discarded hard disk of CPUs was dismantled to separate the Nd-Fe-B magnets for recovery of rare earth metals including iron. Further, Nd-Fe-B magnets were demagnetized by heating at 300 °C for 3 h. The demagnetized material was crushed into small sizes (2 × 2 mm) using the mortar pestle to make the particle surface area higher, which will enable effective leaching. The process flow-sheet for the pre-treatment of Nd-Fe-B magnet is presented as Figure 1. The composition of Nd-Fe-B magnet was determined by the chemical analysis method and presented in Table 1.

Figure 1.
Pre-treatment of magnets prior to leaching of REMs.

Table 1.
Composition of metals present in Nd-Fe-B magnet.
2.2. Leaching Procedure
Dissolution experiments were carried out in a leaching reactor of capacity: 100 mL (Borosil, Mumbai, India) equipped with a condenser facility to avoid the loss of liquid through evaporation due to heating. Leaching studies were carried out using a hot plate fitted with a temperature controller and sensor. To optimize the process parameters for the effective dissolution of REMs, the concentration of sulfuric acid leachant and temperature were varied between 0.5 to 2.5 M and 25 to 90 °C, respectively. To study the dissolution behavior of REMs along with other metals, the samples of slurry/leach liquor were collected at regular intervals of time during the leaching experiment. The leach liquor was separated from the leached residue using filtration method. The metals present in the leached residue and leach liquor were checked to see the material balance. Satisfactory material balance was obtained for each set of leaching experiments by calculating the weight of the sample before leaching, which is equal to the amount of metals concentration in solution plus the weight of leached residue. Further, the leach liquor was processed for REMs extraction using the solvent extraction technique.
2.3. Solvent Extraction Procedure
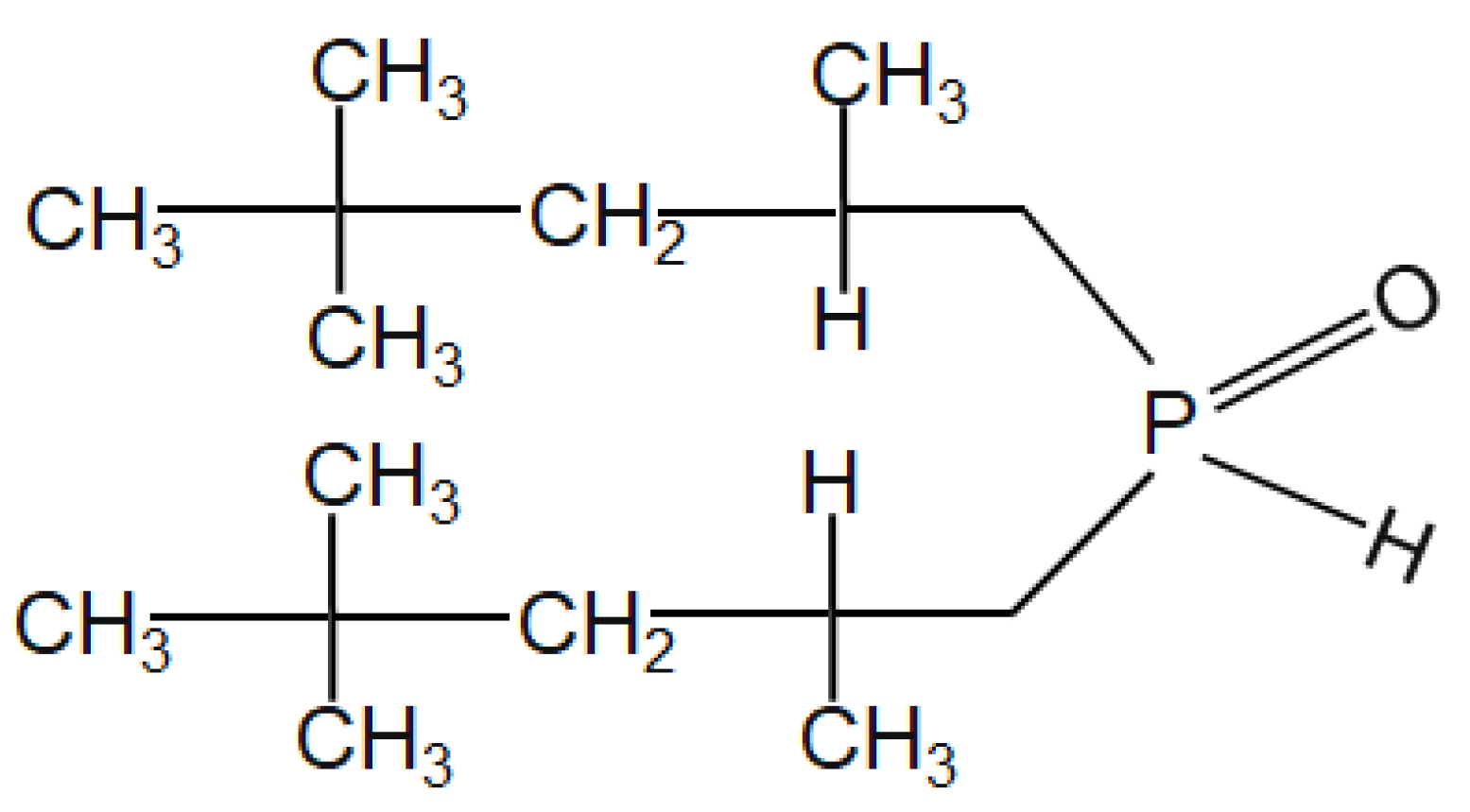
Solvent extraction studies were carried out in a conical flask using a magnetic stirrer (Borosil, Mumbai, India) at room temperature. The equal volume of leach liquor and extractant Cyanex 272 (Figure 2) (50 mL/50 mL) were put in the conical flask for proper mixing. Ammonium hydroxide was used to maintain the eq. pH in the range of 1 to 3.5. When the solution attained the equilibrium, the metal-loaded organic extractant and raffinate were separated using a separating funnel. The metals-loaded organic extractant was stripped using dil. sulfuric acid. The concentration of metal ions in the loaded extractant and raffinate were analyzed by using ICP-OES (VISTA–PMX, CCD Simultaneous, Make: Australia). The material balance was checked by calculating the metal ions present in the strip solution obtained from loaded extractant, raffinate, and the head sample.
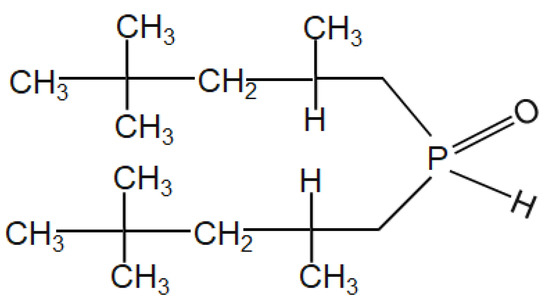
Figure 2.
Structure of Cyanex 272 [bis (2,2,4 trimethylpentyl) phosphinic acid].
2.4. Precipitation Procedure
Precipitation experiments were conducted in a beaker (capacity: 100 mL) under the constant stirring speed (300 rpm) at standard temperature using ammonium hydroxide as a precipitant. The solution pH was varied in the range of 1.0 to 2.5 by the addition of ammonium hydroxide (25.96 M) for the precipitation of REMs. During the precipitation reaction, samples were collected at regular intervals of time to observe the precipitation behavior of metals at various pH and time.
