Cobalt Recovery from Li-Ion Battery Recycling: A Critical Review
Abstract
:1. Introduction
1.1. Cobalt: Critical and Strategic Metal
1.2. Li-Ion Batteries
| Cathode Type | LCO | LFP | LMO | NCA | NMC |
|---|---|---|---|---|---|
| Chemical formula | LiCoO2 | LiFePO4 | LiMn2O4 or LiMnO2 | Li(Ni0.8Co0.15Al0.05)O2 | LiNi0.33Co0.33Mn0.33O2–NMC111 LiNi0.5Co0.3Mn0.2O2–NMC532 LiNi0.6Co0.2Mn0.2O2–NMC622 LiNi0.8Co0.1Mn0.1O2–NMC811 |
| Structure | Layered | Olivine | Spinel | Layered | Layered |
| Year introduced | 1991 | 1996 | 1996 | 1999 | 2008 |
| Safety | Moderate | Excellent | Very good | Good | Good |
| Energy density | Very good | Good | Good | Excellent | Excellent |
| Power density | Good | Very good | Very good | Very good | Good |
| Lifespan | Good | Very good | Very good | Very good | Very good |
| Cycle lifespan | Good | Very good | Good | Very good | Very good |
| Performance | Very good | Very good | Good | Very good | Very good |
| Cost | Poor | Very good | Very good | Good | Good |
| Market share | Obsolete | Electric bikes, buses, and large vehicles | Small | Steady | Growing (from NMC 111 > NMC 532 > NMC 622 > NMC 811 to no-cobalt chemistries |
| Specific density (Wh/g) | 200 | 120 | 140 | 245 | 200 |
| Cycles (charge-discharge) | 1000 | 300 | 820 | 950 | 850 |
1.3. Urban Mining and Challenges for Sustainable Development
2. State-of-Art
2.1. Leaching of Li-Ion Batteries
2.1.1. Inorganic Acid Leaching
| References | Li-Ion Battery Type | Leaching Agents | Conditions | Co Leaching Efficiency |
|---|---|---|---|---|
| Meshram et al. (2015) [43] | NMC | Leaching agent: H2SO4 Reducing agent: NaHSO3 | S/L ratio = 20 g/L; 1 M H2SO4 and 0.075 M NaHSO3; 4 h; 95 °C | 91.60% |
| Chen et al. (2018) [50] | NMC | Leaching agent: H2SO4 Reducing agent: H2O2 | S/L ratio = 30 g/L; 1 M H2SO4; 4% v/v H2O2; 90 min; 70 °C | 98.5% Co |
| Xuan et al. (2021) [51] | NMC | Leaching agent: HCl | S/L ratio = 20 g/L; 4 M HCl; 120 min; 82 °C | ~100% Co |
| Vieceli et al. (2021) [52] | NMC | Leaching agent: H2SO4 | S/L ratio = 1/50; 2.5 M H2SO4; 60 min; 50 °C. Calcination at 500 °C for 90 min | 90% Co |
| He et al. (2022) [47] | NMC | Assisted by ultrasound Leaching agent: H2SO4 | S/L ratio = 10 g/L; 1 M H2SO4; 250 W; 30 min; 90 °C | 30% Co |
| Takahashi et al. (2020) [42] | LCO | Assisted by ultrasound Leaching agent: H2SO4 Reducing agent: H2O2 | S/L ratio = 1/5; pH 3; H2O2 dosage | 99% |
| Chen et al. (2018) [53] | LCO, LMO, LFP, NMC | Leaching agent: H3PO4 Reducing agent: H2O2 | S/L = 1/50; 1 M H3PO4; 4% v/v H2O2; for 10 min; 40 °C | <20% Co (the goal was the Li extraction) |
2.1.2. Organic Acid Leaching
2.2. Consolidated Technologies for Co Separation
2.2.1. Solvent Extraction
2.2.2. Ion Exchange Resins
2.2.3. Precipitation
3. Emerging Technologies for Cobalt Separation
3.1. Ionic Liquids
3.1.1. Deep Eutectic Solvents
3.1.2. Supercritical Fluids
3.1.3. Nanotechnology
3.1.4. Biohydrometallurgy
- redoxolysis (reaction occurs as biooxidation and bioreduction)
- acidolysis (proton promotes dissolution with biogenic inorganic or organic acids)
- complexolysis (complexation promoted dissolution).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Academies Science Advisory Council. Priorities for Critical Materials for a Circular Economy; European Academies Science Advisory Council: Halle (Saale), Germany, 2016. [Google Scholar]
- European Commission. 2017 List of Critical Raw Materials for the EU; EU: Brussels, Belgium, 2017. [Google Scholar]
- European Union. Critical Raw Material List. 2020. Available online: https://rmis.jrc.ec.europa.eu/?page=crm-list-2020-e294f6 (accessed on 30 July 2021).
- U.S. Department of Energy. Critical Materials Strategy; U.S. Department of Energy: Washington, DC, USA, 2010.
- USGS Interior Releases 2018’s Final List of 35 Minerals Deemed Critical to U. S. National Security and the Economy. Available online: https://www.usgs.gov/news/interior-releases-2018-s-final-list-35-minerals-deemed-critical-us-national-security-and (accessed on 25 August 2020).
- MCTIC Plano de Ciência, Tecnologia e Inovação para Minerais Estratéticos. Available online: https://www.inova.rs.gov.br/upload/arquivos/202006/16181825-plano-de-ciencia-tecnologia-e-inovacao-para-minerais-estrategicos.pdf (accessed on 25 August 2020).
