The Effects of Coexisting Copper, Iron, Cobalt, Nickel, and Zinc Ions on Gold Recovery by Enhanced Cementation via Galvanic Interactions between Zero-Valent Aluminum and Activated Carbon in Ammonium Thiosulfate Systems
Abstract
:1. Introduction
2. Materials and Methods
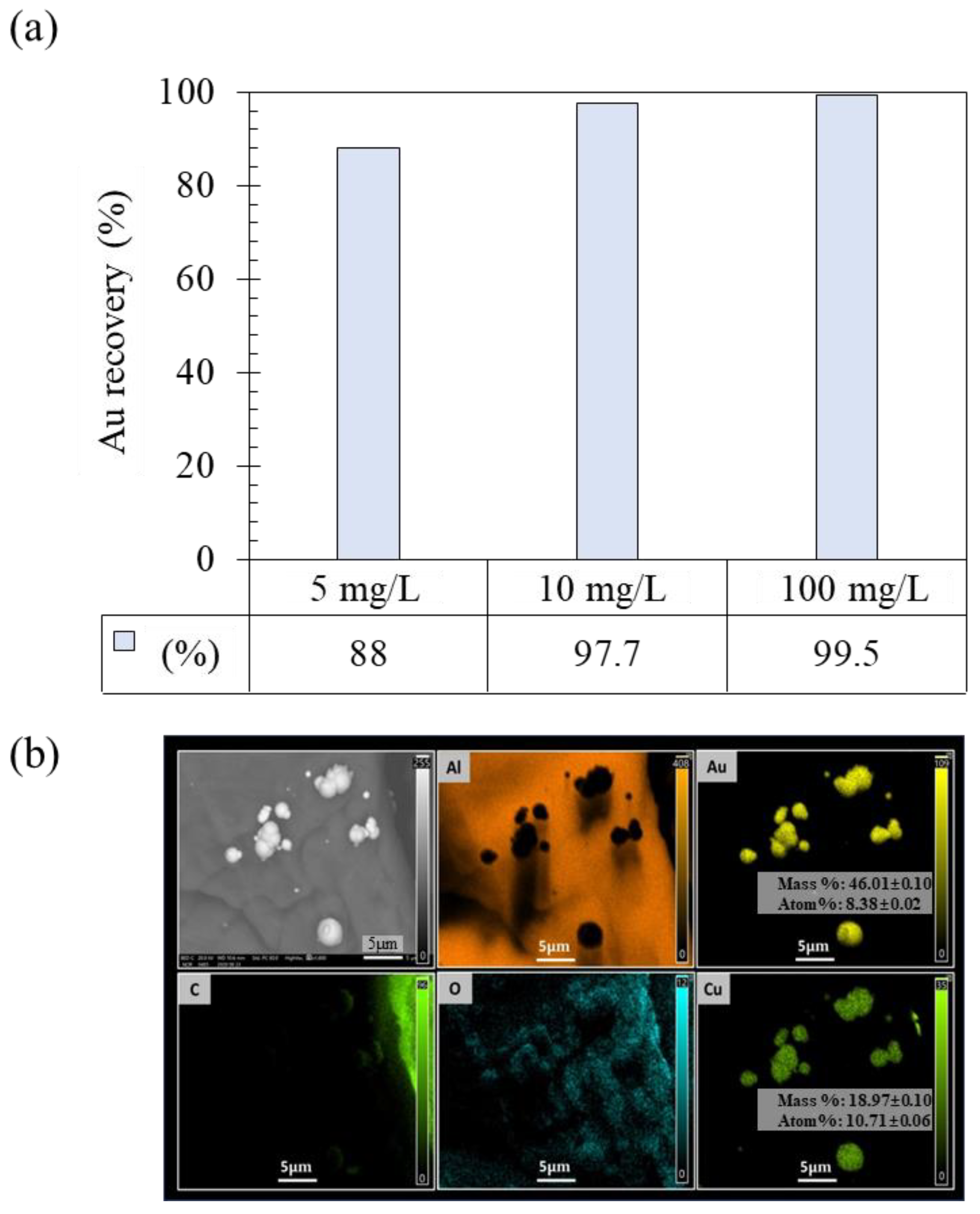
2.1. Recovery of Au Ions from Solutions with a Low Au Concentration
2.2. Recovery of Au Ions from Solutions Containing Coexisting Metal Ions
3. Results
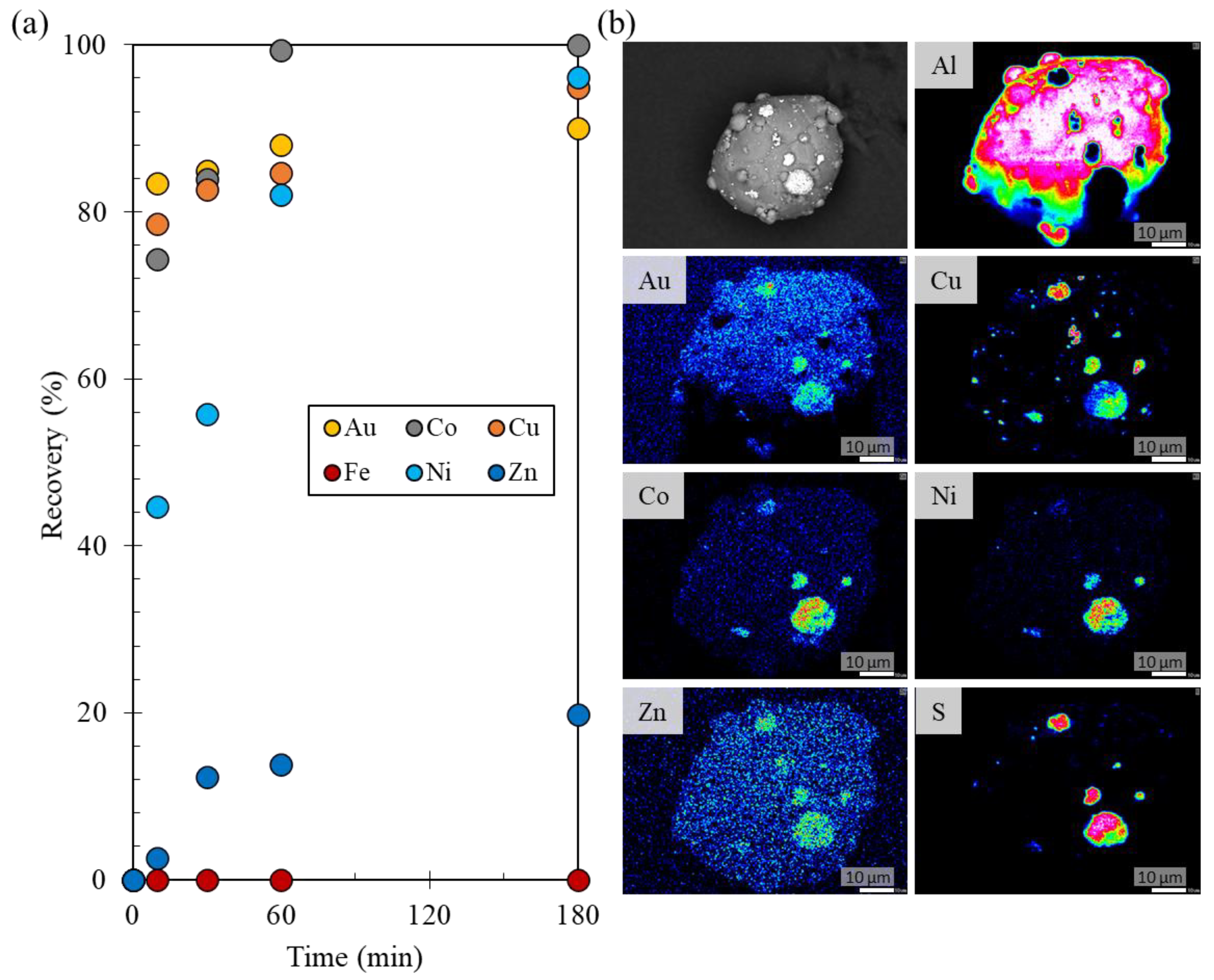
3.1. Recovery of Au Ions from Solutions Containing a Low Au Concentration
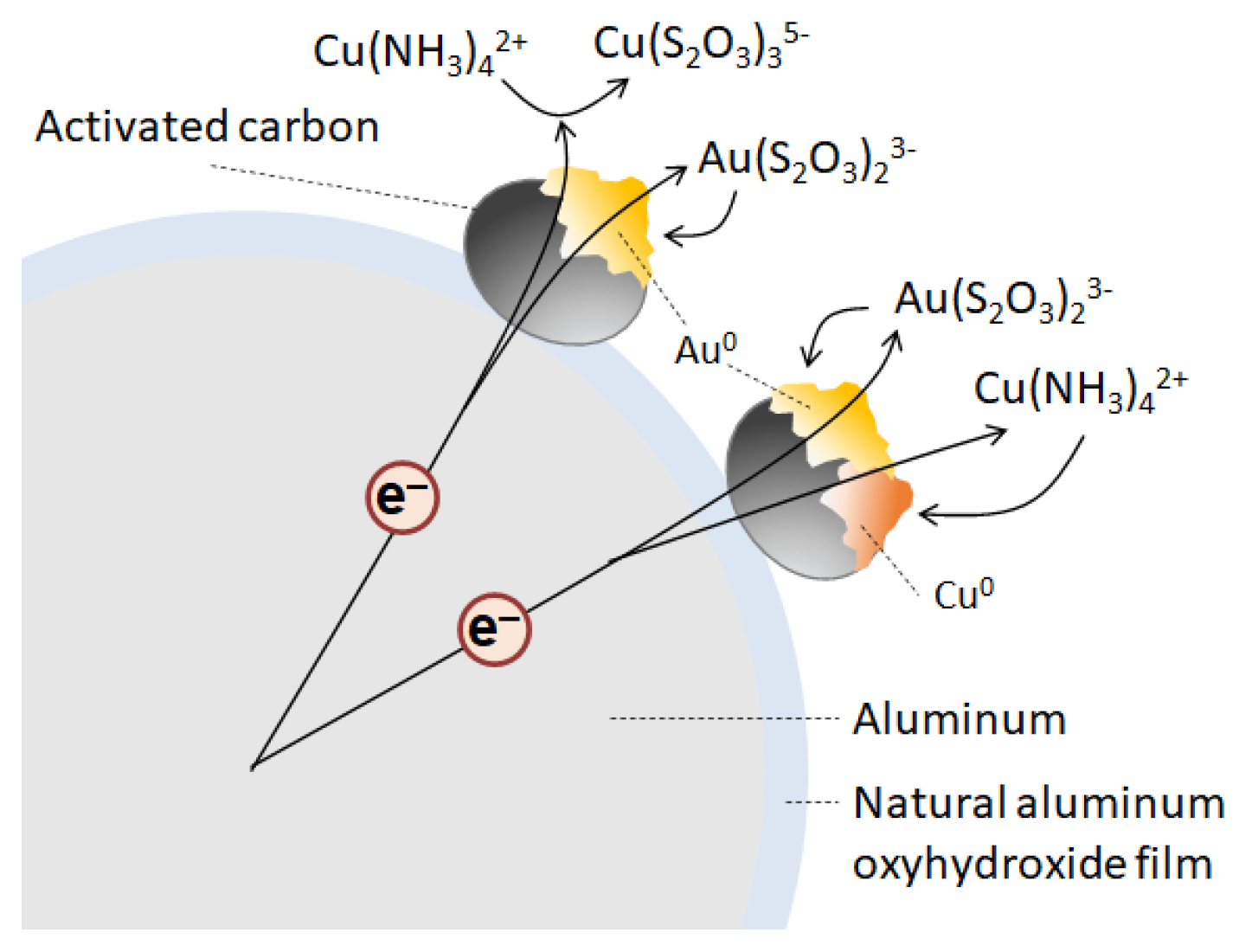
3.2. Recovery of Au Ions from Solutions Containing Coexisting Metal Ions
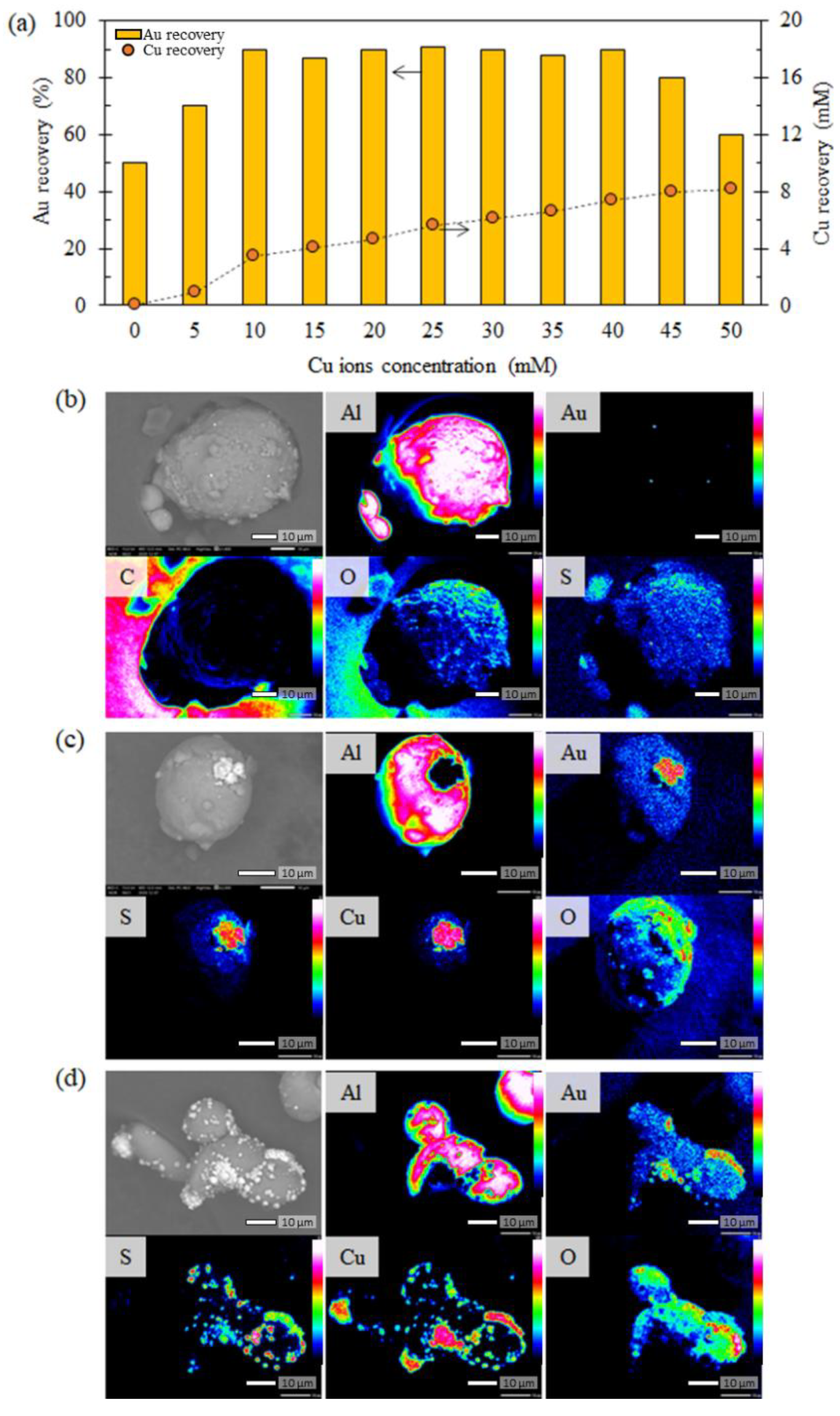
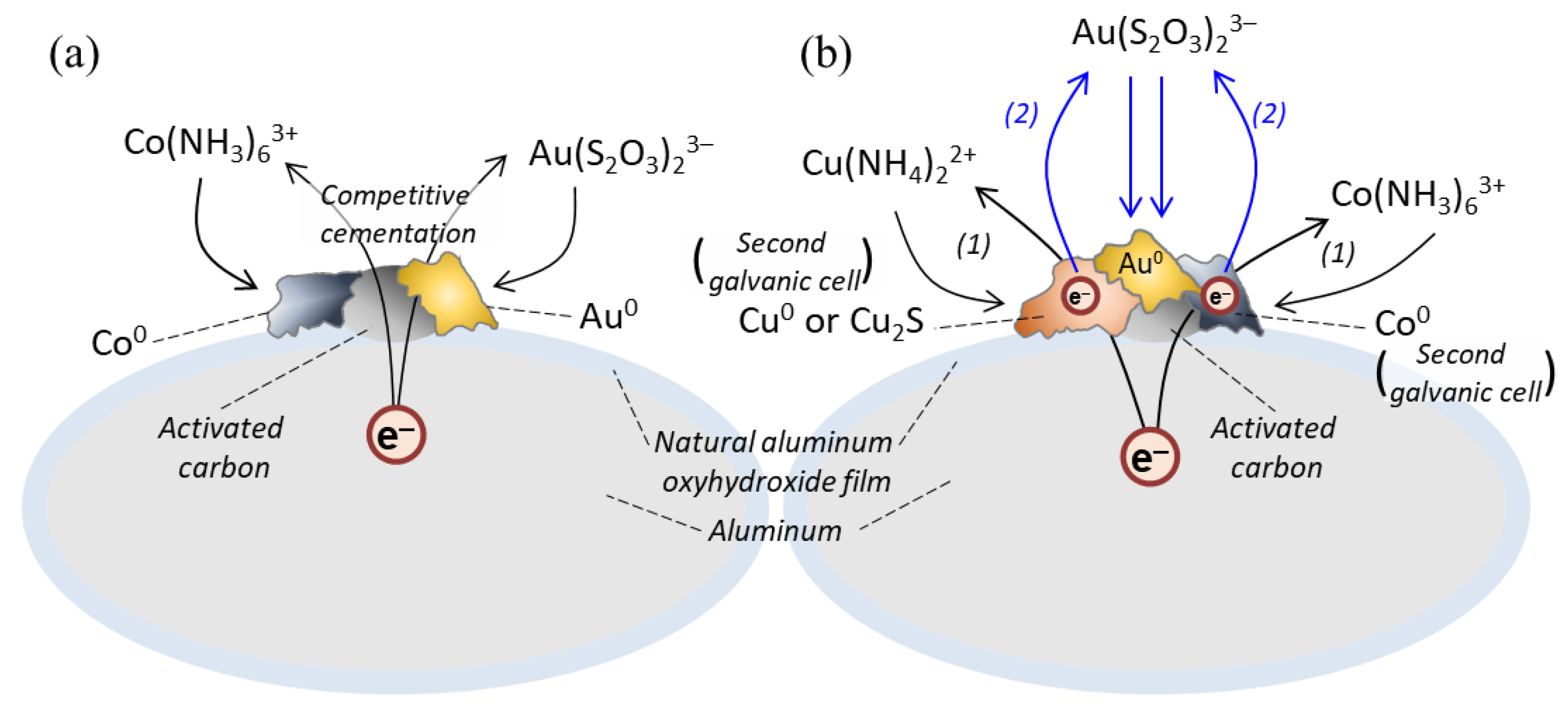
3.2.1. Recovery of Au Ions with Varying Cu Concentrations in the Solution
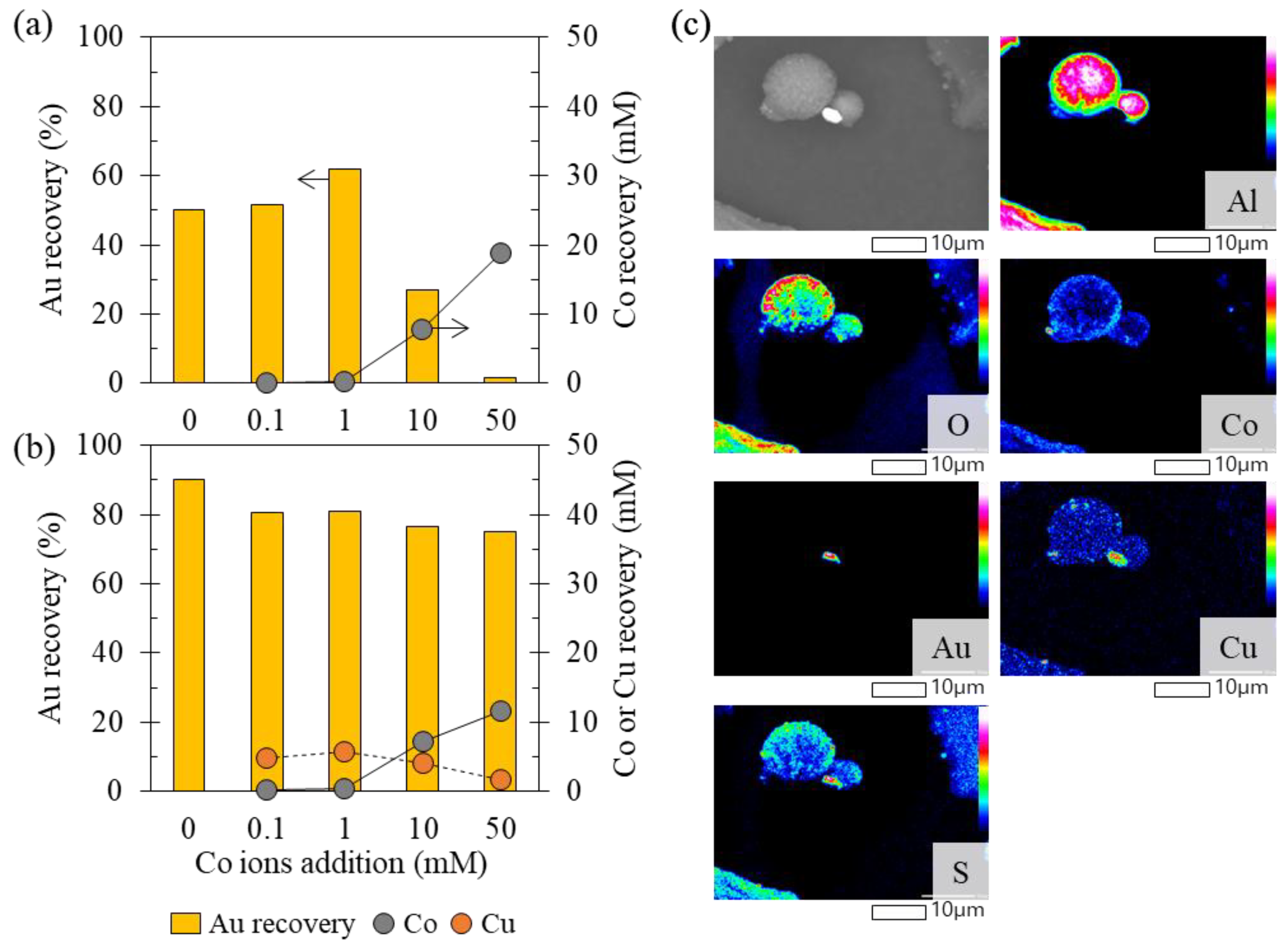
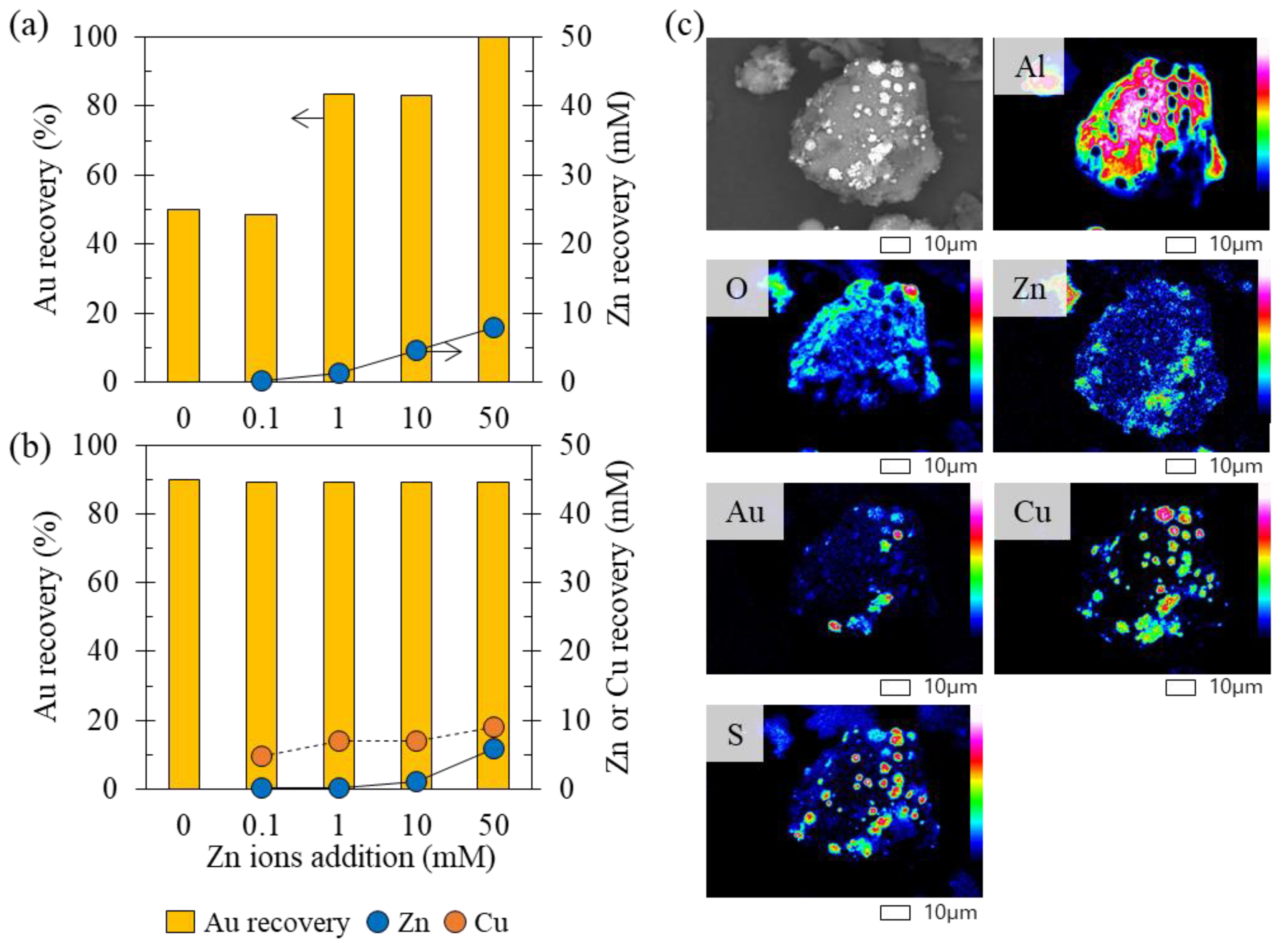
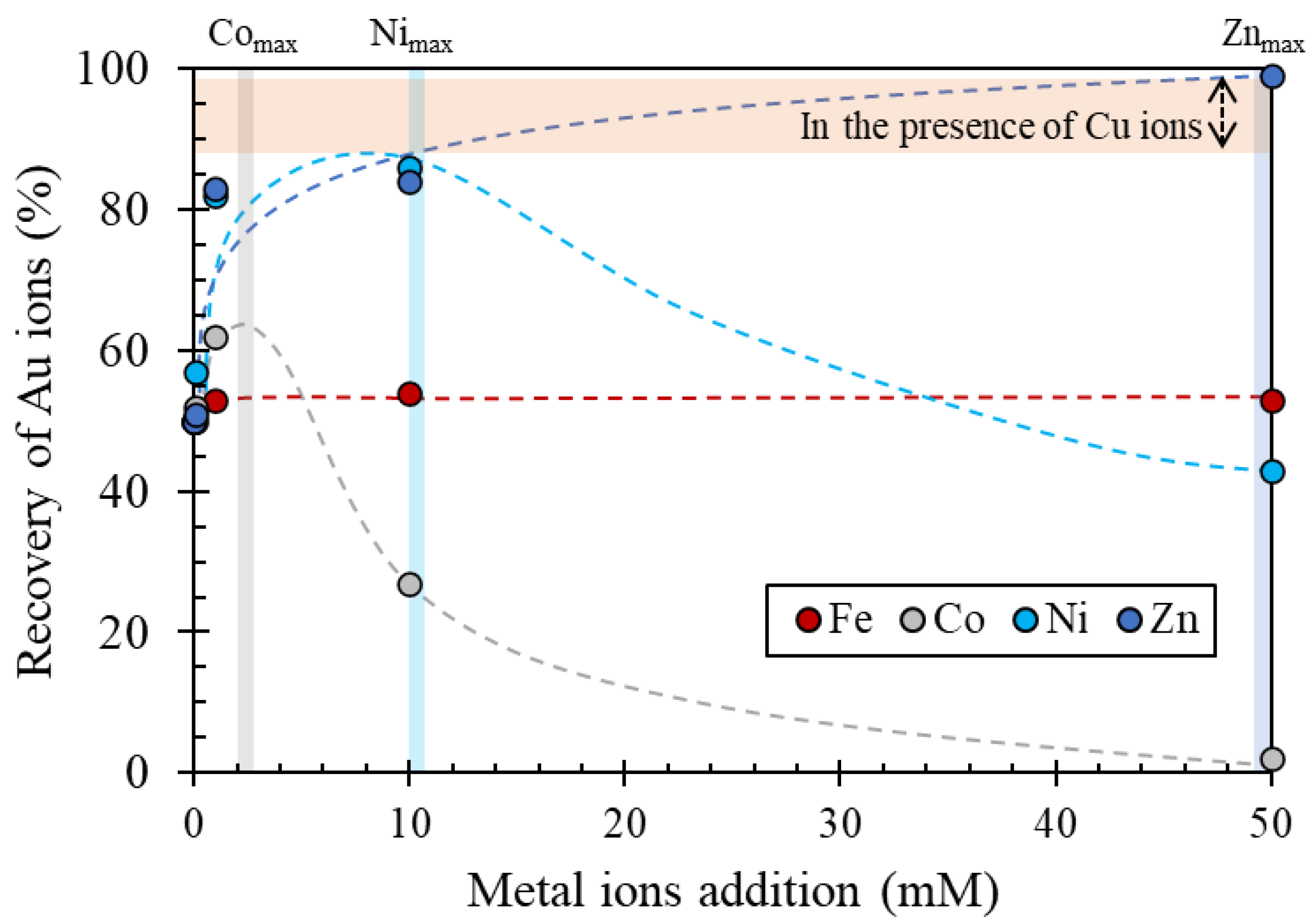
3.2.2. Recovery of Au Ions with Varying Fe, Co, Ni, and Zn Concentrations in the Solution
- Effect 1: Fe ions precipitated from solution phase, and they did not affect Au recovery.
- Effect 2: Zn ions and low concentrations of Co and Ni ions enhanced Au recovery.
- Effect 3: Higher concentrations of Co and Ni ions suppressed Au recovery.