2.5. Characterization and Analysis of Samples
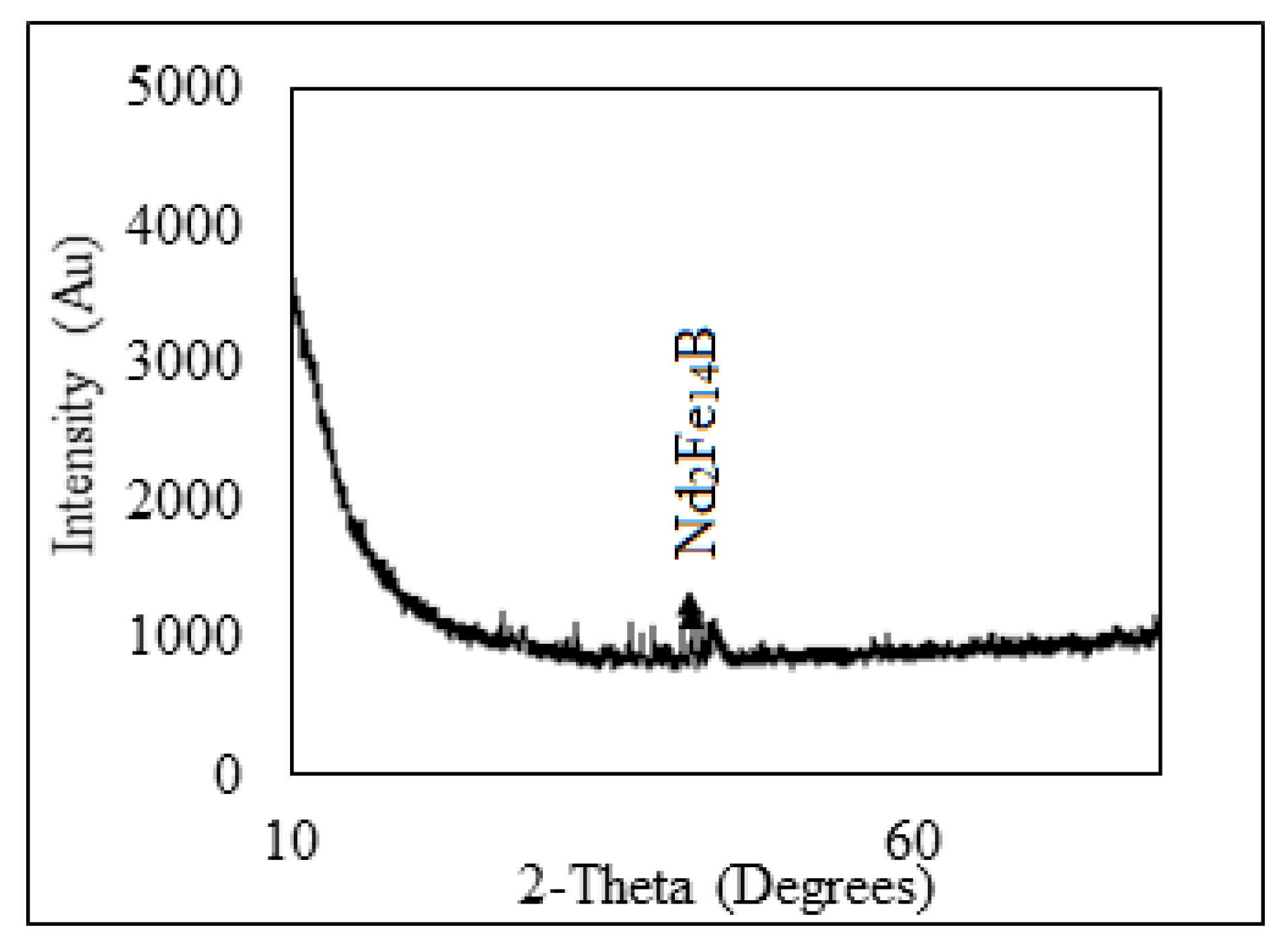
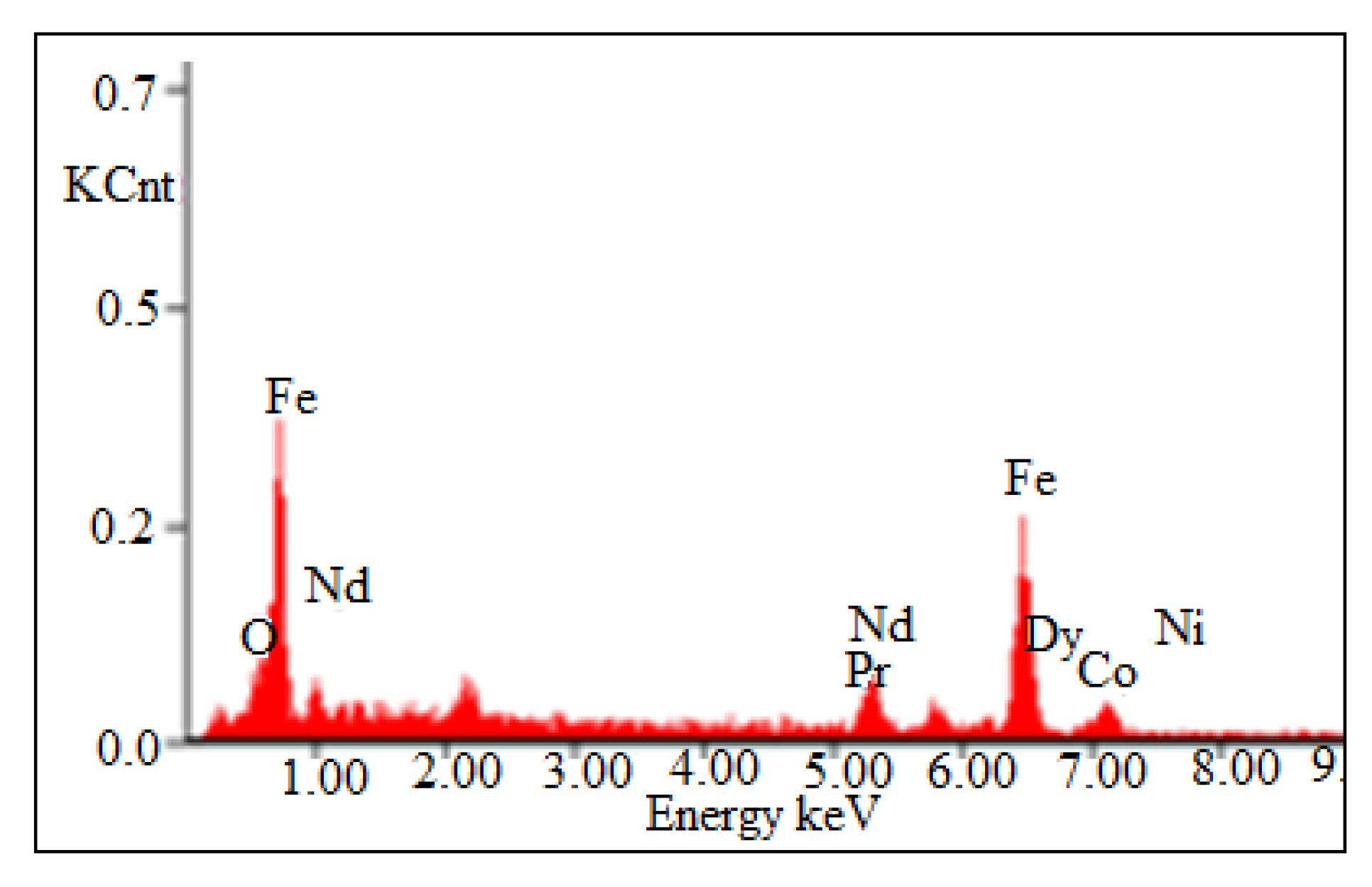
Non-ferrous metals were analyzed using Atomic Absorption Spectrophotometer, AAS, Analyst 200 (Perkin Elmer, Waltham, MA, USA), and REMs were analyzed using Inductively Coupled Plasma-Optical Emission Spectrophotometer, ICP-OES, VISTA-PMX, CCD Simultaneous (Australia). X-ray diffraction (XRD, Bruker AXS D8 instrument) (Bruker, Wisconsin, WI, USA) and scanning electron microscope energy dispersive X-ray spectroscopy (SEM-EDS, JXA-8230 Electron Probe Micro Analyzer, JEOL) (JEOL, Tokyo, Japan) were carried to analyze the phases as well as morphological appearances of the beneficiated magnet. XRD pattern shows that Nd-Fe-B magnet mainly contains the peak of Nd2Fe14B (Figure 3) and was also confirmed by EDS analysis (Figure 4). The Eh- pH of the solution was analyzed (Eh: 0.55 to 1.05 V) using Eutech pH 700 (Thermo Fisher Scientific, 7 Gul Circle, level 2M, Keppel Logistic Building, Singapore). Analytical grade (AR) ammonium hydroxide, sulfuric acid, hydrochloric acid supplied by Rankem, India, were used for experimental purposes.
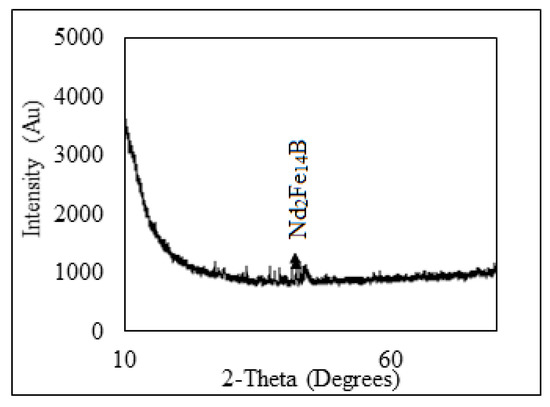
Figure 3.