- Shedd, K.B. Cobalt; USGS: Reston, VA, USA, 2021. [Google Scholar]
- Cheyns, K.; Banza Lubaba Nkulu, C.; Ngombe, L.K.; Asosa, J.N.; Haufroid, V.; De Putter, T.; Nawrot, T.; Kimpanga, C.M.; Numbi, O.L.; Ilunga, B.K.; et al. Pathways of human exposure to cobalt in Katanga, a mining area of the D.R. Congo. Sci. Total Environ. 2014, 490, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Atibu, E.K.; Devarajan, N.; Laffite, A.; Giuliani, G.; Salumu, J.A.; Muteb, R.C.; Mulaji, C.K.; Otamonga, J.P.; Elongo, V.; Mpiana, P.T.; et al. Assessment of trace metal and rare earth elements contamination in rivers around abandoned and active mine areas. The case of Lubumbashi River and Tshamilemba Canal, Katanga, Democratic Republic of the Congo. Chem. Der Erde Geochem. 2016, 76, 353–362. [Google Scholar] [CrossRef]
- Pourret, O.; Lange, B.; Bonhoure, J.; Colinet, G.; Decrée, S.; Mahy, G.; Séleck, M.; Shutcha, M.; Faucon, M.P. Assessment of soil metal distribution and environmental impact of mining in Katanga (Democratic Republic of Congo). Appl. Geochem. 2015, 64, 43–55. [Google Scholar] [CrossRef]
- Squadrone, S.; Burioli, E.; Monaco, G.; Koya, M.K.K.; Prearo, M.; Gennero, S.; Dominici, A.; Abete, M.C.C. Human exposure to metals due to consumption of fish from an artificial lake basin close to an active mining area in Katanga (D.R. Congo). Sci. Total Environ. 2016, 568, 679–684. [Google Scholar] [CrossRef]
- Butsic, V.; Baumann, M.; Shortland, A.; Walker, S.; Kuemmerle, T. Conservation and conflict in the Democratic Republic of Congo: The impacts of warfare, mining, and protected areas on deforestation. Biol. Conserv. 2015, 191, 266–273. [Google Scholar] [CrossRef]
- Cobalt Use. Available online: https://www.cobaltinstitute.org/about-cobalt/the-cobalt-value-chain/cobalt-use/ (accessed on 29 October 2021).
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Martins, L.S.; Guimarães, L.F.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric car battery: An overview on global demand, recycling and future approaches towards sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef]
- Hannan, M.A.; Wali, S.B.; Ker, P.J.; Rahman, M.S.A.; Mansor, M.; Ramachandaramurthy, V.K.; Muttaqi, K.M.; Mahlia, T.M.I.; Dong, Z.Y. Battery energy-storage system: A review of technologies, optimization objectives, constraints, approaches, and outstanding issues. J. Energy Storage 2021, 42, 103023. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garole, D.J.; Hossain, R.; Garole, V.J.; Sahajwalla, V.; Nerkar, J.; Dubal, D.P. Recycle, Recover and Repurpose Strategy of Spent Li-ion Batteries and Catalysts: Current Status and Future Opportunities. ChemSusChem 2020, 13, 3079–3100. [Google Scholar] [CrossRef]
- Piątek, J.; Afyon, S.; Budnyak, T.M.; Budnik, S.; Sipponen, M.H.; Slabon, A. Sustainable Li-Ion Batteries: Chemistry and Recycling. Adv. Energy Mater. 2020, 11, 2003456. [Google Scholar] [CrossRef]
- Mansur, M.B.; Guimarães, A.S.; Petraniková, M. An Overview on the Recovery of Cobalt from End-of-life Lithium-Ion Batteries. Miner. Process. Extra. Metall. Rev. 2021, 1–21. [Google Scholar] [CrossRef]
- Jena, K.K.; AlFantazi, A.; Mayyas, A.T. Comprehensive Review on Concept and Recycling Evolution of Lithium-Ion Batteries (LIBs). Energy Fuels 2021, 35, 18257–18284. [Google Scholar] [CrossRef]
- European Commission Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability. Available online: https://ec.europa.eu/docsroom/documents/42849 (accessed on 7 October 2020).
- Center, L.J.; Ali, S.H.; Watson, J.E.M. Mining and biodiversity: Key issues and research needs in conservation science. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181926. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Lee, J.-H.; Seo, J.K.; Jo, W.Y.; Whang, D.; Hwang, S.M.; Kim, Y.-J. Graphene collage on Ni-rich layered oxide cathodes for advanced lithium-ion batteries. Nat. Commun. 2021, 12, 2145. [Google Scholar] [CrossRef]
- Elwert, T.; Goldmann, D.; Römer, F.; Buchert, M.; Merz, C.; Schueler, D.; Sutter, J. Current developments and challenges in the recycling of key components of (Hybrid) electric vehicles. Recycling 2016, 1, 25–60. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li-Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, C.; Zhou, T.; Li, H.; Dai, A. Recovery of valuable metals from LiNi0.5Co0.2Mn0.3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leaching. Waste Manag. 2019, 85, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. E-Waste Recycling and Resource Recovery: A Review on Technologies, Barriers and Enablers with a Focus on Oceania. Metals 2021, 11, 1313. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Baars, J.; Domenech, T.; Bleischwitz, R.; Melin, H.E.; Heidrich, O. Circular economy strategies for electric vehicle batteries reduce reliance on raw materials. Nat. Sustain. 2021, 4, 71–79. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Selective separation of Sc(III) and Zr(IV) from the leaching of bauxite residue using trialkylphosphine acids, tertiary amine, tri-butyl phosphate and their mixtures. Sep. Purif. Technol. 2021, 279, 119798. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Träger, T.; Friedrich, B.; Weyhe, R. Recovery Concept of Value Metals from Automotive Lithium-Ion Batteries. Chem. Ing. Tech. 2015, 87, 1550–1557. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Mansur, M.B. Recycling Batteries; Elsevier Ltd.: Cham, Switzerland, 2019; ISBN 9780081021583. [Google Scholar]
- Pavez, P.; Honores, J.; Millán, D.; Isaacs, M. UN sustainable development goals: How can sustainable/green chemistry contribute? Curr. Opin. Green Sustain. Chem. 2018, 13, 154–157. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Vaughan, J.; Tenório, J.A.S. Recovery of scandium from various sources: A critical review of the state of the art and future prospects. Miner. Eng. 2021, 172, 107148. [Google Scholar] [CrossRef]
- National Waste Policy: Less Waste, More Resources. Available online: http://www.elgaronline.com/view/9781784715878.xml (accessed on 8 February 2021).