- Effect 4: In the presence of Cu ions, the effects of other coexisting metal ions were hindered, i.e., Au recovery was almost constant (the orange section illustrated in Figure 8), regardless of the presence of other coexisting metal ions.
3.2.3. Recovery of Au Ions from Solutions Containing Various Coexisting Metal Ions
4. Conclusions
- Recovery of Au ions from the solution with low Au concentrations of about less than 10 mg/L;
- Investigation of the effects of various coexisting metal ions that could be present in ore for Au recovery.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aylmore, M.; Muir, D. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and e-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Opiso, E.M.; Aseneiro, J.P.J.; Banda, M.H.T.; Tabelin, C.B. Solid-phase partitioning of mercury in artisanal gold mine tailings from selected key areas in Mindanao, Philippines, and its implications for mercury detoxification. Waste Manag. Res. 2018, 36, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.; Tabelin, C.B.; Takahashi, H.; Park, I.; Ito, M.; Hiroyoshi, N. Interference of coexisting copper and aluminum on the ammonium thiosulfate leaching of gold from printed circuit boards of waste mobile phones. Waste Manag. 2018, 81, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Aazami, M.; Lapidus, G.; Azadeh, A. The effect of solution parameters on the thiosulfate leaching of Zarshouran refractory gold ore. Int. J. Miner. Process. 2014, 131, 43–50. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.-C.; Huynh, T.H.; Jeong, J.; Pandey, B. Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone. Hydrometallurgy 2014, 149, 118–126. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Park, I.; Nagata, Y.; Ito, M.; Hiroyoshi, N. Ammonium thiosulfate extraction of gold from printed circuit boards (PCBs) of end-of-life mobile phones and its recovery from pregnant leach solution by cementation. Hydrometallurgy 2020, 191, 105214. [Google Scholar] [CrossRef]
- Molleman, E.; Dreisinger, D. The treatment of copper–gold ores by ammonium thiosulfate leaching. Hydrometallurgy 2002, 66, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Ficeriová, J.; Baláz, P.; Villachica, C.L. Thiosulfate leaching of silver, gold and bismuth from a complex sulfide concentrates. Hydrometallurgy 2005, 77, 35–39. [Google Scholar] [CrossRef]
- Fleming, C.A.; Mezei, A.; Bourricaudy, E.; Canizares, M.; Ashbury, M. Factors influencing the rate of gold cyanide leaching and adsorption on activated carbon, and their impact on the design of CIL and CIP circuits. Miner. Eng. 2011, 24, 484–494. [Google Scholar] [CrossRef]
- Navarro, P.; Vargas, C.; Alonso, M.; Alguacil, F. The adsorption of gold on activated carbon from thiosulfate-ammoniacal solutions. Gold Bull. 2006, 39, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Arima, H.; Fujita, T.; Yen, W.