XRD pattern of crushed magnet.
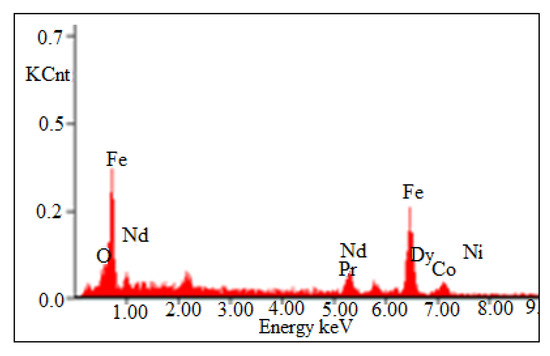
Figure 4.
EDS pattern of demagnetized crushed Nd-Fe-B magnet.
3. Results and Discussion
In order to recover the REMs from discarded Nd-Fe-B magnets, leaching, solvent extraction, and precipitation experiments were carried out. At first, Nd-Fe-B magnets were demagnetized by heat treatment. The demagnetized material was crushed to get fine powder for making it suitable for the effective leaching of REMs. The leaching studies were conducted varying different experimental parameters to get suitable conditions for metals dissolution. Further, from the leach liquor, solvent extraction studies were made to extract the REMs, particularly, Nd from the leach liquor. The loaded organic was stripped to get Nd enriched solution. Finally, the precipitation studies were carried out to recover the Nd as precipitate with other elements as minor/trace. The obtained results are discussed below.
3.1. Leaching Study
For the effective dissolution of metals from waste Nd-Fe-B magnets, the various experimental parameters, i.e., acid concentration, reaction time, temperature, pulp density, etc. were studied to leach the REMs effectively from the demagnetized and crushed powder of magnets.
3.1.1. Effect of Acid Concentration
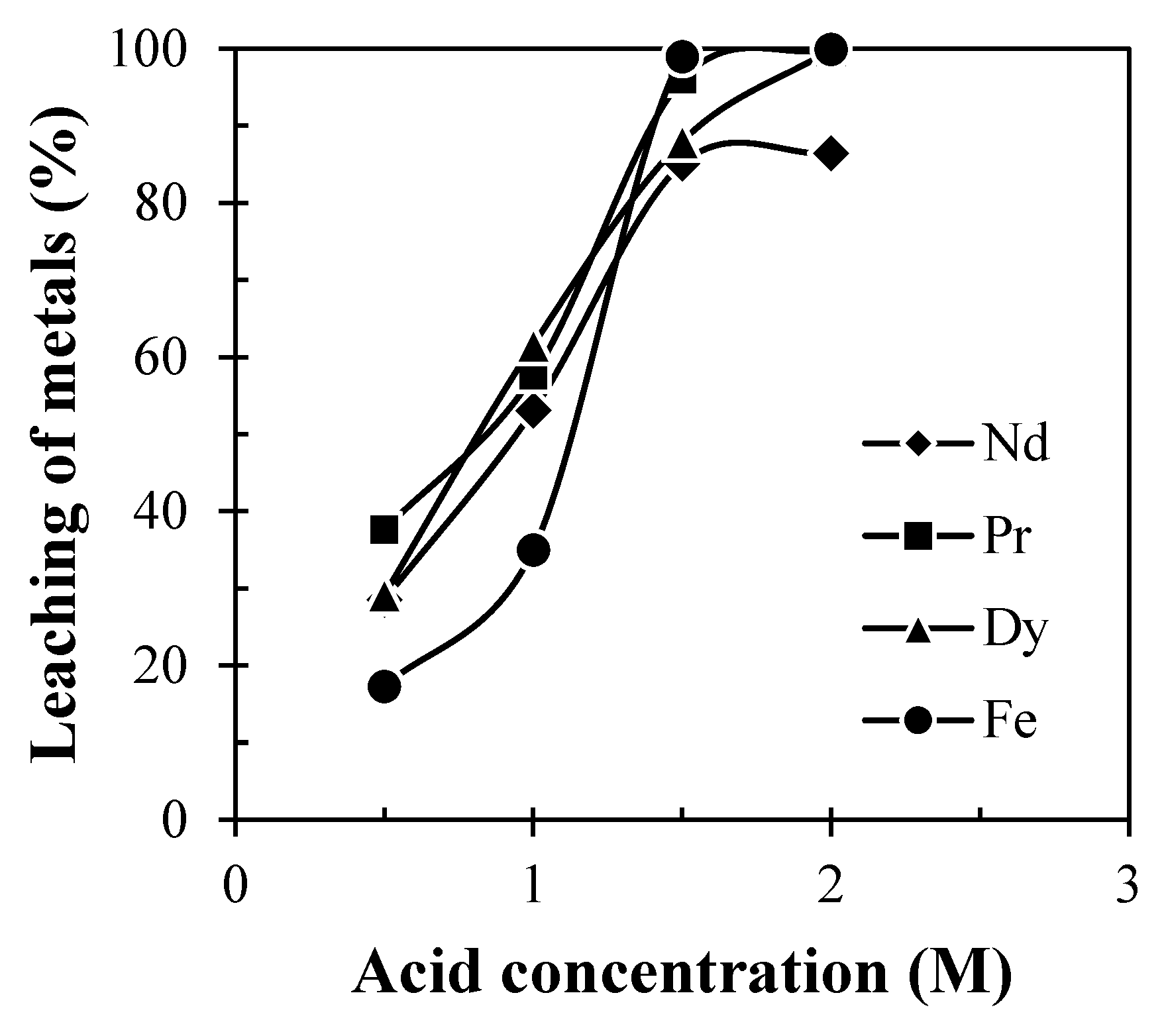
Leaching experiments were performed using different acid concentrations of leachant varied from 0.5 to 2.5 M at temp. 60 °C and mixing time 60 min maintaining pulp density 100 g/L. Results (Figure 5) show that the dissolution of REMs (Nd, Pr and Dy) was found to be increased with increasing acid concentration due to the increase in the acidic strength of the leachant. But, above the acid concentration of 2 M, the leaching efficiency of REMs remained the same. This indicates that 2 M H2SO4 has enough acidity to leach the REMs. Hence, 2 M H2SO4 was chosen as the optimum concentration of acid for the dissolution of REMs from spent Nd-Fe-B magnets. The chemical reaction involved during the leaching of REMs is presented in Equation (1):
2Nd2Fe14B + 37H2SO4 → 2Nd2(SO4)3 + 28FeSO4 + B2(SO4)3 + 18.5H2

Figure 5.
Effect of acid concentration on leaching of REMs from Nd-Fe-B magnets (Solid: Crushed magnet; Liquid: 0.5 to 2 M H2SO4; Pulp density: 100 g/L; Temp.: 60 °C; Time: 60 min).