- Zeng, Y.; Maxwell, S.; Runting, R.K.; Venter, O.; Watson, J.E.M.; Carrasco, L.R. Environmental destruction not avoided with the Sustainable Development Goals. Nat. Sustain. 2020, 3, 795–798. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; Franklin, J.A., Ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium-Ion Batteries, Part II: Laboratory-Scale Research Developments in Mechanical, Thermal, and Leaching Treatments. J. Sustain. Metall. 2020, 6, 142–160. [Google Scholar] [CrossRef]
- Takahashi, V.C.I.; Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Enhancing cobalt recovery from Li-ion batteries using grinding treatment prior to the leaching and solvent extraction process. J. Environ. Chem. Eng. 2020, 8, 103801. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical processing of spent lithium-ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Jiménez Correa, M.M.; Espinosa, D.C.R.; Tenório, J.A.S. Study of the reduction process of iron in leachate from nickel mining waste. Braz. J. Chem. Eng. 2018, 35, 1241–1248. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Dreisinger, D.; Tenório, J.A.S. Recovery of nickel and cobalt from nickel laterite leach solution using chelating resins and pre-reducing process. Can. J. Chem. Eng. 2019, 97, 1181–1190. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Pinheiro, É.F.; Espinosa, D.C.R.; Tenório, J.A.S.; Baltazar, M.d.P.G. Adsorption of lanthanum and cerium on chelating ion exchange resins: Kinetic and thermodynamic studies. Sep. Sci. Technol. 2021, 57, 60–69. [Google Scholar] [CrossRef]
- He, H.; Feng, J.; Gao, X.; Fei, X. Selective separation and recovery of lithium, nickel, MnO2, and Co2O3 from LiNi0.5Mn0.3Co0.2O2 in spent battery. Chemosphere 2022, 286, 131897. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wilson, B.P.; Lundström, M.; Liu, Z. Clean and efficient recovery of spent LiCoO2 cathode material: Water-leaching characteristics and low-temperature ammonium sulfate calcination mechanisms. J. Clean. Prod. 2020, 268, 122299. [Google Scholar] [CrossRef]
- Kumar, A.; Holuszko, M.; Espinosa, D.C.R. E-waste: An overview on generation, collection, legislation and recycling practices. Resour. Conserv. Recycl. 2017, 122, 32–42. [Google Scholar] [CrossRef]
- Chen, W.S.; Ho, H.J. Recovery of valuable metals from lithium-ion batteries NMC cathode waste materials by hydrometallurgical methods. Metals 2018, 8, 321. [Google Scholar] [CrossRef] [Green Version]
- Xuan, W.; de Souza Braga, A.; Korbel, C.; Chagnes, A. New insights in the leaching kinetics of cathodic materials in acidic chloride media for lithium-ion battery recycling. Hydrometallurgy 2021, 204, 105705. [Google Scholar] [CrossRef]
- Vieceli, N.; Casasola, R.; Lombardo, G.; Ebin, B.; Petranikova, M. Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid. Waste Manag. 2021, 125, 192–203. [Google Scholar] [CrossRef]
- Chen, X.; Cao, L.; Kang, D.; Li, J.; Zhou, T.; Ma, H. Recovery of valuable metals from mixed types of spent lithium-ion batteries. Part II: Selective extraction of lithium. Waste Manag. 2018, 80, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Meng, X.; Cao, H.; Lin, X.; Liu, C.; Sun, Y.; Zhang, Y.; Sun, Z. Selective recovery of lithium from spent lithium iron phosphate batteries: A sustainable process. Green Chem. 2018, 20, 3121–3133. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kim, J.; Kim, S.-K.; Yun, Y.-S. Simple, green organic acid-based hydrometallurgy for waste-to-energy storage devices: Recovery of NiMnCoC2O4 as an electrode material for pseudocapacitor from spent LiNiMnCoO2 batteries. J. Hazard. Mater. 2021, 407, 127481. [Google Scholar] [CrossRef] [PubMed]
- Urias, P.M.; dos Reis Menêzes, L.H.; Cardoso, V.L.; de Resende, M.M.; de Souza Ferreira, J. Leaching with mixed organic acids and sulfuric acid to recover cobalt and lithium from lithium-ion batteries. Environ. Technol. 2020, 42, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium-ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Esmaeili, M.; Rastegar, S.O.; Beigzadeh, R.; Gu, T. Ultrasound-assisted leaching of spent lithium-ion batteries by natural organic acids and H2O2. Chemosphere 2020, 254, 126670. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Recovery of cobalt as cobalt oxalate from spent lithium-ion batteries by using glycine as leaching agent. J. Environ. Chem. Eng. 2016, 4, 2378–2383. [Google Scholar] [CrossRef]
- Santhosh, G.; Nayaka, G.P. Cobalt recovery from spent Li-ion batteries using lactic acid as dissolution agent. Clean. Eng. Technol. 2021, 3, 100122. [Google Scholar] [CrossRef]
- Sun, C.; Xu, L.; Chen, X.; Qiu, T.; Zhou, T. Sustainable recovery of valuable metals from spent lithium-ion batteries using DL-malic acid: Leaching and kinetics aspect. Waste Manag. Res. 2018, 36, 113–120. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- Kislik, V.S. Solvent Extraction: Classical and Novel Approaches; Elsevier: Amsterdan, The Netherlands, 2014; ISBN 9780444537782. [Google Scholar]
- de Oliveira, G.F.R.; Botelho, A.B.; Tenório, J.A.S. Separation of Cobalt from the Nickel-Rich Solution from Hpal Process by Synergism Using Organic Extracts Cyanex 272 and Ionquest 290. Tecnol. Em Metal. Mater. E Min. 2019, 16, 464–469. [Google Scholar] [CrossRef]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum-Group Metals, 1st ed.; Elsevier: Oxford, UK, 2011; ISBN 9780080968094. [Google Scholar]
- Alvial-Hein, G.; Mahendra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardo, S. Solvent extraction of aluminium in the presence of cobalt, nickel and magnesium from sulphate solutions by Cyanex 272. Hydrometallurgy 2005, 80, 90–97. [Google Scholar] [CrossRef]
- CYTEC CYANEX 272 Extractant. Available online: http://www.cytec.com/sites/default/files/datasheets/CYANEX272Brochure.pdf (accessed on 17 January 2018).