-T. Gold Cementation from Ammonium Thiosulfate Solution by Zinc, Copper and Aluminium Powders. Mater. Trans. 2002, 43, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Hiskey, J.B.; Lee, J. Kinetics of gold cementation on copper in ammoniacal thiosulfate solutions. Hydrometallurgy 2003, 69, 45–56. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, Y.; Li, Q. Recovery of Gold from Pregnant Thiosulfate Solutions by the Resin Adsorption Technique. Metals 2017, 7, 555. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.; Tabelin, C.B.; Takahashi, H.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced cementation of gold via galvanic interactions using activated carbon and zero-valent aluminum: A novel approach to recover gold ions from ammonium thiosulfate medium. Hydrometallurgy 2020, 191, 105165. [Google Scholar] [CrossRef]
- Vaughan, J.P. The process mineralogy of gold: The classification of ore types. JOM 2004, 56, 46–48. [Google Scholar] [CrossRef]
- Cho, K.; Kim, H.; Myung, E.; Purev, O.; Choi, N.; Park, C. Recovery of Gold from the Refractory Gold Concentrate Using Microwave Assisted Leaching. Metal 2020, 10, 571. [Google Scholar] [CrossRef]
- Qin, H.; Guo, X.; Tian, Q.; Yu, D.; Zhang, L. Recovery of gold from sulfide refractory gold ore: Oxidation roasting pretreatment and gold extraction. Miner. Eng. 2021, 164, 106822. [Google Scholar] [CrossRef]
