Solid-Solid Phase Transformations and Their Kinetics in Ti-Al-Nb Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
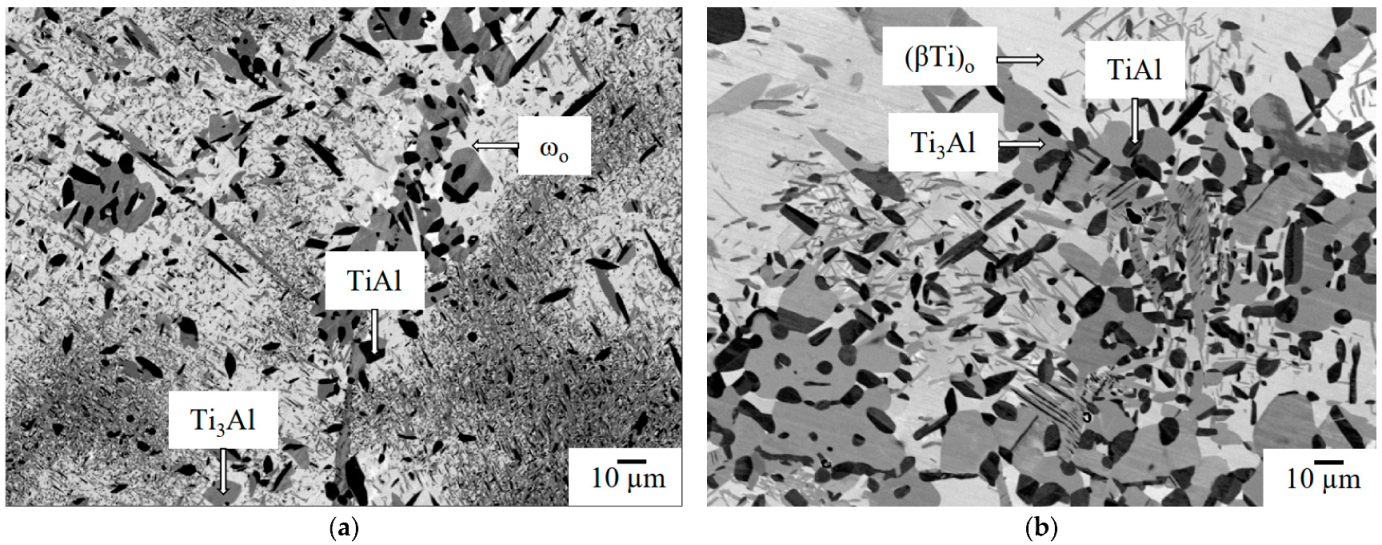
3.1. Alloy Characterisation
3.2. Thermal Analysis of Phase Transformations
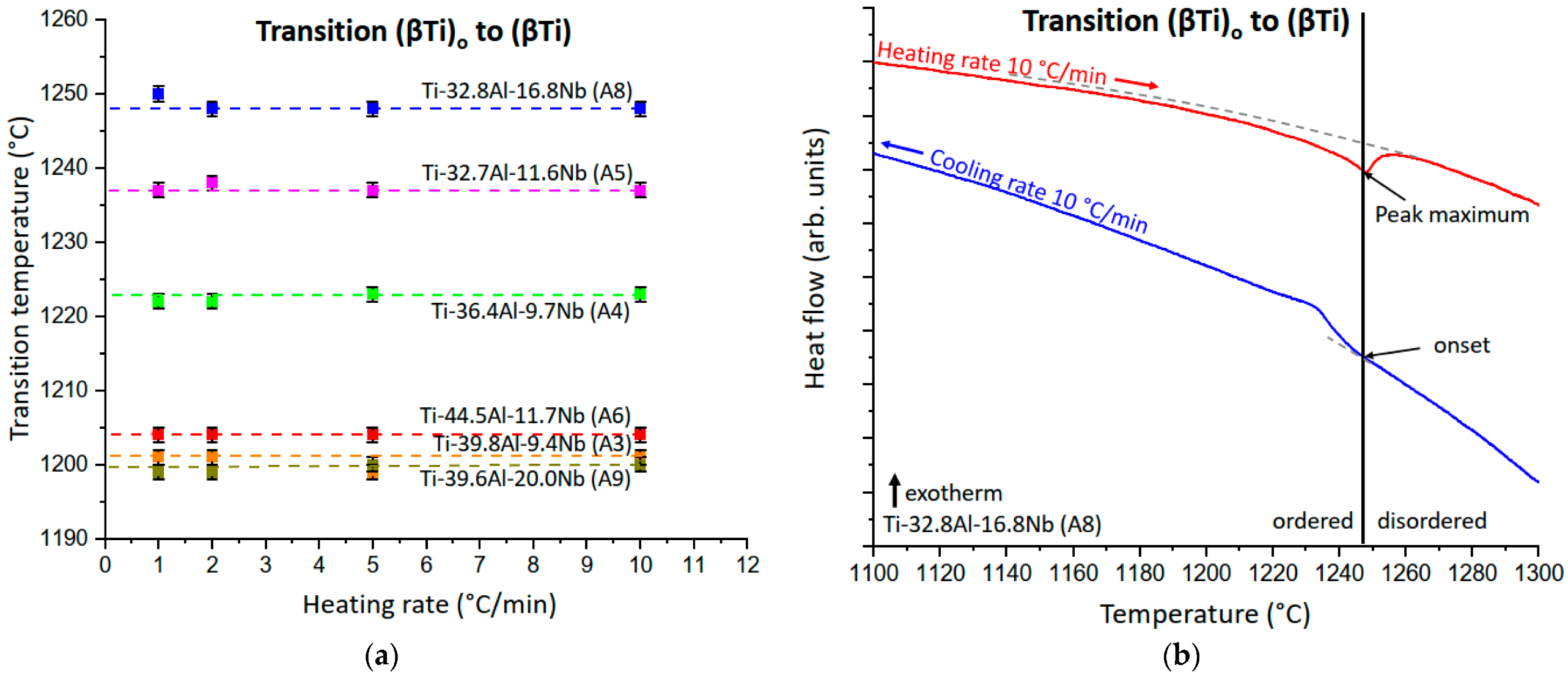
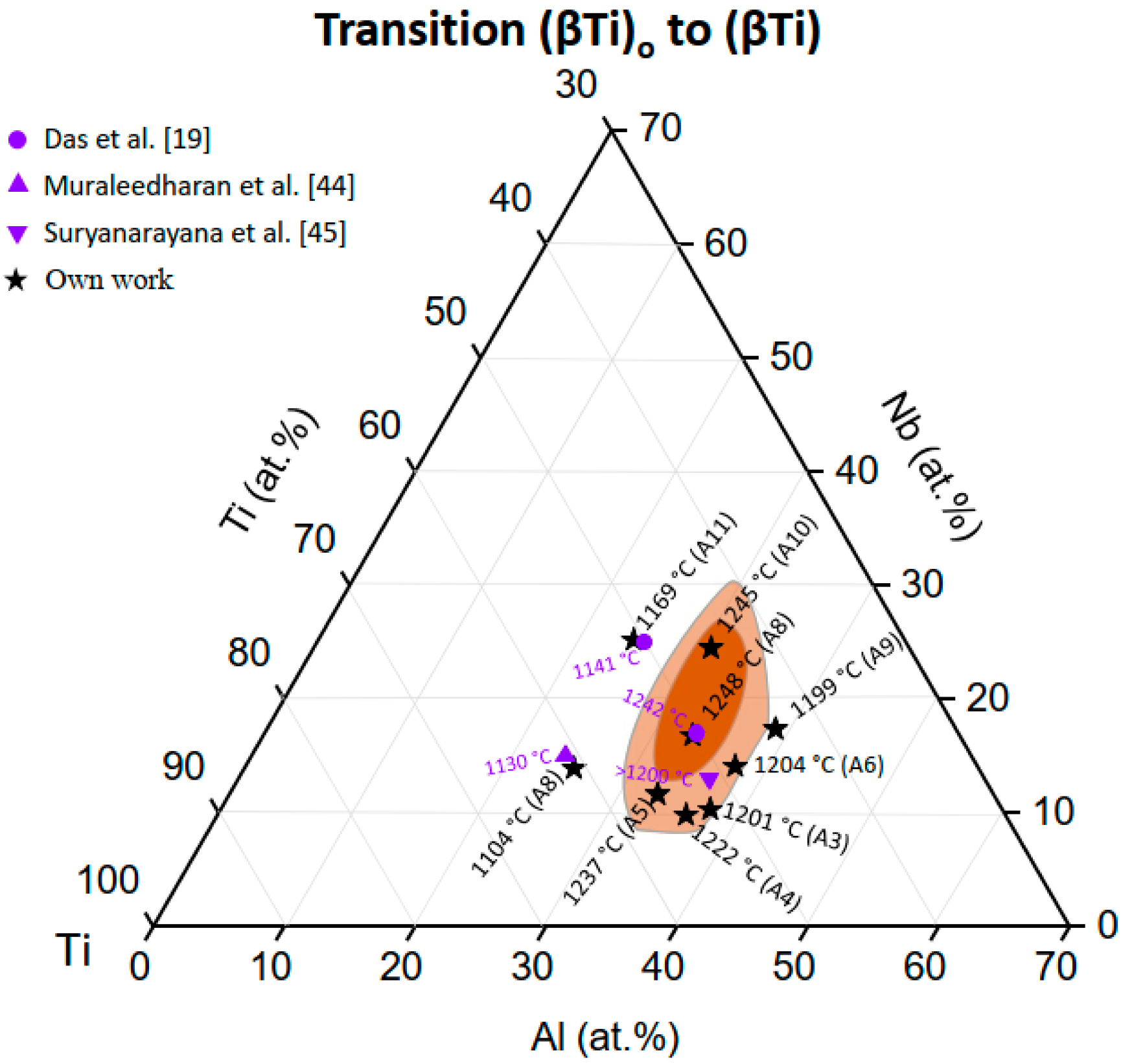
3.2.1. The (βTi)o to (βTi) Order/Disorder Transition
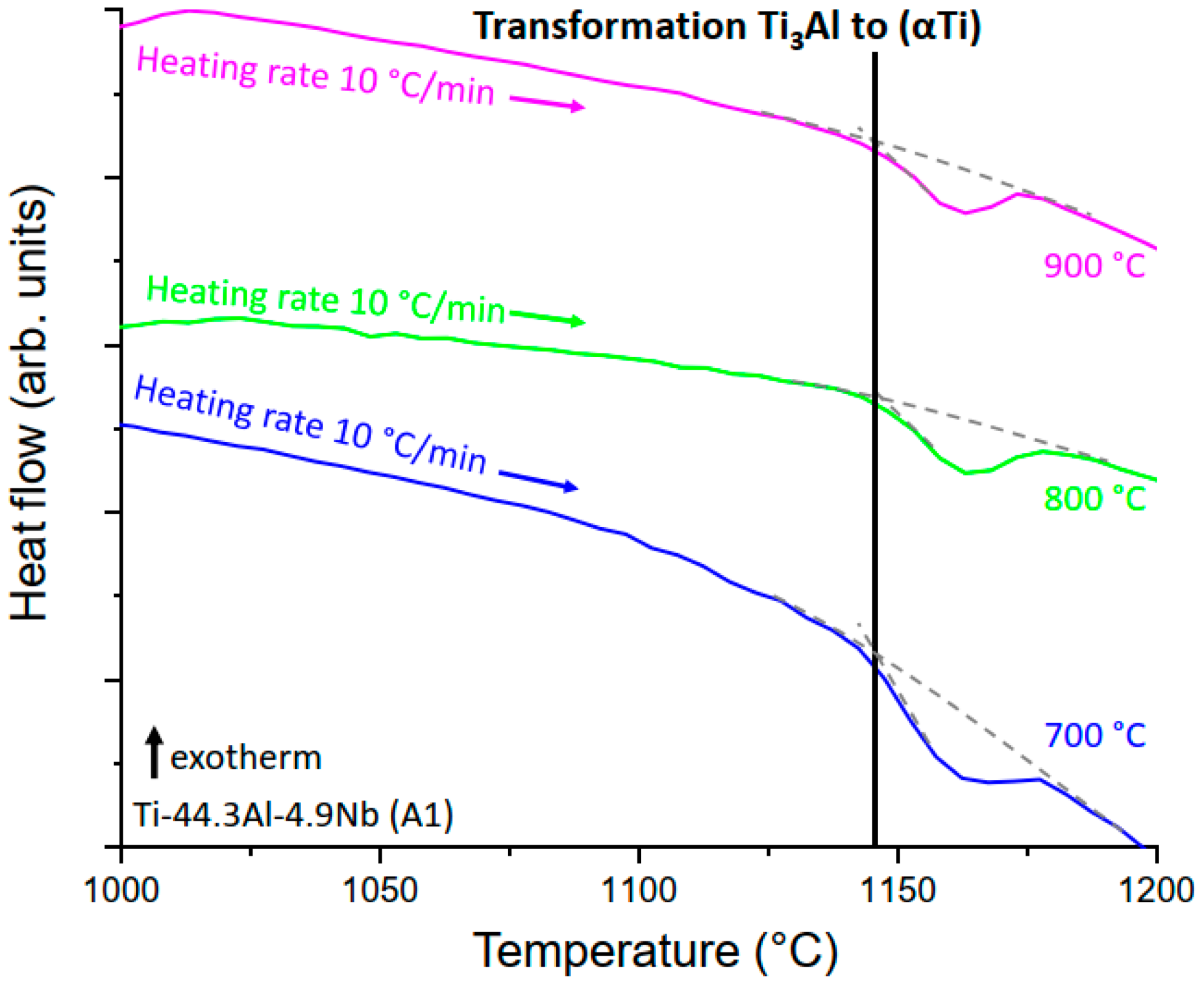
3.2.2. The Ti3Al to (αTi) Transformation
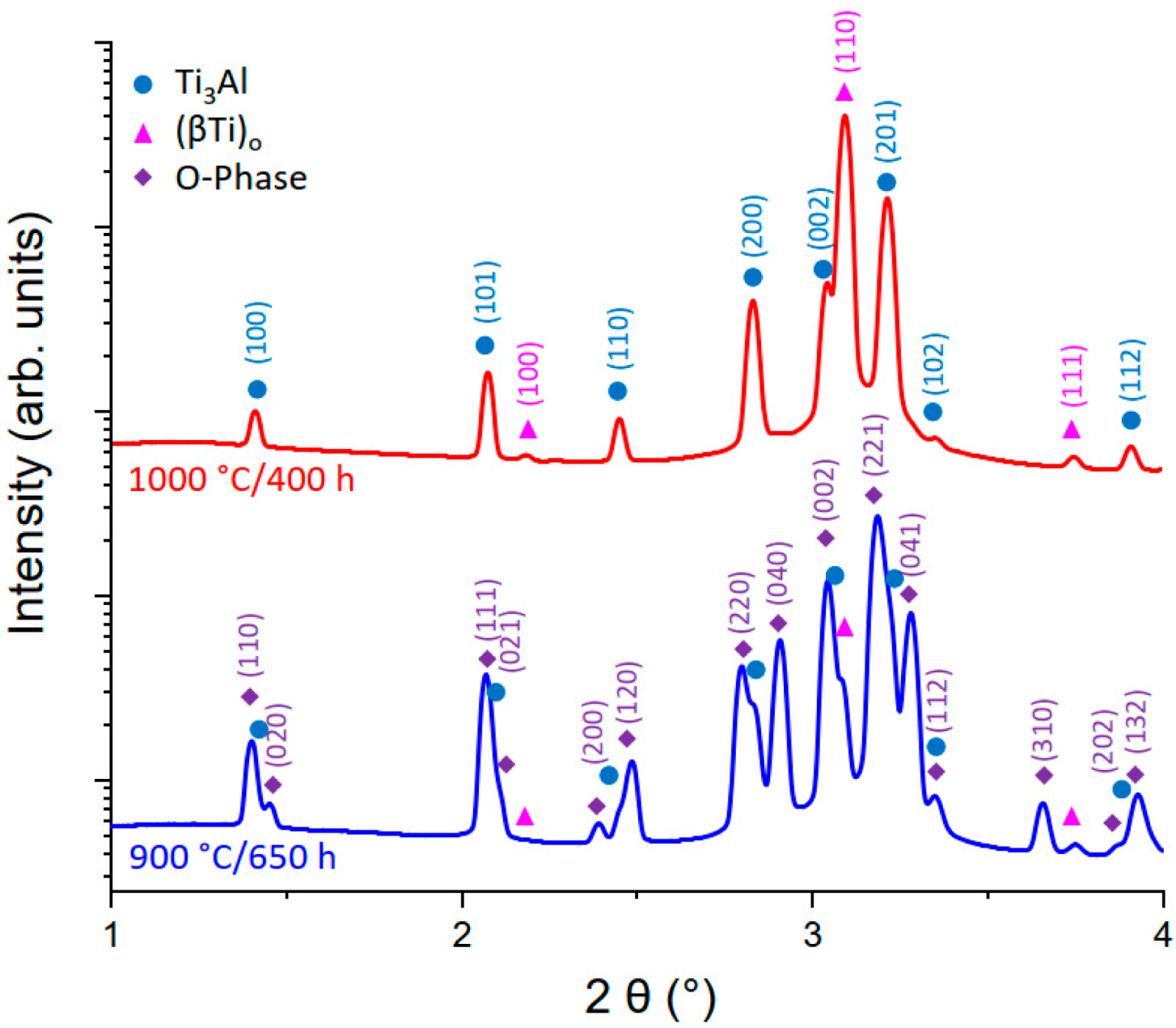
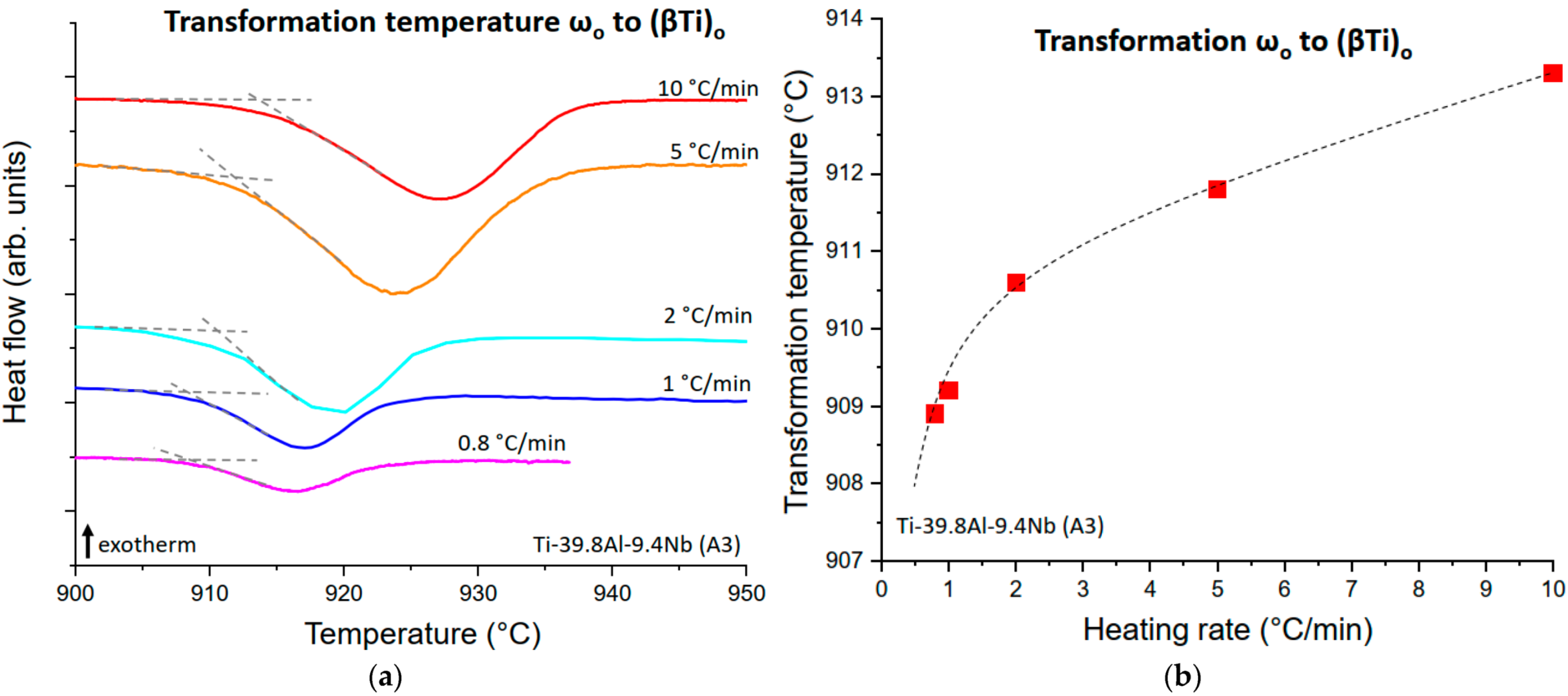
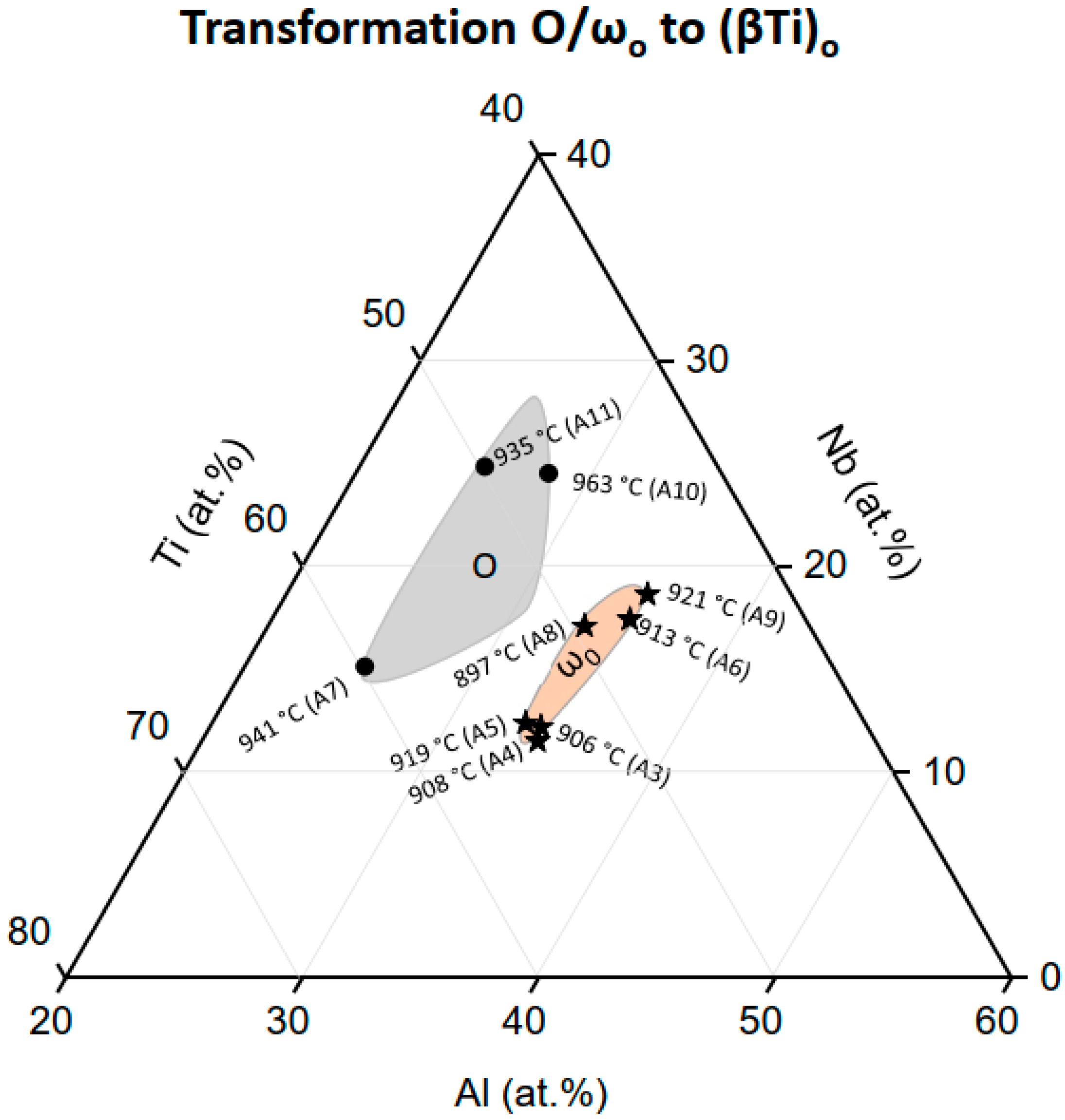
3.2.3. The ωo and O Phase Decomposition Reactions
4. Discussion
4.1. The Heating Rate Independent Transition (βTi)o to (βTi)
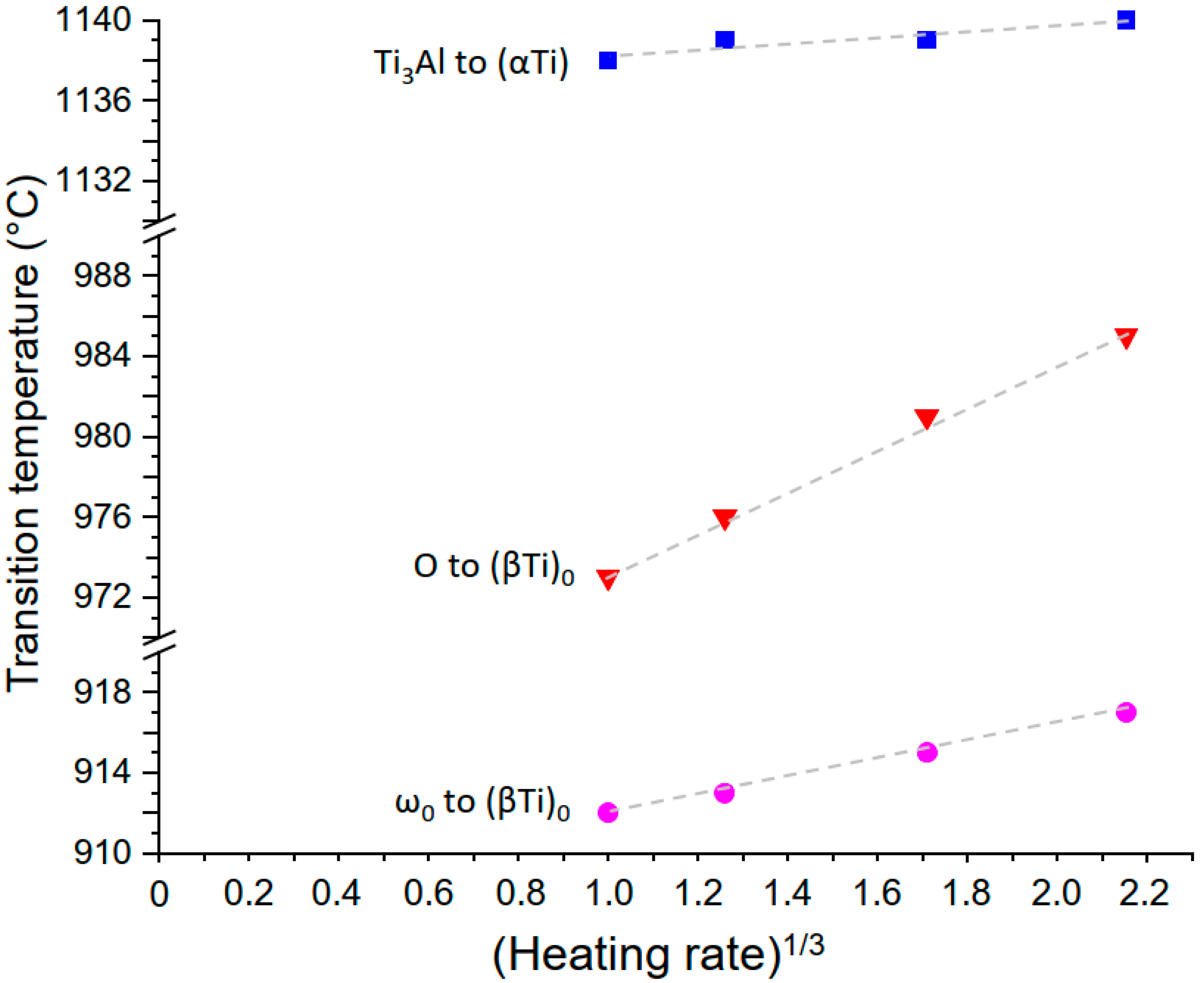
4.2. Heating Rate Dependent Transformations
4.2.1. The Ti3Al to (αTi) Transformation
4.2.2. The ωo to (βTi)o Transformation
4.2.3. The O Phase to (βTi)o Transformation and Comparison of the Heating Rate Dependent Transformations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Clemens, H.; Mayer, S. Design, Processing, Microstructure, Properties, and Applications of Advanced Intermetallic TiAl Alloys. Adv. Eng. Mater. 2012, 15, 191–215. [Google Scholar] [CrossRef]
- Mayer, S.; Erdely, P.; Fischer, F.D.; Holec, D.; Kastenhuber, M.; Klein, T.; Clemens, H. Intermetallic β-Solidifying γ-TiAl Based Alloys—From Fundamental Research to Application. Adv. Eng. Mater. 2017, 19, 1600735. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Inui, H.; Ito, K. High-temperature structural intermetallics. Acta Mater. 2000, 48, 307–322. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, M.; Ye, T.; Liang, Y.; Ren, Y.; Dong, C.; Lin, J. Microstructure stability and micro-mechanical behavior of as-cast gamma-TiAl alloy during high-temperature low cycle fatigue. Acta Mater. 2018, 145, 504–515. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, N.; Liang, H.; Liu, Y. Phase transformation and microstructure control of Ti2AlNb-based alloys: A review. J. Mater. Sci. Technol. 2020, 80, 203–216. [Google Scholar] [CrossRef]