3.1.2. Effect of Temperature
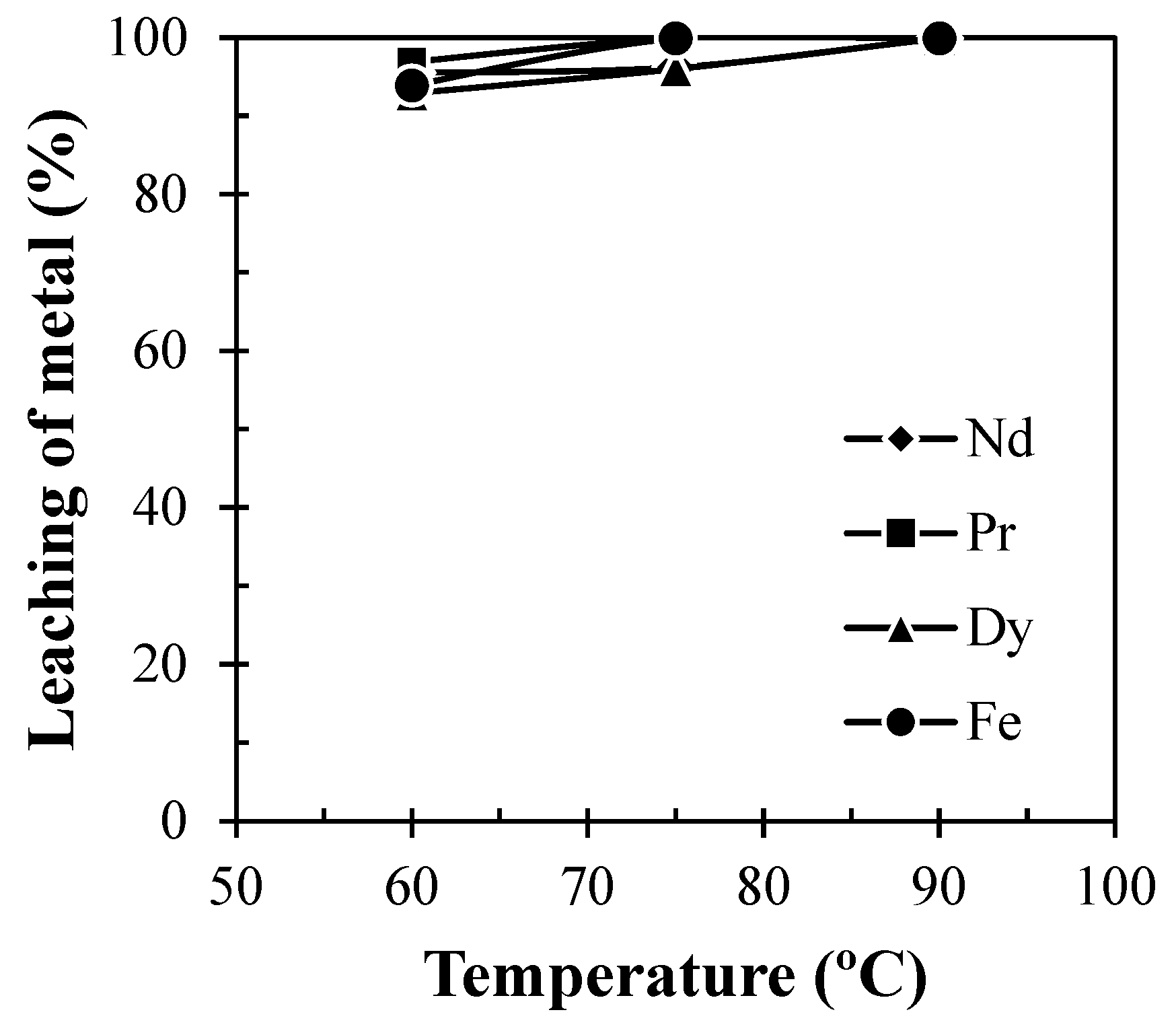
To optimize the temperature for leaching of REMs, experiments were performed at various experimental temperatures varying in the range of 60 to 90 °C at a pulp density of 100 g/L. Figure 6 showed that leaching efficiency of Nd, Pr, and Dy enhanced with the increase in solution temperature due to the increase in the rate of reaction. A total of ~95.5% Nd was found to be leached at 75 °C and a further increase in solution temperature above 75 °C had no significant enhancement on the dissolution of REMs. Therefore, 75 °C was chosen as the optimum temperature for leaching of REMs for further sets of leaching experiments.
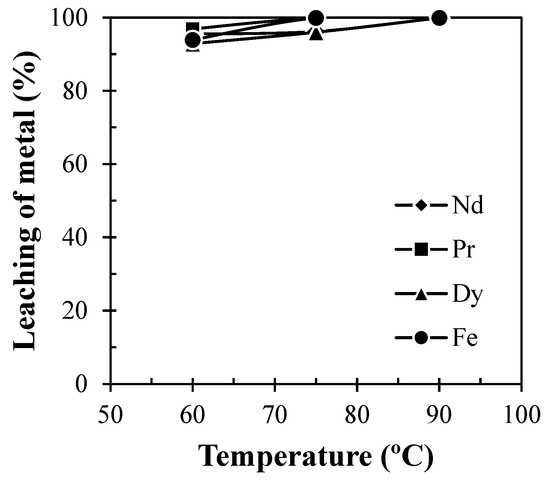
Figure 6.
Effect of temperature on leaching of REMs from Nd-Fe-B magnets (Solid: Crushed magnet; Liquid: 2 M H2SO4; Pulp density: 100 g/L; Time: 60 min).
3.1.3. Effect of Pulp Density
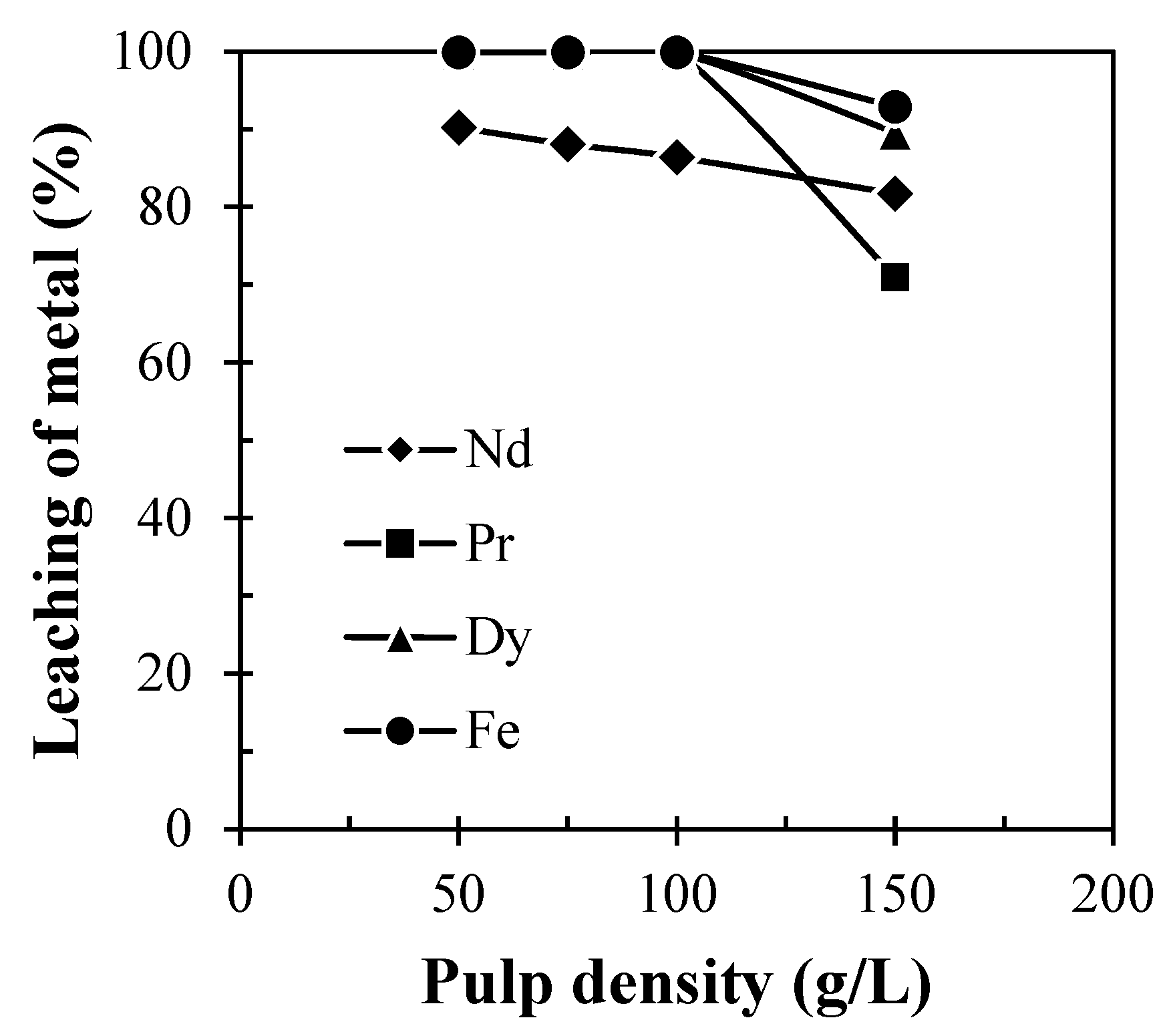
For the optimization of solid to liquid ratio, experiments were performed in a range of pulp density variations from 25 to 200 g/L at 60 °C in 60 min. It was found that leaching of REMs remains the same in the range of pulp density 25 to 100 g/L (Figure 7), but leaching efficiency of REMs was found to be decreased at the pulp density above 100 g/L. This indicates that the number of moles of metallic constitutes (Nd, Pr, and Dy) of REMs became higher than that of leachant molecules, resulting in the decrease in the leaching efficiency of REMs above the pulp density of 100 g/L. Therefore, 100 g/L pulp density was chosen as the optimum condition for the dissolution of REMs from the discarded magnet.