- Ichlas, Z.T.; Ibana, D.C. Process development for the direct solvent extraction of nickel and cobalt from nitrate solution: Aluminum, cobalt, and nickel separation using Cyanex 272. Int. J. Miner. Metall. Mater. 2017, 24, 37–46. [Google Scholar] [CrossRef]
- Nayl, A.A.; Hamed, M.M.; Rizk, S.E. Selective extraction and separation of metal values from leach liquor of mixed spent Li-ion batteries. J. Taiwan Inst. Chem. Eng. 2015, 55, 119–125. [Google Scholar] [CrossRef]
- Wang, F.; Sun, R.; Xu, J.; Chen, Z.; Kang, M. Recovery of cobalt from spent lithium-ion batteries using sulphuric acid leaching followed by solid-liquid separation and solvent extraction. RSC Adv. 2016, 6, 85303–85311. [Google Scholar] [CrossRef]
- Vieceli, N.; Reinhardt, N.; Ekberg, C.; Petranikova, M. Optimization of Manganese Recovery from a Solution Based on Lithium-Ion Batteries by Solvent Extraction with D2EHPA. Metals 2020, 11, 54. [Google Scholar] [CrossRef]
- Keller, A.; Hlawitschka, M.W.; Bart, H.J. Manganese recycling of spent lithium-ion batteries via Solvent extraction. Sep. Purif. Technol. 2021, 275, 119166. [Google Scholar] [CrossRef]
- Nadimi, H.; Karazmoudeh, N.J. Selective Separation and Purification of Mn from Co and Ni in Waste Mobile Phone Lithium-Ion Batteries Using D2EHAP via Solvent Extraction Method. J. Sustain. Metall. 2021, 7, 653–663. [Google Scholar] [CrossRef]
- Yoshimi, T.; Narusako, M.; Nakamura, Y. Method of Separating/Recovering Current Collector and Positive Electrode Active Material from Positive Electrode Member for Lithium-Ion Battery. 2013-139592A, 2013. [Google Scholar]
- Yamaguchi, Y.; Hino, J. Method for Recovering Valuable Metals from Lithium Batteries Containing Co, Ni, Mn. Patent JP-2009193778-A, 27 August 2009. [Google Scholar]
- Abdou, T.R.; Junior, A.B.B.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of polymeric composites from industrial waste by pyrolysis: Deep evaluation for carbon fibers reuse. Waste Manag. 2021, 120, 1–9. [Google Scholar] [CrossRef]
- Zainol, Z.; Nicol, M.J. Ion-exchange equilibria of Ni2+, Co2+, Mn2+ and Mg2+ with iminodiacetic acid chelating resin Amberlite IRC 748. Hydrometallurgy 2009, 99, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Mendes, F.D.; Martins, A.H. Selective nickel and cobalt uptake from pressure sulfuric acid leach solutions using column resin sorption. Int. J. Miner. Process. 2005, 77, 53–63. [Google Scholar] [CrossRef]
- Mendes, F.D.; Martins, A.H. Recovery of nickel and cobalt from acid leach pulp by ion-exchange using chelating resin. Miner. Eng. 2005, 18, 945–954. [Google Scholar] [CrossRef]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Dreisinger, D.B.; Espinosa, D.C.R.; Tenório, J.A.S. Pre-Reducing Process Kinetics to Recover Metals from Nickel Leach Waste Using Chelating Resins. Int. J. Chem. Eng. 2018, 2018, 9161323. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; de Vicente, A.; Espinosa, D.C.R.; Tenório, J.A.S. Recovery of metals by ion exchange process using chelating resin and sodium dithionite. J. Mater. Res. Technol. 2019, 8, 4464–4469. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Jiménez Correa, M.M.; Espinosa, D.C.R.; Dreisinger, D.; Tenório, J.A.S. Recovery of Cu(II) from nickel laterite leach using prereduction and chelating resin extraction: Batch and continuous experiments. Can. J. Chem. Eng. 2019, 97, 924–929. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. The use of the chelating resin of a new generation Lewatit MonoPlus TP-220 with the bis-picolylamine functional groups in the removal of selected metal ions from acidic solutions. Chem. Eng. J. 2012, 197, 493–508. [Google Scholar] [CrossRef]
- Littlejohn, P.; Vaughan, J. Recovery of nickel and cobalt from laterite leach tailings through resin-in-pulp scavenging and selective ammoniacal elution. Miner. Eng. 2013, 54, 14–20. [Google Scholar] [CrossRef]
- Strauss, M.L.; Diaz, L.A.; McNally, J.; Klaehn, J.; Lister, T.E. Separation of cobalt, nickel, and manganese in leach solutions of waste lithium-ion batteries using Dowex M4195 ion exchange resin. Hydrometallurgy 2021, 206, 105757. [Google Scholar] [CrossRef]
- Deepatana, A.; Tang, J.A.; Valix, M. Comparative study of chelating ion exchange resins for metal recovery from bioleaching of nickel laterite ores. Miner. Eng. 2006, 19, 1280–1289. [Google Scholar] [CrossRef]
- Silva, R.A.; Hawboldt, K.; Zhang, Y. Application of resins with functional groups in the separation of metal ions/species–a review. Miner. Process. Extra. Metall. Rev. 2018, 39, 395–413. [Google Scholar] [CrossRef]
- Perez, I.D.; Botelho Junior, A.B.; Aliprandini, P.; Espinosa, D.C.R. Copper recovery from nickel laterite with high-iron content: A continuous process from mining waste. Can. J. Chem. Eng. 2020, 98, 957–968. [Google Scholar] [CrossRef]