- Li, W.; Yin, Y.; Xu, Q.; Zhou, J.; Nan, H.; Ji, X.; Shen, X.; Feng, X.; Yu, W.; Tu, Z.; et al. Tensile behavior of γ/α2 interface system in lamellar TiAl alloy via molecular dynamics. Comput. Mater. Sci. 2018, 159, 397–402. [Google Scholar] [CrossRef]
- Gamsjäger, E.; Liu, Y.; Rester, M.; Puschnig, P.; Draxl, C.; Clemens, H.; Dehm, G.; Fischer, F. Diffusive and massive phase transformations in Ti–Al–Nb alloys—Modelling and experiments. Intermetallics 2013, 38, 126–138. [Google Scholar] [CrossRef]
- Kenel, C.; Leinenbach, C. Influence of Nb and Mo on microstructure formation of rapidly solidified ternary Ti–Al-(Nb, Mo) alloys. Intermetallics 2016, 69, 82–89. [Google Scholar] [CrossRef]
- Rackel, M.W.; Stark, A.; Gabrisch, H.; Schell, N.; Schreyer, A.; Pyczak, F. Orthorhombic phase formation in a Nb-rich γ-TiAl based alloy—An in situ synchrotron radiation investigation. Acta Mater. 2016, 121, 343–351. [Google Scholar] [CrossRef]
- Bolz, S.; Oehring, M.; Lindemann, J.; Pyczak, F.; Paul, J.; Stark, A.; Lippmann, T.; Schrüfer, S.; Roth-Fagaraseanu, D.; Schreyer, A.; et al. Microstructure and mechanical properties of a forged β-solidifying γ TiAl alloy in different heat treatment conditions. Intermetallics 2014, 58, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Banumathy, S.; Neelam, N.S.; Chandravanshi, V.; Bhattacharjee, A.; Ravi, K. The Effect of Nb addition on microstructure, oxidation behavior and strength of some γ-TiAl alloys. Mater. Today Proc. 2018, 5, 5514–5520. [Google Scholar] [CrossRef]
- Bean, G.E.; Kesler, M.S.; Manuel, M.V. Effect of Nb on phase transformations and microstructure in high Nb titanium aluminides. J. Alloys Compd. 2014, 613, 351–356. [Google Scholar] [CrossRef]
- Tetsui, T. Effects of high niobium addition on the mechanical properties and high-temperature deformability of gamma TiAl alloy. Intermetallics 2002, 10, 239–245. [Google Scholar] [CrossRef]
- Braun, J.; Ellner, M.; Predel, B. Experimental investigations of the structure and stability of the TiAl phase. Z. Fuer Met. 1995, 86, 870–876. [Google Scholar]