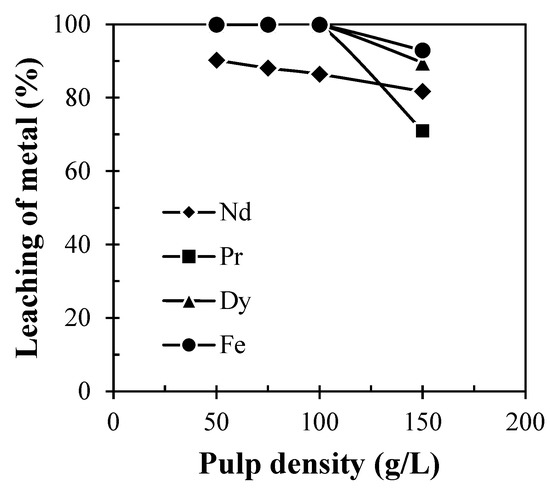
Figure 7.
Effect of pulp density on leaching of REMs from Nd-Fe-B magnets (Solid: Crushed magnet; Acid concentration: 2 M H2SO4; Temp.: 60 °C; Time: 60 min).
3.2. Solvent Extraction of Nd and Pr
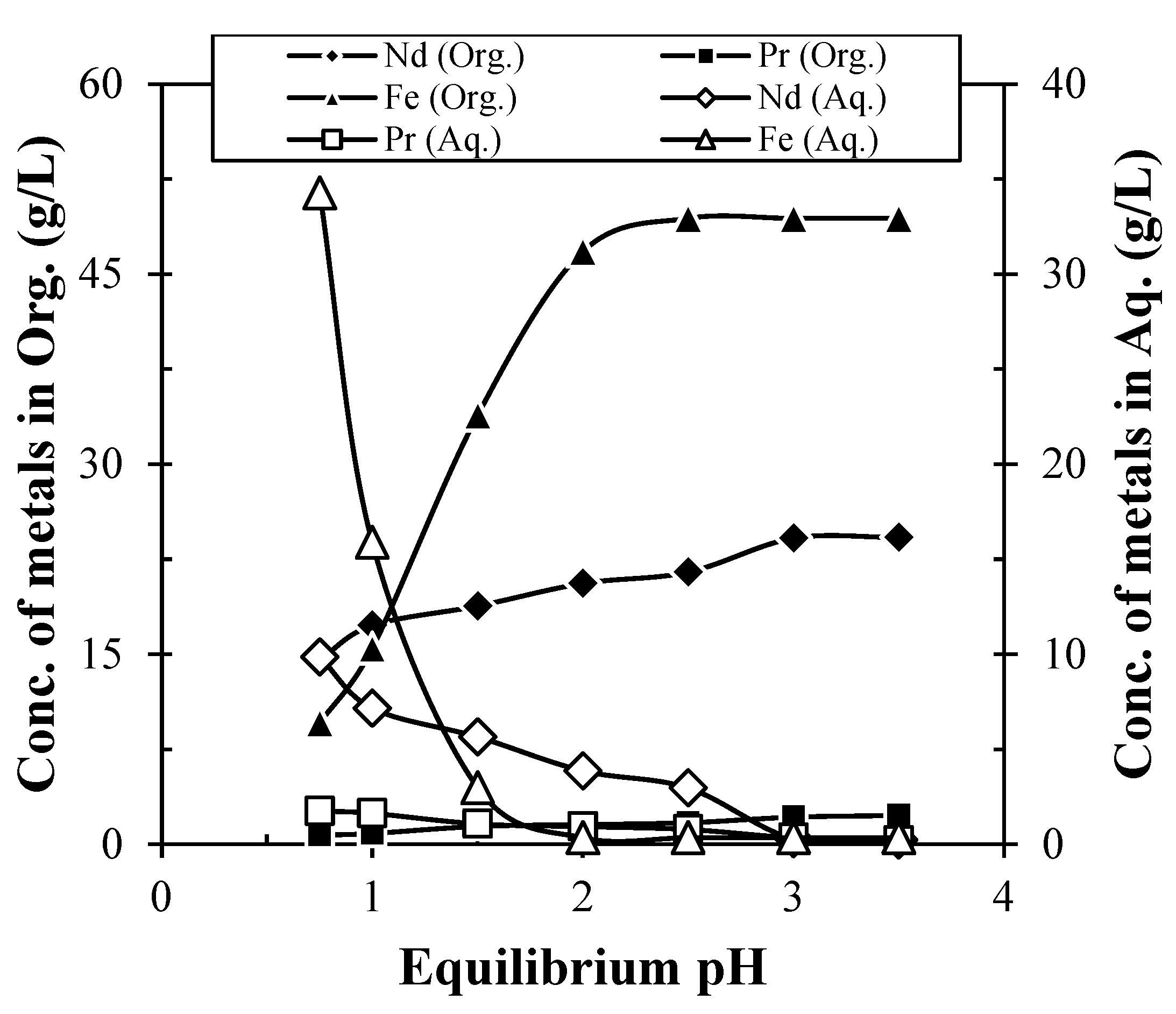
Selective extraction and stripping of REMs/Fe using solvent extraction technique were tried. Experiments were carried out to recover Nd from the obtained leach liquor containing 24.45 g/L Nd, 1.2 g/L Pr, 2.49 g/L Dy, and 49.76 g/L Fe using Cyanex 272. It was found that extraction of Nd increases with the increase in eq. pH, while Pr and Fe also co-extracted (Figure 8). About 15.59 g/L Nd were extracted using 35% Cyanex 272 at eq. pH 0.75 in 10 min mixing time, while complete extraction of Nd occurred at eq. pH 3. Further increase in eq. pH had no significant effect on the extraction of Nd. Further increase in eq. pH had no significant effect on the extraction of Nd. Hence, eq. pH 3 was considered as optimum pH for the extraction of Nd from leach liquor. Further, extraction co-efficient (Kd) (Equation (2)) and separation factor (βNd/Pr) (Equation (3)) of Nd were calculated and found to be 1.5 and 4.42, respectively. The low value of separation factor and extraction co-efficient for Nd indicates that the separation can be possible using multistage extraction. The pH was adjusted during the solvent extraction experiment. The pH was not adjusted separately for aqueous feed, therefore, no precipitation occurred. As per the Eh-pH diagram, the REMs (Nd, Pr) were precipitated as their hydroxides at or above pH 1.5. However, at the same pH, REMs form their complexes and had a tendency to be transferred into the organic phase during solvent extraction. The solvent extraction reaction is very fast in comparison to the slow precipitation reaction. Hence, no precipitation was observed during the solvent extraction of REMs under the studied conditions.
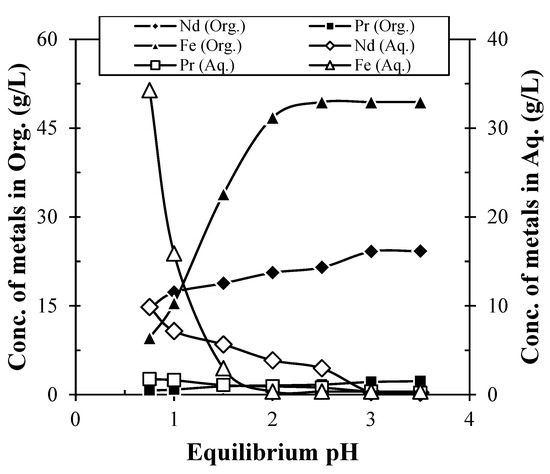
Figure 8.
Extraction of Nd and Pr from leach liquor of spent Nd-Fe-B magnet (Extractant: 35% Cyanex 272; Solution: Leach liquor containing 24.45 g/L Nd, 1.2 g/L Pr, 2.49 g/L Dy, and 49.76 g/L Fe; Time: 10 min; O/A ratio: 1/1).
The multistage solvent extraction process for the separation of REMs and Fe will increase the production cost. Therefore, to make the process feasible and economical, direct precipitation studies were carried out.