- Oxley, A.; Barcza, N. Hydro-pyro integration in the processing of nickel laterites. Miner. Eng. 2013, 54, 2–13. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Vicente, A.D.A.; Espinosa, D.C.R.; Tenório, J.A.S. Effect of iron oxidation state for copper recovery from nickel laterite leach solution using chelating resin. Sep. Sci. Technol. 2020, 55, 788–798. [Google Scholar] [CrossRef]
- Perez, I.D.; Anes, I.A.; Botelho Junior, A.B.; Espinosa, D.C.R. Comparative study of selective copper recovery techniques from nickel laterite leach waste towards a competitive sustainable extractive process. Clean. Eng. Technol. 2020, 1, 100031. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Sofińska-Chmiel, W.; Mendyk, E.; Hubicki, Z. DOWEX M 4195 and LEWATIT® MonoPlus TP 220 in Heavy Metal Ions Removal from Acidic Streams. Sep. Sci. Technol. 2014, 49, 2003–2015. [Google Scholar] [CrossRef]
- Havlík, T. Hydrometallurgy: Principles and APPLICATION; Cambridge International Science Publishing Limited: Cambridge, UK, 2008; Volume 61, ISBN 9781845694074. [Google Scholar]
- Jackson, E. Hydrometallurgical Extraction and Reclamation, 1st ed.; Ellis Horwood Limited: Southampton, UK, 1986; ISBN 0853125686. [Google Scholar]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Vemic, M.; Bordas, F.; Comte, S.; Guibaud, G.; Lens, P.N.L.L.; van Hullebusch, E.D. Recovery of molybdenum, nickel and cobalt by precipitation from the acidic leachate of a mineral sludge. Environ. Technol. 2016, 37, 2231–2242. [Google Scholar] [CrossRef]
- Zhu, S.G.; He, W.Z.; Li, G.M.; Zhou, X.; Zhang, X.J.; Huang, J.W. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans. Nonferrous Met. Soc. China Engl. Ed. 2012, 22, 2274–2281. [Google Scholar] [CrossRef]
- Duarte Castro, F.; Vaccari, M.; Cutaia, L. Valorization of resources from end-of-life lithium-ion batteries: A review. Crit. Rev. Environ. Sci. Technol. 2021, 1–44. [Google Scholar] [CrossRef]
- Cai, G.; Fung, K.Y.; Ng, K.M.; Wibowo, C. Process development for the recycle of spent lithium-ion batteries by chemical precipitation. Ind. Eng. Chem. Res. 2014, 53, 18245–18259. [Google Scholar] [CrossRef]
- He, S.; Xiang, W.; He, W.; Yu, F.; Liu, Z. Recovery of spent LiCoO2 cathode material: Thermodynamic analysis and experiments for precipitation and separation of elements. Chem. Eng. J. 2022, 429, 132371. [Google Scholar] [CrossRef]
- Oruê, B.P.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R.; dos Baltazar, M.P.G. Kinetic Study of Manganese Precipitation of Nickel Laterite Leach Based-solution by Ozone Oxidation. Ozone Sci. Eng. 2020, 00, 1–15. [Google Scholar] [CrossRef]
- Choubey, P.K.; Dinkar, O.S.; Panda, R.; Kumari, A.; Jha, M.K.; Pathak, D.D. Selective extraction and separation of Li, Co and Mn from leach liquor of discarded lithium-ion batteries (LIBs). Waste Manag. 2021, 121, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Guo, J.; Du, Y.; Xu, H.H.; He, Y.H. Effects of synthesis conditions on layered Li[Ni1/3Co 1/3Mn1/3]O2 positive-electrode via hydroxide co-precipitation method for lithium-ion batteries. Trans. Nonferr. Met. Soc. China Engl. Ed. 2011, 21, 114–120. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, H.; Svensson, A.M.; Fossdal, A.; Sheridan, E.; Lu, S.; Vullum-Bruer, F. High capacity Li[Ni0.8Co0.1Mn0.1]O2 synthesized by sol-gel and co-precipitation methods as cathode materials for lithium-ion batteries. Solid State Ion. 2013, 249–250, 105–111. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Paiva, A.P.; Nogueira, C.A. Ionic Liquids in the Extraction and Recycling of Critical Metals from Urban Mines. Waste Biomass Valorization 2021, 12, 1725–1747. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Domańska, U. Separation of cobalt, lithium and nickel from the “black mass” of waste Li-ion batteries by ionic liquids, DESs and organophosphorous-based acids extraction. J. Mol. Liq. 2021, 343, 117694. [Google Scholar] [CrossRef]
- Morizono, H.; Oshima, T.; Baba, Y. Extraction Mechanism of Cobalt(II) Using an Alkylated Histidine Extractant in an Ionic Liquid System. Solvent Extra. Res. Dev. Jpn. 2011, 18, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Wellens, S.; Thijs, B.; Binnemans, K. An environmentally friendlier approach to hydrometallurgy: Highly selective separation of cobalt from nickel by solvent extraction with undiluted phosphonium ionic liquids. Green Chem. 2012, 14, 1657–1665. [Google Scholar] [CrossRef] [Green Version]
- Parmentier, D.; Paradis, S.; Metz, S.J.; Wiedmer, S.K.; Kroon, M.C. Continuous process for selective metal extraction with an ionic liquid. Chem. Eng. Res. Des. 2016, 109, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Chen, C.; Fu, M.L. Separation of cobalt and lithium from spent lithium-ion battery leach liquors by ionic liquid extraction using Cyphos IL-101. Hydrometallurgy 2020, 197, 105439. [Google Scholar] [CrossRef]