- Braun, J.; Ellner, M. On the partial atomic volume of aluminium in the titanium-rich phases of the binary system Ti-Al. Z. Fuer Met. 2000, 91, 389–392. [Google Scholar]
- Cahn, R.W. Binary Alloy Phase Diagrams, 2nd ed.; Massalski, T.B., Okamoto, H., Subramanian, P.R., Kacprzak, L., Eds.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Kononikhina, V.; Stark, A.; Gan, W.; Schreyer, A.; Pyczak, F. Ordering and disordering of β/βo-phase in γ-TiAl based alloys investigated by neutron diffraction. MRS Adv. 2017, 2, 1399–1404. [Google Scholar] [CrossRef]
- Das, K.; Das, S. Order-disorder transformation of the body centered cubic phase in the Ti-Al-X (X = Ta, Nb, or Mo) system. J. Mater. Sci. 2003, 38, 3995–4002. [Google Scholar] [CrossRef]
- Bendersky, L.; Boettinger, W.; Burton, B.; Biancaniello, F.; Shoemaker, C. The formation of ordered ω-related phases in alloys of composition Ti4Al3Nb. Acta Met. Mater. 1990, 38, 931–943. [Google Scholar] [CrossRef]
- Banerjee, D.P.K.; Gogia, A.; Nandi, T.; Joshi, V. A new ordered orthorhombic phase in a Ti3Al-Nb alloy. Acta Met. 1988, 36, 871–882. [Google Scholar] [CrossRef]
- Hsiung, L.; Cai, W.; Wadley, H. Mechanisms of isothermal phase transformations in rapidly solidified Ti-24Al-11Nb. Acta Met. Mater. 1992, 40, 3035–3049. [Google Scholar] [CrossRef]
- Bendersky, L.; Roytburd, A.; Boettinger, W. Phase transformations in the (Ti, Al)3 Nb section of the Ti-Al-Nb system—I. Microstructural predictions based on a subgroup relation between phases. Acta Met. Mater. 1994, 42, 2323–2335. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Stark, A.; Oehring, M.; Pyczak, F.; Lippmann, T.; Lottermoser, L.; Schreyer, A. The Transformation Mechanism of β Phase to ω-Related Phases in Nb-Rich γ-TiAl Alloys Studied by In Situ High-Energy X-ray Diffraction. Mater. Sci. Forum 2013, 772, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Rackel, M.W.; Stark, A.; Gabrisch, H.; Pyczak, F. Screening for O phase in advanced γ–TiAl alloys. Intermetallics 2021, 131, 107086. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, W.; Li, D.; Xu, J.; Ma, X.; Liang, X.; Zhang, J. Orthorhombic precipitate variant selection in a Ti2AlNb based alloy. Mater. Des. 2018, 158, 46–61. [Google Scholar] [CrossRef]