- Islam, K.; Vilaysouk, X.; Murakami, S. Integrating remote sensing and life cycle assessment to quantify the environmental impacts of copper-silver-gold mining: A case study from Laos. Resour. Conserv. Recycl. 2020, 154, 104630. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W.; Xu, B.; Li, Q.; Jiang, T. Study on oxygen pressure thiosulfate leaching of gold without the catalysis of copper and ammonia. Hydrometallurgy 2019, 187, 71–80. [Google Scholar] [CrossRef]
- Gorji, M.; Hosseini, M.R.; Ahmadi, A. Comparison and optimization of the bio-cyanidation potentials of B. megaterium and P. aeruginosa for extracting gold from an oxidized copper-gold ore in the presence of residual glycine. Hydrometallurgy 2020, 191, 105218. [Google Scholar] [CrossRef]
- Agorhom, E.A.; Owusu, C. The Effects of Pulp Rheology on Gravity Gold Recovery in Free Milling Gold Ore of the Tarkwaian Systems of Ghana. Miner. Process. Extr. Met. Rev. 2020, 1–6. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Silwamba, M.; Paglinawan, F.C.; Mondejar, A.J.S.; Duc, H.G.; Resabal, V.J.; Opiso, E.M.; Igarashi, T.; Tomiyama, S.; Ito, M.; et al. Solid-phase partitioning and release-retention mechanisms of copper, lead, zinc and arsenic in soils impacted by artisanal and small-scale gold mining (ASGM) activities. Chemosphere 2020, 260, 127574. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. A Review of Recent Advances in Depression Techniques for Flotation Separation of Cu–Mo Sulfides in Porphyry Copper Deposits. Metal 2020, 10, 1269. [Google Scholar] [CrossRef]