- Zante, G.; Masmoudi, A.; Barillon, R.; Trébouet, D.; Boltoeva, M. Separation of lithium, cobalt and nickel from spent lithium-ion batteries using TBP and imidazolium-based ionic liquids. J. Ind. Eng. Chem. 2020, 82, 269–277. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Selective separation of cobalt and nickel using a stable supported ionic liquid membrane. Sep. Purif. Technol. 2020, 252, 117477. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Partition studies on cobalt and recycling of valuable metals from waste Li-ion batteries via solvent extraction and chemical precipitation. J. Clean. Prod. 2019, 225, 820–832. [Google Scholar] [CrossRef]
- Zante, G.; Braun, A.; Masmoudi, A.; Barillon, R.; Trébouet, D.; Boltoeva, M. Solvent extraction fractionation of manganese, cobalt, nickel and lithium using ionic liquids and deep eutectic solvents. Miner. Eng. 2020, 156, 106512. [Google Scholar] [CrossRef]
- Othman, E.A.; van der Ham, A.G.J.; Miedema, H.; Kersten, S.R.A. Recovery of metals from spent lithium-ion batteries using ionic liquid [P8888][Oleate]. Sep. Purif. Technol. 2020, 252, 117435. [Google Scholar] [CrossRef]
- Yang, S.; Liu, G.; Wang, J.; Cui, L.; Chen, Y. Recovery of lithium from alkaline brine by solvent extraction with functionalized ionic liquid. Fluid Phase Equilib. 2019, 493, 129–136. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Singh, M.B.; Kumar, V.S.; Chaudhary, M.; Singh, P. A Mini Review on Synthesis, Properties and Applications of Deep Eutectic Solvents. J. Indian Chem. Soc. 2021, 98, 100210. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Ma, Z.; Yan, L. Deep eutectic solvents eutectogels: Progress and challenges. Green Chem. Eng. 2021, 2, 359–367. [Google Scholar] [CrossRef]
- Yu, D.; Huang, Z.; Makuza, B.; Guo, X.; Tian, Q. Pretreatment options for the recycling of spent lithium-ion batteries: A comprehensive review. Miner. Eng. 2021, 173, 107218. [Google Scholar] [CrossRef]
- Tran, M.K.; Rodrigues, M.T.F.; Kato, K.; Babu, G.; Ajayan, P.M. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 2019, 4, 339–345. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Liu, L.; Li, J. A low-toxicity and high-efficiency deep eutectic solvent for the separation of aluminum foil and cathode materials from spent lithium-ion batteries. J. Hazard. Mater. 2019, 380, 120846. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Lu, Z.; Xu, Z. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chem. 2020, 22, 4473–4482. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Bianchi, M.; Zanoni, R.; Simonetti, G.; Navarra, M.A.; Pagnanelli, F. Selective recovery of cobalt from mixed lithium-ion battery wastes using deep eutectic solvent. Chem. Eng. J. 2021, 417, 129249. [Google Scholar] [CrossRef]
- Roldán-Ruiz, M.J.; Ferrer, M.L.; Gutiérrez, M.C.; Del Monte, F. Highly Efficient p-Toluenesulfonic Acid-Based Deep-Eutectic Solvents for Cathode Recycling of Li-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 5437–5445. [Google Scholar] [CrossRef]
- Chen, L.; Chao, Y.; Li, X.; Zhou, G.; Lu, Q.; Hua, M.; Li, H.; Ni, X.; Wu, P.; Zhu, W. Engineering a tandem leaching system for the highly selective recycling of valuable metals from spent Li-ion batteries. Green Chem. 2021, 23, 2177–2184. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Amado, F.R.; Cruz, E.D.; Tanabe, E.H. Metal recovery using supercritical carbon dioxide. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Supercritical Carbon Dioxide as Green Solvent; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–103. ISBN 9780128173886. [Google Scholar]
- Chaudhary, A.; Dwivedi, A.; Upadhyayula, S. Volume 2: Handbook of Greener Synthesis of Nanomaterials and Compounds. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Xu, Z. A review of current progress of supercritical fluid technologies for e-waste treatment. J. Clean. Prod. 2019, 227, 794–809. [Google Scholar] [CrossRef]
- Lin, F.; Liu, D.; Das, S.M.; Prempeh, N.; Hua, Y.; Lu, J. Recent progress in heavy metal extraction by supercritical CO2 fluids. Ind. Eng. Chem. Res. 2014, 53, 1866–1877. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Machado, C.M.; Silva, M.L.; Calgaro, C.O.; Dotto, G.L.; Tanabe, E.H. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction. Waste Manag. 2016, 51, 245–251. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, D.; Dai, Y.; Ma, Q.; Zheng, R.; Dai, C. Analysis on extraction behaviour of lithium-ion battery electrolyte solvents in supercritical CO2 by gas chromatography. Int. J. Electrochem. Sci. 2016, 11, 7594–7604. [Google Scholar] [CrossRef]
- Grützke, M.; Kraft, V.; Weber, W.; Wendt, C.; Friesen, A.; Kramer, S.; Winter, M.; Nowak, S. Supercritical carbon dioxide extraction of lithium-ion battery electrolytes. J. Supercrit. Fluids 2014, 94, 216–222. [Google Scholar] [CrossRef]
- Fu, Y.; Schuster, J.; Petranikova, M.; Ebin, B. Innovative recycling of organic binders from electric vehicle lithium-ion batteries by supercritical carbon dioxide extraction. Resour. Conserv. Recycle. 2021, 172, 105666. [Google Scholar] [CrossRef]