- Bendersky, L.; Boettinger, W.; Roytburd, A. Coherent precipitates in the b.c.c./orthorhombic two-phase field of the Ti-Al-Nb system. Acta Met. Mater. 1991, 39, 1959–1969. [Google Scholar] [CrossRef]
- Schell, N.; King, A.; Beckmann, F.; Fischer, T.; Müller, M.; Schreyer, A. The High Energy Materials Science Beamline (HEMS) at PETRA III. Mater. Sci. Forum 2013, 772, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Hammersley, A.P. FIT2D: A multi-purpose data reduction, analysis and visualization program. J. Appl. Crystallogr. 2016, 49, 646–652. [Google Scholar] [CrossRef]
- Höhne, G.; Cammenga, H.; Eysel, W.; Gmelin, E.; Hemminger, W. The temperature calibration of scanning calorimeters. Thermochim. Acta 1990, 160, 1–12. [Google Scholar] [CrossRef]
- Höhne, G.W.H. Remarks on the calibration of differential scanning calorimeters. J. Therm. Anal. Calorim. 1991, 37, 1987–2000. [Google Scholar] [CrossRef]
- Gmelin, E.; Sarge, S.M. Calibration of differential scanning calorimeters. Pure Appl. Chem. 1995, 67, 1789–1800. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.T.; Devletian, J.H. Determination of equilibrium solid-phase transition temperature using DTA. Met. Mater. Trans. A 1991, 22, 1993–1998. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Devletian, J.H.; Manthiram, A. Application of differential thermal analysis to solid-solid transitions in phase diagram determination. J. Phase Equilibria Diffus. 1994, 15, 37–41. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary Aluminum-Titanium phase diagram. J. Phase Equilibria Diffus. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Transformations in Metals and Alloys, 2nd ed.; Pergamon Press: Oxford, UK, 1975; p. 549. [Google Scholar]
- Malinov, S.; Novoselova, T.; Sha, W. Experimental and modelling studies of the thermodynamics and kinetics of phase and structural transformations in a gamma TiAl-based alloy. Mater. Sci. Eng. A 2004, 386, 344–353. [Google Scholar] [CrossRef]