- Park, I.; Higuchi, K.; Tabelin, C.B.; Jeon, S.; Ito, M.; Hiroyoshi, N. Suppression of arsenopyrite oxidation by microencapsulation using ferric-catecholate complexes and phosphate. Chemosphere 2021, 269, 129413. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Venkatesh, A. Constraints of mineralogical characterization of gold ore: Implication for genesis, controls and evolution of gold from Kundarkocha gold deposit, eastern India. J. Asian Earth Sci. 2015, 97, 136–149. [Google Scholar] [CrossRef]
- Wu, J.; Ahn, J.; Lee, J. Gold deportment and leaching study from a pressure oxidation residue of chalcopyrite concentrate. Hydrometallurgy 2021, 201, 105583. [Google Scholar] [CrossRef]
- Adams, M.; Lawrence, R.; Bratty, M. Biogenic sulphide for cyanide recycle and copper recovery in gold–copper ore processing. Miner. Eng. 2008, 21, 509–517. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.; Chen, L. Application of fluoride to enhance aluminum cementation of gold from acidic thiocyanate solution. Hydrometallurgy 2007, 89, 196–206. [Google Scholar] [CrossRef]
- Nguyen, H.; Tran, T.; Wong, P. A kinetic study of the cementation of gold from cyanide solutions onto copper. Hydrometallurgy 1997, 46, 55–69. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; Nyambe, I.; et al. Detoxification of lead-bearing zinc plant leach residues from Kabwe, Zambia by coupled extraction-cementation method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- Arima, H.; Fujita, T.; Yen, W.-T. Using Nickel as a Catalyst in Ammonium Thiosulfate Leaching for Gold Extraction. Mater. Trans. 2004, 45, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jiang, T.; Xu, B.; Zhang, Y.; Li, Q.; Yang, Y.; He, Y. Thiosulphate leaching of gold in Cu-NH3-S2O32−—H2O system: An updated thermodynamic analysis using predominance area and species distribution diagrams. Miner. Eng. 2020, 151, 106336. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; He, Y. Thermodynamic analysis of ammoniacal thiosulfate leaching of gold catalyzed by Co(III)/Co(II) using Eh-pH and speciation diagrams. Hydrometallurgy 2018, 178, 240–249. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Li, Q.; Yang, Y.; Liu, X.; Jiang, T. Kinetic studies of gold leaching from a gold concentrate calcine by thiosulfate with cobalt-ammonia catalysis and gold recovery by resin adsorption from its pregnant solution. Sep. Purif. Technol. 2019, 213, 368–377. [Google Scholar] [CrossRef]
- BC Campus, Appendix: Standard Reduction Potentials by Value. Available online: https://opentextbc.ca/introductorychemistry/back-matter/appendix-standard-reduction-potentials-by-value-2/ (accessed on 1 April 2021).