- Toma, H.E. Developing nanotechnological strategies for green industrial processes. Pure Appl. Chem. 2013, 85, 1655–1669. [Google Scholar] [CrossRef]
- Toma, H.E. Magnetic nanohydrometallurgy: A nanotechnological approach to elemental sustainability. Green Chem. 2015, 17, 2027–2041. [Google Scholar] [CrossRef]
- Condotti, U.; Almeida, S.N.; Silveira, A.T.; De Melo, F.M.; Toma, H.E. Green processing of strategic elements based on magnetic nanohydrometallurgy. J. Braz. Chem. Soc. 2018, 29, 948–959. [Google Scholar] [CrossRef]
- Dhiman, V.; Kondal, N. ZnO Nanoadsorbents: A potent material for removal of heavy metal ions from wastewater. Colloids Interface Sci. Commun. 2021, 41, 100380. [Google Scholar] [CrossRef]
- Le, A.T.; Pung, S.Y.; Sreekantan, S.; Matsuda, A.; Huynh, D.P. Mechanisms of removal of heavy metal ions by ZnO particles. Heliyon 2019, 5, e01440. [Google Scholar] [CrossRef] [Green Version]
- Melo, F.M.; Silveira, A.T.; Quartarolli, L.F.; Kaid, F.F.; Cornejo, D.R.; Toma, H.E. Magnetic behavior of superparamagnetic nanoparticles containing chelated transition metal ions. J. Magn. Magn. Mater. 2019, 487, 165324. [Google Scholar] [CrossRef]
- Lobato, N.C.C.; de Mello Ferreira, A.; Weidler, P.G.; Franzreb, M.; Mansur, M.B. Improvement of magnetic solvent extraction using functionalized silica-coated Fe3O4 nanoparticles. Sep. Purif. Technol. 2019, 229, 115839. [Google Scholar] [CrossRef]
- Lobato, N.C.C.; Ferreira, Â.D.M.; Mansur, M.B. Evaluation of magnetic nanoparticles coated by oleic acid applied to solvent extraction processes. Sep. Purif. Technol. 2016, 168, 93–100. [Google Scholar] [CrossRef]
- Lobato, N.C.C.; Mansur, M.B.; De Mello Ferreira, A. Characterization and chemical stability of hydrophilic and hydrophobic magnetic nanoparticles. Mater. Res. 2017, 20, 736–746. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, A.; Baran, M.F.; Acay, H. Kinetic and isotherm investigation into the removal of heavy metals using a fungal-extract-based bio-nanosorbent. Environ. Technol. Innov. 2020, 20, 101076. [Google Scholar] [CrossRef]
- Matar, W.; Boufi, S. Poly(methacrylic acid-co-maleic acid) grafted nanofibrillated cellulose as a reusable novel heavy metal ions adsorbent. Carbohydr. Polym. 2015, 126, 199–207. [Google Scholar] [CrossRef]
- He, K.; Zhang, Z.Y.; Zhang, F.S. Synthesis of graphene and recovery of lithium from lithiated graphite of spent Li-ion battery. Waste Manag. 2021, 124, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Bozo, L.; Godoy-Faúndez, A.; Herrera-Urbina, R.; Higueras, P.; Salazar, J.L.; Valdés-González, H.; Vyhmeister, E.; Antizar-Ladislao, B. Greening chilean copper mining operations through industrial ecology strategies. J. Clean. Prod. 2014, 84, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Silvas, F.P.C.; Jiménez Correa, M.M.; Caldas, M.P.K.; de Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Printed circuit board recycling: Physical processing and copper extraction by selective leaching. Waste Manag. 2015, 46, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Yamane, L.H.; de Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Comparative assessment of metallurgical recovery of metals from electronic waste with special emphasis on bioleaching. Environ. Sci. Pollut. Res. 2017, 24, 6989–7008. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Tucker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterisation. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Uchimura, S.K.; Arevalo, S.J.; Rosario, C.G.A.; Aguilar, M.Q.; Tenório, J.A.S.; Espinosa, D.C.R. Bioleaching of metal from waste stream using a native strain of Acidithiobacillus isolated from a coal mine drainage. Can. J. Chem. Eng. 2019, 97, 2920–2927. [Google Scholar] [CrossRef]
- Utimura, S.K.; Rosario, C.G.A.; Botelho, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Bioleaching process for metal recovery from waste materials. In Minerals, Metals and Materials Series; Springer: Cham, Switzerland, 2017; pp. 283–290. [Google Scholar]
- Kaksonen, A.H.; Deng, X.; Bohu, T.; Zea, L.; Khaleque, H.N.; Gumulya, Y.; Boxall, N.J.; Morris, C.; Cheng, K.Y. Prospective directions for biohydrometallurgy. Hydrometallurgy 2020, 195, 105376. [Google Scholar] [CrossRef]
- Komnitsas, K.A. Recent Advances in Hydro- and Biohydrometallurgy; MDPI: Basel, Switzerland, 2019; ISBN 9783039212996. [Google Scholar]
- Roy, J.J.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.J.; Ralph, D.E.; Ahn, J.G.; Rhee, Y.H. Bioleaching of metals from spent lithium-ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Manag. 2008, 28, 333–338. [Google Scholar] [CrossRef]