- Böhm, H.; Löhberg, K.A. Superlattice of the CsCl-Type in the System Ti-Mo-Al. Z. Fuer Met. 1958, 49, 173–178. [Google Scholar]
- Hamajima, T.; Luetjering, G.; Weissmann, S. Microstructure and phase relations for Ti-Mo-Al alloys. Met. Mater. Trans. A 1972, 3, 2805–2810. [Google Scholar] [CrossRef]
- Banerjee, D.; Nandy, T.; Gogia, A. Site occupation in the ordered beta phase of ternary Ti-Al-Nb alloys. Scr. Met. 1987, 21, 597–600. [Google Scholar] [CrossRef]
- Leonard, K.J.; Vasudevan, V.K. Site occupancy preferences in the B2 ordered phase in Nb–rich Nb–Ti–Al alloys. Mater. Sci. Eng. A 2002, 329-331, 461–467. [Google Scholar] [CrossRef]
- Holec, D.; Reddy, R.K.; Klein, T.; Clemens, H. Preferential site occupancy of alloying elements in TiAl-based phases. J. Appl. Phys. 2016, 119, 205104. [Google Scholar] [CrossRef] [Green Version]
- Muraleedharan, K.; Gogia, A.K.; Nandy, T.K.; Banerjee, D.; Lele, S. Transformations in a Ti-24AI-15Nb alloy: Part I. Phase equilibria and microstructure. Met. Mater. Trans. A 1992, 23, 401–415. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Lee, D. Phase relations in Ti-Al-Nb alloys at 1200 °C. Scr. Met. Mater. 1992, 26, 919–924. [Google Scholar] [CrossRef]
- Asta, M.; Ormeci, A.; Wills, J.M.; Albers, R.C. First-Principles Study of Intermetallic Phase Stability in the Ternary Ti-Al-Nb Alloy System. MRS Proc. 1994, 364. [Google Scholar] [CrossRef] [Green Version]
- Kestner-Weykamp, H.; Ward, C.; Broderick, T.; Kaufman, M. Microstructures and phase relationships in the Ti3Al + Nb system. Scr. Met. 1989, 23, 1697–1702. [Google Scholar] [CrossRef]
- Cupid, D.M.; Fabrichnaya, O.; Rios, O.; Ebrahimi, F.; Seifert, H.J. Thermodynamic re-assessment of the Ti–Al–Nb system. Int. J. Mater. Res. 2009, 100, 218–233. [Google Scholar] [CrossRef]
- Witusiewicz, V.; Bondar, A.; Hecht, U.; Velikanova, T. The Al–B–Nb–Ti system: IV. Experimental study and thermodynamic re-evaluation of the binary Al–Nb and ternary Al–Nb–Ti systems. J. Alloys Compd. 2009, 472, 133–161. [Google Scholar] [CrossRef]
- Jones, S.; Shull, R.; McAlister, A.; Kaufman, M. Microstructural studies of Ti-Al alloys in the vicinity of the “eutectoid” reaction (α→α2 + γ). Scr. Met. 1988, 22, 1235–1240. [Google Scholar] [CrossRef]