3.3. Recovery of REMs from Leach Liquor by Precipitation
Further, the precipitation studies were carried out to recover REMs from the obtained leach liquor 24.45 g/L Nd, 1.2 g/L Pr, 2.49 g/L Dy and 49.76 g/L Fe using the precipitation technique. The pH of the solution was varied in the range of 0.5 and 2.0 using ammonium hydroxide to precipitate selectively the REMs at room temperature, where REEs represents the rare earth elements (Equations (4) and (5)):
REE2(SO4)3 + 6NH4OH → 2REE(OH)3 + 3(NH4)2SO4
2REE(OH)3 → REE2O3 + 3H2O
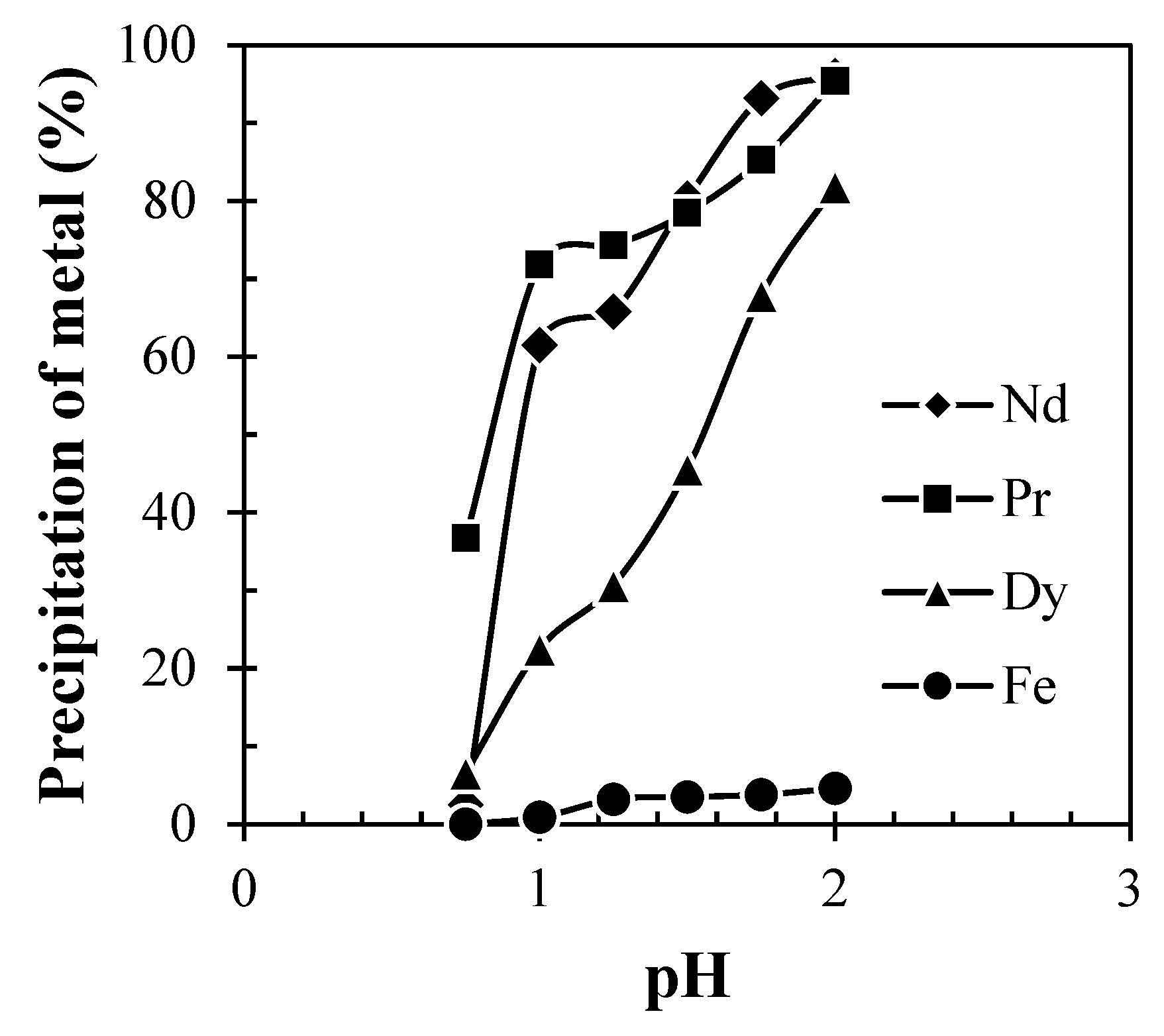
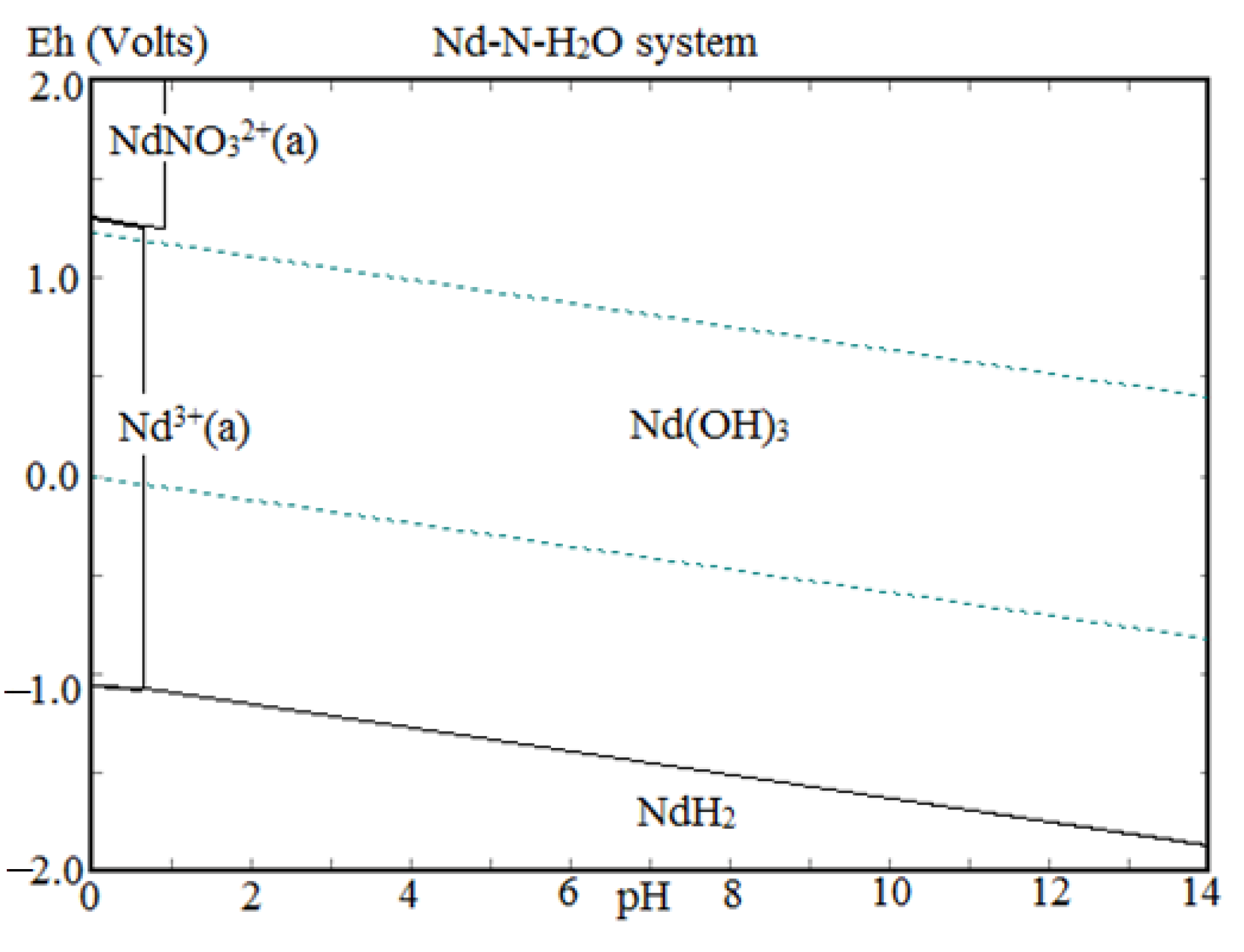
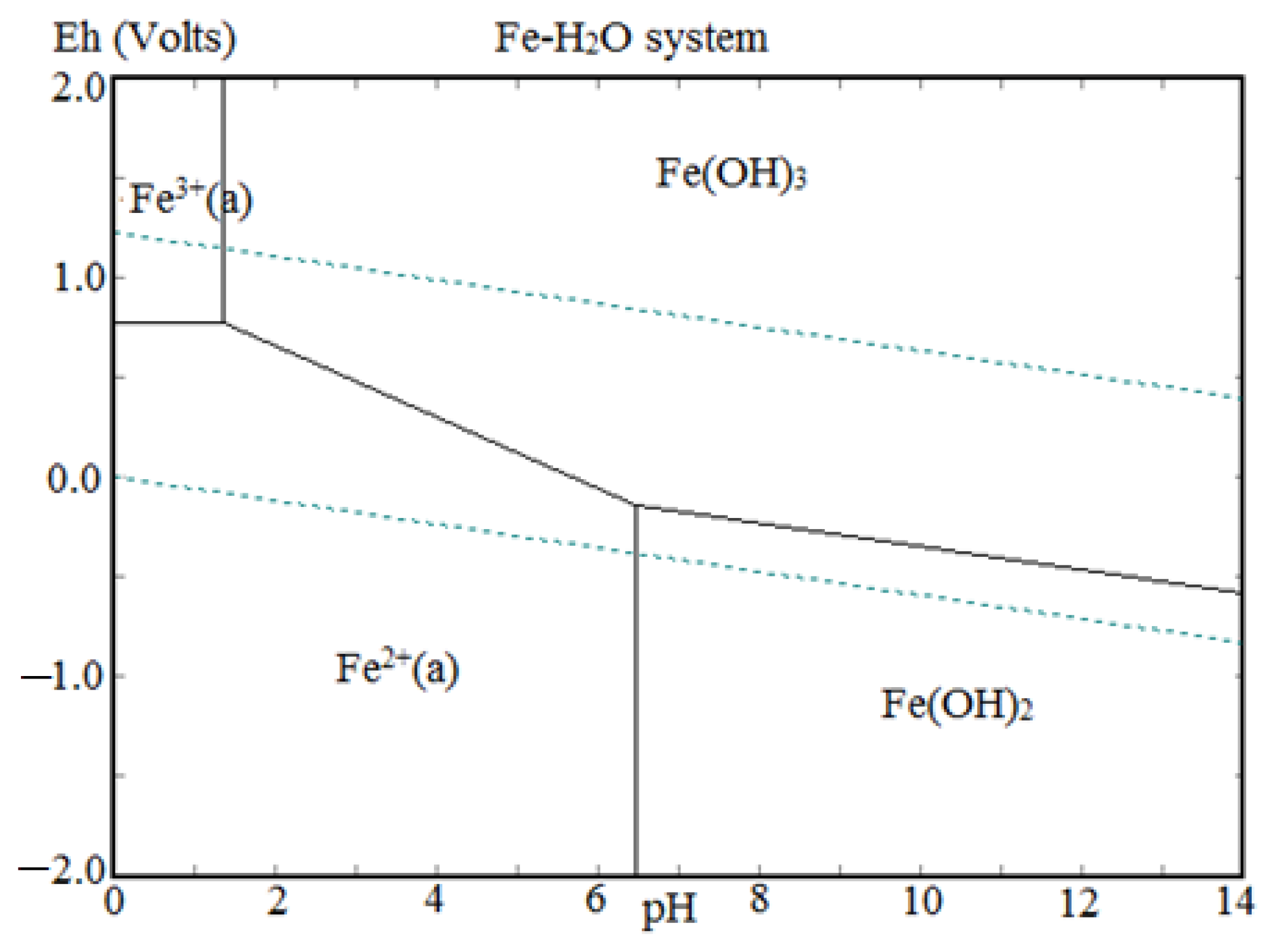
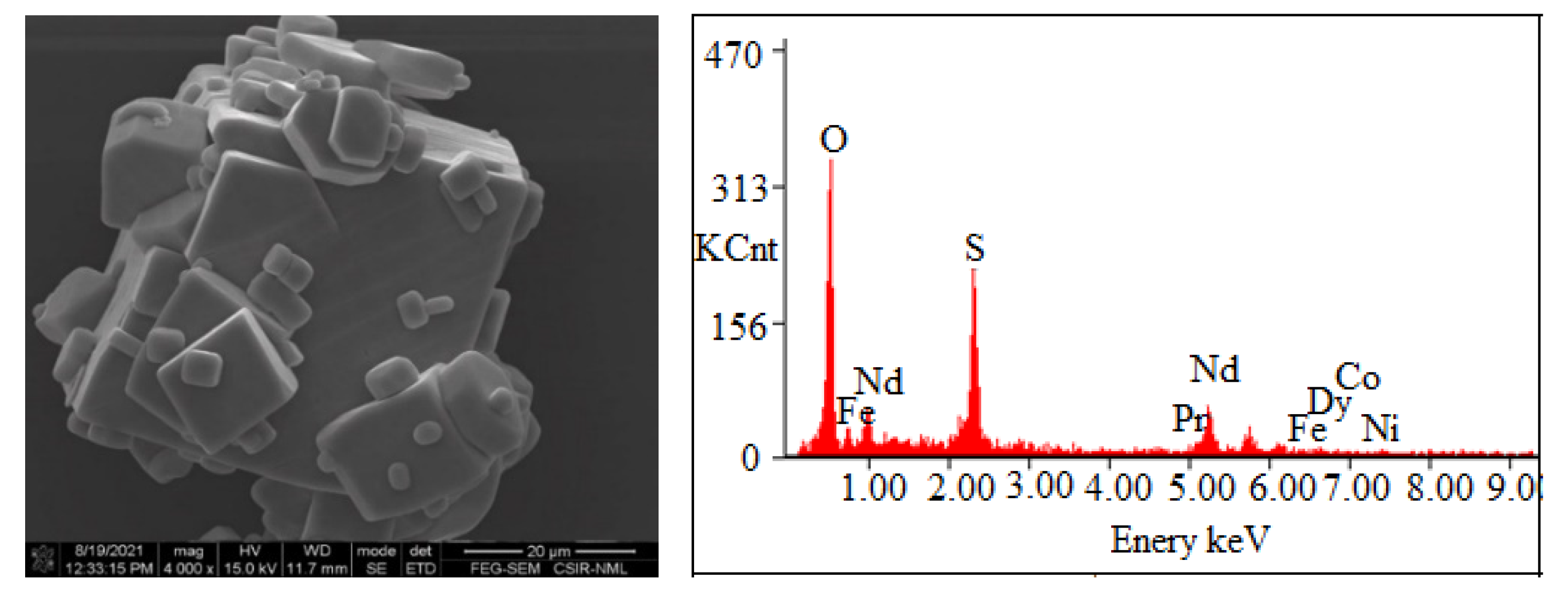
The precipitation of Nd, Pr, and Dy (Figure 9) was found to be increased with the increase in pH from 0.5 to 2.0 due to the low solubility of REMs at eq. pH 2. Eh-pH diagram drawn using the HSC chemistry version 6.0, which shows that