- Senanayake, G.; Senaputra, A.; Nicol, M.J. Effect of thiosulfate, sulfide, copper(II), cobalt(II)/(III) and iron oxides on the ammoniacal carbonate leaching of nickel and ferronickel in the caron process. Hydrometallurgy 2010, 105, 60–68. [Google Scholar] [CrossRef]
- Djokic, S.S. Electroless Depositiore on of Metals and Alloys, Modern Aspects of Electrochemistry; Brian, E.C., Conway, E., Ralph, E.W., White, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 51–133. [Google Scholar]
- Senanayake, G.; Zhang, X.M. Gold leaching by copper (II) in ammoniacal thiosulfate solutions in the presence of additives. Part II: Effects of residual Cu(II), pH and redox potentials on reactivity of colloidal gold. Hydrometallurgy 2012, 115-116, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Compare Metals. Available online: https://metals.comparenature.com/en/copper-vs-cobalt/comparison-6-30-0 (accessed on 15 April 2021).
- New Medical Device Shielding Requirements and Die Casting (IEC 60601-0-2: 2014—4th Edition). Available online: https://www.abdiecasting.com/new-medical-device-shielding-requirements-and-die-casting/ (accessed on 26 May 2021).
- Örgül, S.; Atalay, Ü. Reaction chemistry of gold leaching in thiourea solution for a Turkish gold ore. Hydrometallurgy 2002, 67, 71–77. [Google Scholar] [CrossRef]
- Soltani, F.; Darabi, H.; Badri, R.; Zamankhan, P. Improved recovery of a low-grade refractory gold ore using flotation-preoxidation-cyanidation methods. Int. J. Min. Sci. Technol. 2014, 24, 537–542. [Google Scholar]
- Melashvili, M.; Fleming, C.; Dymov, I.; Matthews, D.; Dreisinger, D. Dissolution of gold during pyrite oxidation reaction. Miner. Eng. 2016, 87, 2–9. [Google Scholar] [CrossRef]
- Murthy, D.S.R.; Kumar, V.; Rao, K.V. Extraction of gold from an Indian low-grade refractor gold ore through physical beneficiation and thiourea leaching. Hydrometallurgy 2003, 68, 125–130. [Google Scholar] [CrossRef]


| Metals | E0/V |
|---|---|
| Au(S2O3)23−/Au | 0.27 |
| Cu(NH3)42+/Cu (S2O3)23− | 0.22 |
| Co(NH3)63+/Co(NH3)x2+ (x is mainly 5 under the current conditions) | 0.21 |
| Co(NH3)63+/Co | 0.1 |
| Cu(NH3)42+/Cu | –0.05 |
| Cu(NH3)42+/Cu2S | –0.2 |
| Ni(NH3)62+/Ni | –0.49 |
| Zn(NH3)42+/Zn | –1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Bright, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. The Effects of Coexisting Copper, Iron, Cobalt, Nickel, and Zinc Ions on Gold Recovery by Enhanced Cementation via Galvanic Interactions between Zero-Valent Aluminum and Activated Carbon in Ammonium Thiosulfate Systems. Metals 2021, 11, 1352. https://doi.org/10.3390/met11091352
Jeon S, Bright S, Park I, Tabelin CB, Ito M, Hiroyoshi N. The Effects of Coexisting Copper, Iron, Cobalt, Nickel, and Zinc Ions on Gold Recovery by Enhanced Cementation via Galvanic Interactions between Zero-Valent Aluminum and Activated Carbon in Ammonium Thiosulfate Systems. Metals. 2021; 11(9):1352. https://doi.org/10.3390/met11091352
Chicago/Turabian StyleJeon, Sanghee, Sharrydon Bright, Ilhwan Park, Carlito Baltazar Tabelin, Mayumi Ito, and Naoki Hiroyoshi. 2021. "The Effects of Coexisting Copper, Iron, Cobalt, Nickel, and Zinc Ions on Gold Recovery by Enhanced Cementation via Galvanic Interactions between Zero-Valent Aluminum and Activated Carbon in Ammonium Thiosulfate Systems" Metals 11, no. 9: 1352. https://doi.org/10.3390/met11091352
APA StyleJeon, S., Bright, S., Park, I., Tabelin, C. B., Ito, M., & Hiroyoshi, N. (2021). The Effects of Coexisting Copper, Iron, Cobalt, Nickel, and Zinc Ions on Gold Recovery by Enhanced Cementation via Galvanic Interactions between Zero-Valent Aluminum and Activated Carbon in Ammonium Thiosulfate Systems. Metals, 11(9), 1352. https://doi.org/10.3390/met11091352