- Appel, F.; Oehring, M.; Wagner, R. Novel design concepts for gamma-base titanium aluminide alloys. Intermetallics 2000, 8, 1283–1312. [Google Scholar] [CrossRef]
- Chladil, H.F.; Clemens, H.; Zickler, G.A.; Takeyama, M.; Kozeschnik, E.; Bartels, A.; Buslaps, T.; Gerling, R.; Kremmer, S.; Yeoh, L.; et al. Experimental studies and thermodynamic simulation of phase transformations in high Nb containing γ-TiAl based alloys. Int. J. Mater. Res. 2007, 98, 1131–1137. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, Y.; Song, L.; Hao, G.; Tian, B.; Xu, R.; Lin, J. Experimental Phase Equilibria and Isopleth Section of 8Nb-TiAl Alloys. Metals 2021, 11, 1229. [Google Scholar] [CrossRef]
- Chladil, H.; Clemens, H.; Leitner, H.; Bartels, A.; Gerling, R.; Schimansky, F.-P.; Kremmer, S. Phase transformations in high niobium and carbon containing γ-TiAl based alloys. Intermetallics 2006, 14, 1194–1198. [Google Scholar] [CrossRef]
- Waterstrat, R.M. Effect of Interstitial Elements on Phase Relationships in the Titanium-Aluminium System; NISTIR 88-3856, U.S. Department of Commerce, National Institute of Standards and Technology: Gaithersburg, MD, USA, 1988; p. 53. [Google Scholar]
- Liss, K.-D.; Bartels, A.; Clemens, H.; Bystrzanowski, S.; Stark, A.; Buslaps, T.; Schimansky, F.-P.; Gerling, R.; Scheu, C.; Schreyer, A. Recrystallization and phase transitions in a γ-TiAl-based alloy as observed by ex situ and in situ high-energy X-ray diffraction. Acta Mater. 2006, 54, 3721–3735. [Google Scholar] [CrossRef]
- Beran, P.; Petrenec, M.; Heczko, M.; Smetana, B.; Žaludová, M.; Šmíd, M.; Kruml, T.; Keller, L. In-situ neutron diffraction study of thermal phase stability in a γ-TiAl based alloy doped with Mo and/or C. Intermetallics 2014, 54, 28–38. [Google Scholar] [CrossRef]
- Yeoh, L.A.; Liss, K.-D.; Bartels, A.; Chladil, H.; Avdeev, M.; Clemens, H.; Gerling, R.; Buslaps, T. In situ high-energy X-ray diffraction study and quantitative phase analysis in the α + γ phase field of titanium aluminides. Scr. Mater. 2007, 57, 1145–1148. [Google Scholar] [CrossRef] [Green Version]