Nd exists as Nd(OH)3 above pH 0.5 (Figure 10), while iron hydroxide formed at pH above 1.5 (Figure 11). Therefore, the pH of the solution was kept between 0.5 to 2 to avoid the co-precipitation of iron during the recovery of REMs hydroxides. The precipitated product was characterized by SEM-EDS (Figure 12), which shows that a strong peak of Nd appeared for the precipitated products. The composition of the precipitated product is shown in Table 2. It confirmed the formation of Nd product in the precipitated sample leaving iron in the solution.
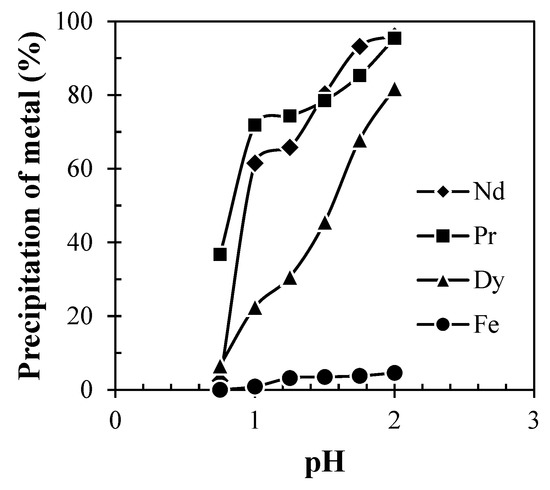
Figure 9.
Effect of pH on precipitation of REMs from the liquor of Nd-Fe-B magnets (Solution: Leach liquor containing 24.45 g/L Nd, 1.2 g/L Pr, 2.49 g/L Dy and 49.76 g/L Fe; Precipitant: Dil. Ammonium hydroxide; Time: 15 min).
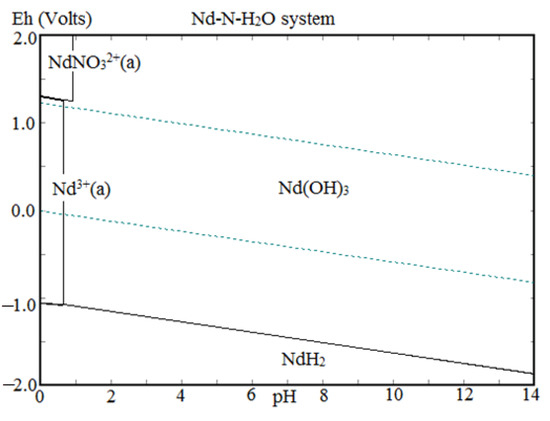
Figure 10.
Eh-pH diagram of neodymium.

Figure 11.
Eh-pH diagram of iron.