- Niu, Z.; Zou, Y.; Xin, B.; Chen, S.; Liu, C.; Li, Y. Process controls for improving bioleaching performance of both Li and Co from spent lithium-ion batteries at high pulp density and its thermodynamics and kinetics exploration. Chemosphere 2014, 109, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ijadi Bajestani, M.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of heavy metals from spent household batteries using Acidithiobacillus ferrooxidans: Statistical evaluation and optimization. Sep. Purif. Technol. 2014, 132, 309–316. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery. J. Clean. Prod. 2016, 116, 249–258. [Google Scholar] [CrossRef]
- Roy, J.J.; Madhavi, S.; Cao, B. Metal extraction from spent lithium-ion batteries (LIBs) at high pulp density by environmentally friendly bioleaching process. J. Clean. Prod. 2021, 280, 124242. [Google Scholar] [CrossRef]
- Chen, X.; Luo, C.; Zhang, J.; Kong, J.; Zhou, T. Sustainable Recovery of Metals from Spent Lithium-Ion Batteries: A Green Process. ACS Sustain. Chem. Eng. 2015, 3, 3104–3113. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag. Res. 2014, 32, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M.; Baniasadi, M. Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. J. Clean. Prod. 2018, 197, 1546–1557. [Google Scholar] [CrossRef]
- Docker, T.; Pant, D. Chemical-biological hybrid systems for the metal recovery from waste lithium-ion battery. J. Environ. Manag. 2019, 248, 109270. [Google Scholar] [CrossRef]
- Stopic, S.; Friedrich, B. Recovery of cobalt from primary and secondary materials: An overview. Mil. Tech. Cour. 2020, 68, 321–327. [Google Scholar]



| References | Li-Ion Battery Type | Leaching Agents | Conditions | Co Leaching Efficiency |
|---|---|---|---|---|
| Musariri et al. (2019) [57] | NMC | Leaching agents: citric acid and DL-malic acid Reducing agents: H2O2 | 1.5 M citric acid, 2% v/v, 30 min, 95 °C; 1.0 M DL-malic acid, 2% v/v, 30 min, 95 °C. | Citric acid: 95%; DL-malic acid: 98% |
| Esmaeili et al. (2020) [58] | NMC | Assisted by ultrasound: 37 kHz Leaching agent: lemon juice (citric acid = 90 mg/g, malic acid = 0.86 mg/g, and ascorbic acid 1.24 mg/g); Reducing agent: H2O2 | S/L ratio = 0.98% (w/v); 57.8% v/v lemon juice; 8.1% v/v H2O2 | 96% |
| Sun et al. (2018) [62] | NMC (111) | Leaching agent: DL-malic Reducing agent: H2O2 | S/L ratio = 40 g/L; 1.2 M DL-malic; 1.5% v/v H2O2; 30 min 80 °C. | 94.3% |
| Urias et al. (2020) [56] | LCO | Leaching agents: H2SO4, lactic, butyric, acetic and propionic; Reducing agents: H2O2 (6%, v/v), and glucose (0.09 mol/L) and lactose (0.09 mol/L) | S/L ratio = 20 g/L; 1.25 M H2SO4 + organic acids (from a fermentation effluent by an anaerobic microbial consortium) 0.75 M; 18.5 g/L; 300 rpm and 0.09 M lactose from MWP; 86 °C | 93.4% |
| Golmohammadzadeh et al. (2017) [63] | LCO | Assisted by ultrasound Leaching agent: acetic acid Reducing agent: H2O2 | S/L ratio ratio = 30 g/L, citric acid = 2 M, 1.25% v/v H2O2; 2 h; 60 °C | 81% |
| Nayaka et al. (2016a) [60] | LCO | Leaching agent: glycine Reducing agent: ascorbic acid | S/L ratio = 0.2 g LiCoO2/100 mL; 0.5 M glycine; 0.02 M ascorbic acid; 360 min; 80 °C | 95% |
| Nayaka et al. (2016b) [64] | LCO | Leaching agent: tartaric acid Reducing agent: ascorbic acid | S/L ratio = 0.2 g LiCoO2/100 mL; 0.4 M tartaric acid; 0.02 M ascorbic acid; 5 h; 80 °C | >95% |
| Ionic Liquid | Chemical Structure |
|---|---|
| Alkylammonium | 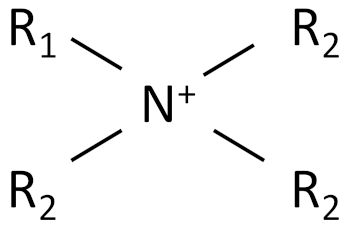 |
| Phosphonium | 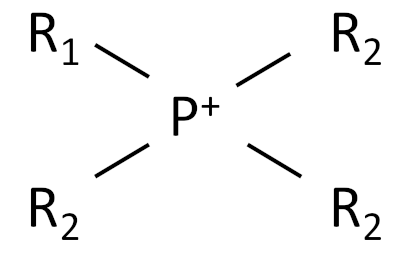 |
| Dialkylimidazolium |  |
| N-alkyl pyridinium | 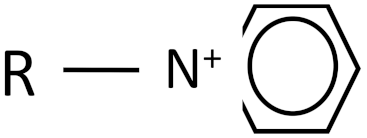 |
| Trifluoroacetate | 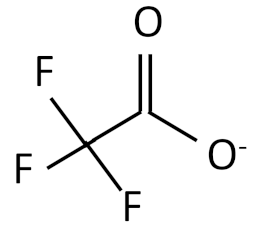 |
| Trifluorosulfonate | 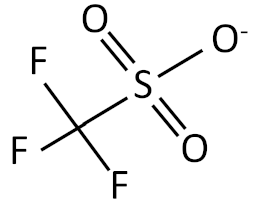 |
| Tetrafluoroborate |  |
| Hexafluorophosphate | 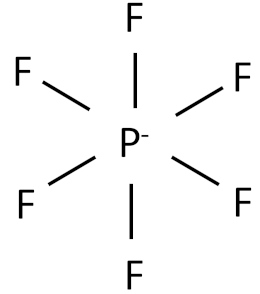 |
| Bis(trifluoromethanesulfonyl)imide | 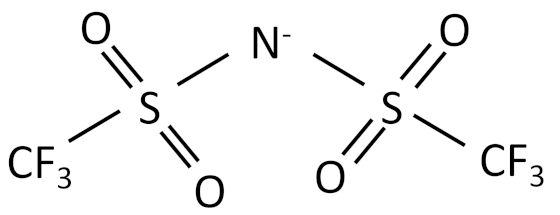 |
| Halides: chloride, bromide, iodide | Cl-, Br-, I- |
| Nitrate, hydrogensulfate, sulfate | [NO3]-, [HSO4]-, [SO4]−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botelho Junior, A.B.; Stopic, S.; Friedrich, B.; Tenório, J.A.S.; Espinosa, D.C.R. Cobalt Recovery from Li-Ion Battery Recycling: A Critical Review. Metals 2021, 11, 1999. https://doi.org/10.3390/met11121999
Botelho Junior AB, Stopic S, Friedrich B, Tenório JAS, Espinosa DCR. Cobalt Recovery from Li-Ion Battery Recycling: A Critical Review. Metals. 2021; 11(12):1999. https://doi.org/10.3390/met11121999
Chicago/Turabian StyleBotelho Junior, Amilton Barbosa, Srecko Stopic, Bernd Friedrich, Jorge Alberto Soares Tenório, and Denise Crocce Romano Espinosa. 2021. "Cobalt Recovery from Li-Ion Battery Recycling: A Critical Review" Metals 11, no. 12: 1999. https://doi.org/10.3390/met11121999
APA StyleBotelho Junior, A. B., Stopic, S., Friedrich, B., Tenório, J. A. S., & Espinosa, D. C. R. (2021). Cobalt Recovery from Li-Ion Battery Recycling: A Critical Review. Metals, 11(12), 1999. https://doi.org/10.3390/met11121999