- Distl, B.; Hauschildt, K.; Rashkova, B.; Pyczak, F.; Stein, F. Low-temperature phase equilibria between 700 °C and 900 °C in the Ti-rich part of the Ti-Al-Nb system. 2021; in preparation. [Google Scholar]
- Sadi, F.-A.; Servant, C. In Situ Neutron Diffraction on the Alloy 50.6Ti-36.5Al-12.9 Nb (at.%). Z. Fuer Met. 2000, 91, 504–509. [Google Scholar]
- Sadi, F.-A. Contribution à L’étude du Diagramme de Phases du Système Al–Nb–Ti au Voisinage des Composés AlNbTi2 et Al3NbTi4. Ph.D. Thesis, Université de Paris-Sud, Paris, France, 1997. [Google Scholar]
- Song, L.; Xu, X.; You, L.; Liang, Y.; Wang, Y.; Lin, J. Ordered α2 to ωo phase transformations in high Nb-containing TiAl alloys. Acta Mater. 2015, 91, 330–339. [Google Scholar] [CrossRef]
- Song, L.; Lin, J.; Li, J. Phase transformation mechanisms in a quenched Ti-45Al-8.5Nb-0.2W-0.2B-0.02Y alloy after subsequent annealing at 800 °C. J. Alloys Compd. 2017, 691, 60–66. [Google Scholar] [CrossRef]



| Alloy | Ti (at.%) | Al (at.%) | Nb (at.%) | ωo to (βTi)o | O to (βTi)o | Ti3Al to (αTi) | (βTi)o to (βTi) |
|---|---|---|---|---|---|---|---|
| A0 | 57.1 | 42.9 | – | – | – | x | – |
| A1 | 50.8 | 44.3 | 4.9 | – | – | x | – |
| A2 | 49.0 | 45.8 | 5.2 | – | – | x | – |
| A3 | 50.8 | 39.8 | 9.4 | x | – | x | x |
| A4 | 53.9 | 36.4 | 9.7 | x | – | * | x |
| A5 | 55.6 | 32.7 | 11.6 | x | – | – | x |
| A6 | 43.8 | 44.5 | 11.7 | x | – | – | x |
| A7 | 60.9 | 25.2 | 13.9 | – | x | – | x |
| A8 | 50.4 | 32.8 | 16.8 | x | * | – | x |
| A9 | 40.4 | 39.6 | 20.0 | x | – | – | x |
| A10 | 45.4 | 30.7 | 23.9 | – | x | – | x |
| A11 | 50.7 | 24.1 | 25.2 | – | x | – | x |
| Heat Treatment (Temperature/Time) | Disordering Temperature (°C) | |
|---|---|---|
| Ti-36.4Al-9.7Nb (A4) | Ti-44.5Al-11.7Nb (A6) | |
| 700 °C/1500 h | 1222 | 1204 |
| 900 °C/650 h | 1223 | 1204 |
| 1000 °C/400 h | 1223 | 1204 |
| Alloy | Ti (at.%) | Al (at.%) | Nb (at.%) | (βTi)o to (βTi) (°C) |
|---|---|---|---|---|
| A3 | 52.3 | 37.4 | 10.4 | 1201 ± 1 |
| A4 | 54.4 | 35.8 | 9.8 | 1222 ± 1 |