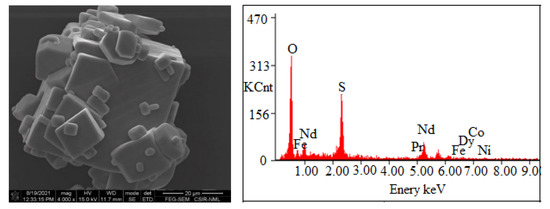
Figure 12.
SEM-EDS of precipitated salts of REMs.

Table 2.
Composition (EDS) of precipitated salts of REMs.
After the separation of REMs, iron (49.7 g) was completely removed by maintaining the pH above 3 using ammonium hydroxide as a precipitant at 60 °C as shown in Equations (6) and (7). It has been assumed that iron was precipitated as Fe3+ due to the high stability of ferric ion. The complete process flow sheet has been developed for the dissolution/extraction of REMs from waste Nd-Fe-B magnets of hard disks as shown in Figure 13. Further, comparative data for extraction of REMs from discarded Nd-Fe-B magnets are summarized in Table 3, which reflects that high temperature is required for pre-treatment (demagnetization, roasting) of magnets prior to hydrometallurgical extraction of REMs. However, demagnetization temperature is comparatively low in the present study, which makes the process feasible from an energy point of view.
Fe2(SO4)3 + 6NH4OH → 2Fe(OH)3 + 3(NH4)2SO4
2Fe(OH)3 → Fe2O3 + 3H2O
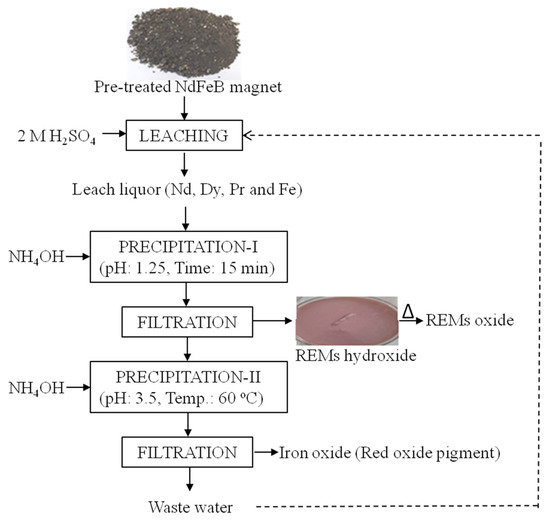
Figure 13.
Developed process flow-sheet to recover REMs from spent Nd-Fe-B magnets.

Table 3.
Literature review on the extraction of rare earth elements from discarded Nd-Fe-B magnets.
4. Conclusions
Based on the laboratory scale studies to recover REMs from discarded Nd-Fe-B magnet of hard disks, the following conclusions have been made and are discussed below.
- It was found that the magnetic strength of Nd-Fe-B hard disk magnet was lost by heat treatment at 300 °C in 2 h.
- The complete leaching of Nd, Pr, Dy, and Fe occurred from the demagnetized magnet using 2 M H2SO4 at 75 °C in 60 min and pulp density 100 g/L.
- It was found that 99.1% Nd, 94% Pr, and 99.9% Fe got extracted from leach liquor using 35% Cyanex 272 at O/A ratio 1/1 in 10 min. The selective extraction of REMs can only be achieved by using multistage solvent extraction. Thus, direct precipitation studies were carried out at different eq. pH for the selective extraction of REMs and Fe.
- Further, ~93.15% Nd, ~85% Pr, and ~68% Dy were precipitated from the leach liquor of discarded magnet at pH 1.75 at room temperature in 15 min. The precipitated hydroxide of REMs was converted to their oxides by heating at 120 °C for 2 h.
- Finally, 96.5% Fe was precipitated as ferric ion through the air sparging between eq. pH 3.5 to 4 at 60 °C.
Author Contributions
Methodology, N.S. and P.K.C.; Validation, P.K.C. and R.P.; Writing—original draft preparation, N.S. and P.K.C.; Writing—review and editing, M.K.J., K.Y. and I.P.; Supervision, M.K.J. and R.K.J.; Project administration, M.K.J. and I.P.; Funding acquisition, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Early-Career Scientists (JP20K15214). The work has been supported by CSIR-NML under Urban Ore Recycling Centre (FTC-0014/MLP-3116) and various Indo-Korean long term collaboration programs.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Authors are grateful to the Director CSIR-National Metallurgical Laboratory, Jamshedpur, India, for giving the permission to publish the paper and work done under CSIR-NML projects and international collaborations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumari, A.; Jha, M.K.; Pathak, D.D. An innovative environmental process for the treatment of scrap Nd-Fe-B magnets. J. Environ. Manag. 2020, 273, 111063. [Google Scholar] [CrossRef] [PubMed]
- Dhir, A.; Malodia, S.; Awan, U.; Sakashita, M.; Kaur, P. Extended valence theory perspective on consumers’ e-waste recycling intentions in Japan. J. Clean. Prod. 2021, 312, 127443. [Google Scholar] [CrossRef]
- Onal, M.A.R.; Aktan, E.; Borra, C.R.; Blanpain, B.; Gerven, T.V.; Guo, M. Recycling of NdFeB magnets using nitration, calcination and water leaching for REE recovery. Hydrometallurgy 2017, 167, 115–123. [Google Scholar] [CrossRef]
- Liu, F.; Porvali, A.; Wang, J.; Wang, H.; Peng, C.; Wilson, B.P.; Lundstrom, M. Recovery and separation of rare earths and boron from spent Nd-Fe-B Magnets. Miner. Eng. 2020, 145, 106097. [Google Scholar] [CrossRef]
- Loy, S.V.; Onal, M.A.R.; Binnemans, K.; Gerven, T.V. Recovery of valuable metals from NdFeB magnets by mechanochemically assisted ferric sulfate leaching. Hydrometallurgy 2020, 191, 105154. [Google Scholar]
- Reisdorfer, G.; Bertuol, D.; Tanabe, E.H. Recovery of neodymium from the magnets of hard disk drives using organic acids. Miner. Eng. 2019, 143, 105938. [Google Scholar] [CrossRef]
- Makarova, I.; Soboleva, E.; Osipenko, M.; Kurilo, I.; Laatikainen, M. Electrochemical leaching of rare-earth elements from spent NdFeB magnets. Hydrometallurgy 2020, 192, 105264. [Google Scholar] [CrossRef]
- Pavon, S.; Fortunya, A.; Collb, M.T.; Sastrec, A.M. Neodymium recovery from NdFeB magnet wastes using Primene 81R·Cyanex 572 IL by solvent extraction. J. Environ. Manag. 2018, 222, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Niam, A.C.; Wang, Y.; Chen, S.; Chang, G.; You, S. Simultaneous recovery of rare earth elements from waste permanent magnet (WPMs) leach liquor by solvent extraction and hollow fiber supported liquid membrane. Chem. Eng. Process. 2020, 148, 107831. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, Y.J.; Liao, C.H.; Popuri, R.S.; Tsai, S.L.; Hung, C.E. Selective leaching process for Neodymium recovery from scrap Nd-Fe-B magnet. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 5825–5833. [Google Scholar] [CrossRef]
- Rabatho, J.P.; Tongamp, W.; Takasaki, Y.; Haga, K.; Shibayama, A. Recovery of Nd and Dy from rare earth magnetic waste sludge by hydrometallurgical process. J. Mater. Cycles Waste Manag. 2013, 15, 171–178. [Google Scholar] [CrossRef]
- Yingnakorn, T.; Laokhen, P.; Sriklang, L.; Patcharawit, T.; Khumkoa, S. Study on Recovery of Rare Earth Elements from NdFeB Magnet Scrap by Using Selective Leaching. Mater. Sci. Forum 2020, 1009, 149–154. [Google Scholar] [CrossRef]
- Feng, L.Y.; Zhu, M.G.; Wei, L.; Dong, Z.; Feng, L.; Lang, C.; Wu, J.Y.; Yan, Q.; An, D. The Impact Induced Demagnetization Mechanism in NdFeB Permanent Magnets. Chin. Phys. Lett. 2013, 30, 097501. [Google Scholar]
- Hoogerstraete, T.V.; Blanpain, B.; Gerven, T.V.; Binnemans, K. From NdFeB magnets towards the rare-earth oxides: A recycling process consuming only oxalic acid. RSC Adv. 2014, 4, 64099–64111. [Google Scholar] [CrossRef] [Green Version]
- Riano, S.; Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015, 17, 2931–2942. [Google Scholar] [CrossRef]
- Onal, M.A.R.; Borra, C.R.; Guo, M.; Blanpain, B.; Van Gerven, T. Recycling of NdFeB magnets using sulfation, selective roasting, and water leaching. J. Sustain. Metall. 2015, 1, 199–215. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).