| A5 | 55.6 | 32.7 | 11.6 | 1237 ± 1 |
| A6 | 48.5 | 37.5 | 14.1 | 1204 ± 1 |
| A7 | 60.9 | 25.2 | 13.9 | 1104 ± 1 |
| A8 | 50.4 | 32.8 | 16.8 | 1248 ± 1 |
| A9 | 42.9 | 38.1 | 19.0 | 1199 ± 1 |
| A10 | 45.4 | 30.7 | 23.9 | 1243 ± 1 |
| A11 | 50.7 | 24.1 | 25.2 | 1169 ± 1 |
| Heating Rate (°C/min) | Ti3Al to (αTi) Transformation Temperatures (°C) | |||
|---|---|---|---|---|
| Ti-42.9Al (A0) | Ti-44.3Al-4.9Nb (A1) | Ti-45.8Al-5.2Nb (A2) | Ti-39.8Al-9.4Nb (A3) | |
| 10 | 1140 | 1147 | 1163 | 1174 |
| 5 | 1139 | 1148 | 1163 | 1173 |
| 2 | 1139 | 1145 | 1161 | 1173 |
| 1 | 1138 | – * | – * | 1172 |
| 0 | 1137 ± 1 | 1143 ± 1 | 1159 ± 1 | 1171 ± 1 |
| Alloy | Ti (at.%) | Al (at.%) | Nb (at.%) | ωo to (βTi)o (°C) |
|---|---|---|---|---|
| A3 | 50.8 | 39.8 | 9.4 | 906 ± 1 |
| A4 | 53.9 | 36.4 | 9.7 | 908 ± 1 |
| A5 | 55.6 | 32.7 | 11.6 | 919 ± 2 |
| A6 | 43.8 | 44.5 | 11.7 | 913 ± 1 |
| A8 | 50.4 | 32.8 | 16.8 | 897 ± 3 |
| A9 | 40.4 | 39.6 | 20.0 | 923 ± 1 |
| Heating Rate (°C/min) | O to (βTi)o Transformation Temperatures (°C) | ||
|---|---|---|---|
| Ti-25.2Al-13.9Nb (A7) | Ti-30.7Al-23.9Nb (A10) | Ti-24.1Al-25.2Nb (A11) | |
| 10 | 964 | 985 | 979 |
| 5 | 960 | 981 | 973 |
| 2 | 956 | 976 | 962 * |
| 1 | 951 | 973 | 955 * |
| 0 | 941 ± 1 | 963 ± 1 | 935 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Distl, B.; Hauschildt, K.; Pyczak, F.; Stein, F. Solid-Solid Phase Transformations and Their Kinetics in Ti-Al-Nb Alloys. Metals 2021, 11, 1991. https://doi.org/10.3390/met11121991
Distl B, Hauschildt K, Pyczak F, Stein F. Solid-Solid Phase Transformations and Their Kinetics in Ti-Al-Nb Alloys. Metals. 2021; 11(12):1991. https://doi.org/10.3390/met11121991
Chicago/Turabian StyleDistl, Benedikt, Katja Hauschildt, Florian Pyczak, and Frank Stein. 2021. "Solid-Solid Phase Transformations and Their Kinetics in Ti-Al-Nb Alloys" Metals 11, no. 12: 1991. https://doi.org/10.3390/met11121991
APA StyleDistl, B., Hauschildt, K., Pyczak, F., & Stein, F. (2021). Solid-Solid Phase Transformations and Their Kinetics in Ti-Al-Nb Alloys. Metals, 11(12), 1991. https://doi.org/10.3390/met11121991









