Environmentally Assisted Cracking Initiation in High-Temperature Water
Abstract
:1. Introduction
2. Materials
2.1. Low-Alloy and Carbon Steels
2.2. Austenitic Stainless Steels
2.3. Ni-Based Alloys
3. High-Temperature Water Environment
3.1. Oxidizing Environment
3.2. Reducing Environment
4. EAC Initiation: Understanding the Mechanism
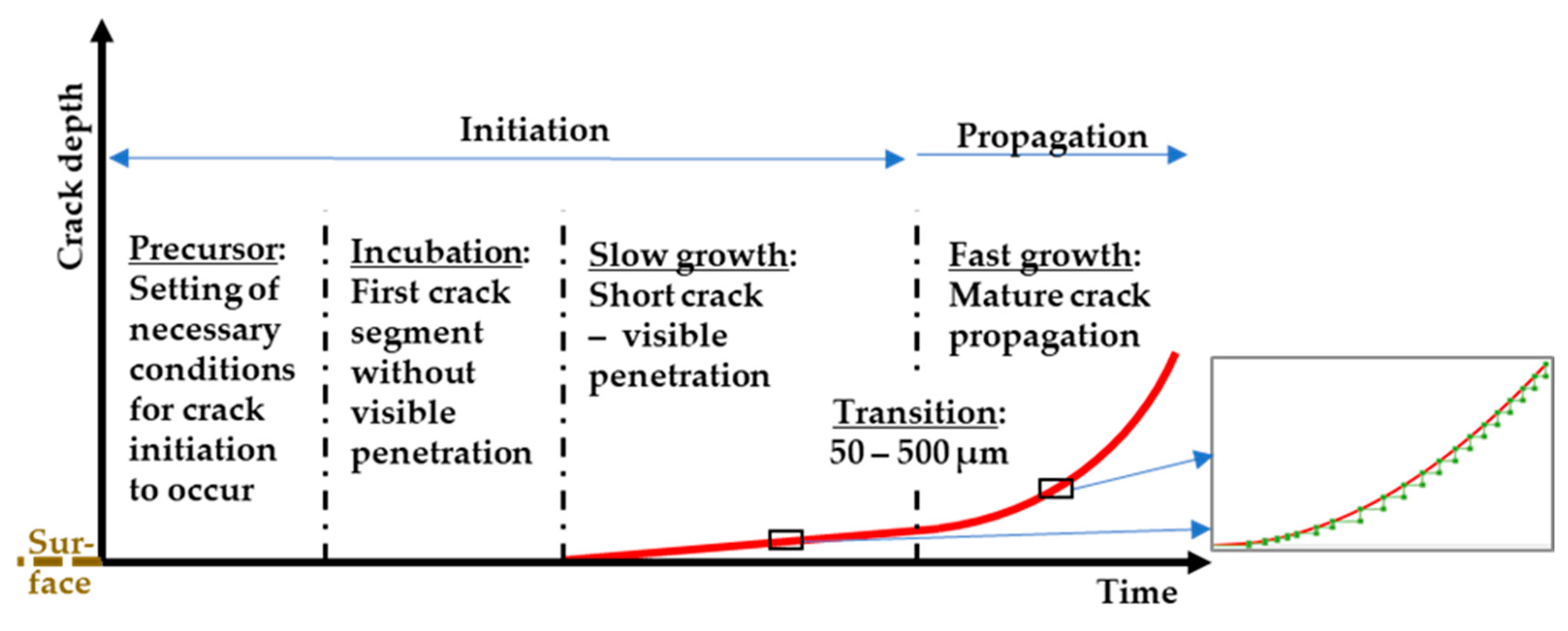
4.1. Low-Alloy and Carbon Steels
4.1.1. Brief Characteristics of the Corrosion System
4.1.2. Description of EAC in the Corrosion System
4.1.3. EAC Initiation Mechanism
4.2. Austenitic Stainless Steels
4.2.1. Brief Characteristics of the Corrosion System
4.2.2. Description of EAC in the Corrosion System
4.2.3. EAC Initiation Mechanism
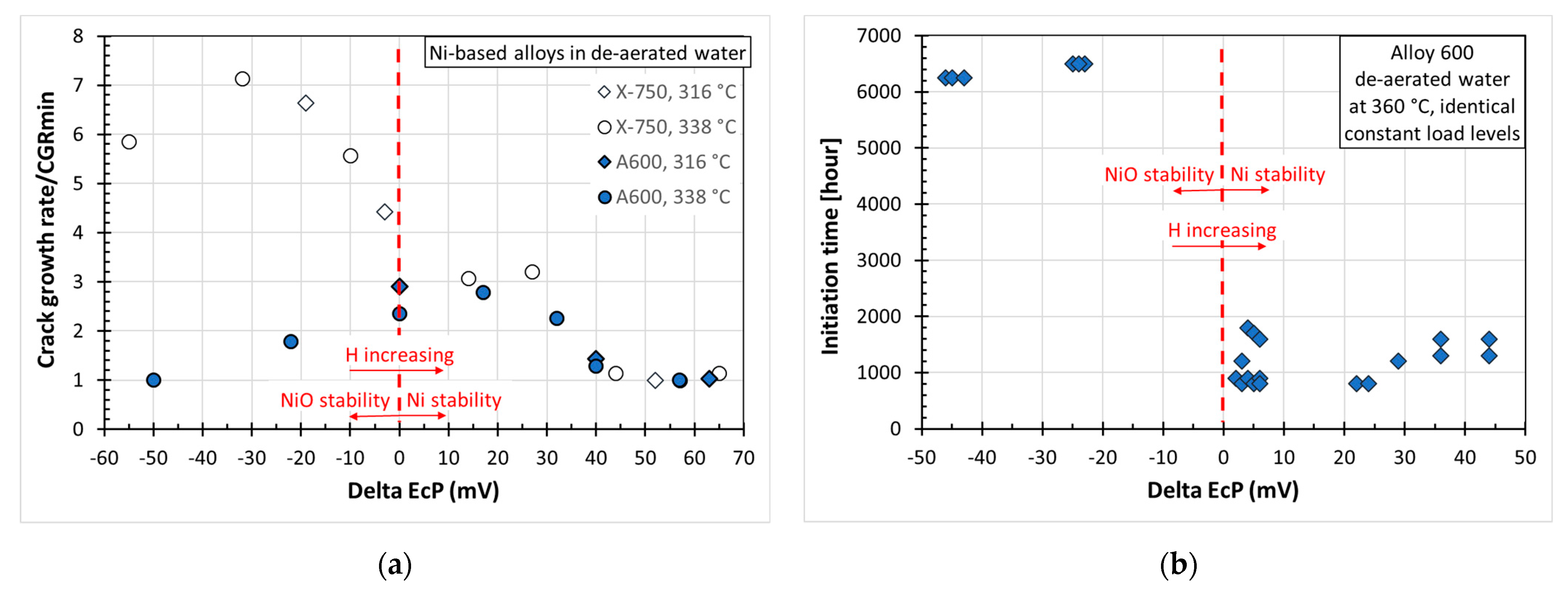
4.3. Ni-Based Alloys
4.3.1. Brief Characteristics of the Corrosion System
4.3.2. Description of EAC in the Corrosion System
4.3.3. EAC Initiation Mechanism
5. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- de Curières, I. Corrosion in PWR Stainless Steel Components: A TSO perspective based on Operating Experience and Expertises. In Proceedings of the International Symposium Fontevraud 8, Avignon, France, 14–18 September 2014. [Google Scholar]
- Ilevbare, G.O.; Cattant, F.; Peat, N.K. EAC of stainless steels under PWR service conditions. In Proceedings of the International Symposium Fontevraud 7, Avignon, France, 26–30 September 2010. [Google Scholar]
- Andresen, P.L. A Brief History of Environmental Cracking in Hot Water. Corrosion 2019, 75, 240–253. [Google Scholar] [CrossRef]
- Konings, R.J.M. Material Performance and Corrosion/Waste Materials. In Comprehensive Nuclear Materials; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-08-056033-5. [Google Scholar]
- Raja, V.S.; Shoji, T. Stress Corrosion Cracking: Theory and Practice; Woodhead Publishing Limited: Cambridge, UK, 2011; ISBN 978-0-85709-376-9. [Google Scholar]
- Turnbull, A.; Mingard, K.; Lord, J.D.; Roebuck, B.; Tice, D.R.; Mottershead, K.J.; Fairweather, N.D.; Bradbury, A.K. Sensitivity of stress corrosion cracking of stainless steel to surface machining and grinding procedure. Corros. Sci. 2011, 53, 3398–3415. [Google Scholar] [CrossRef]
- Chang, L.; Burke, M.G.; Scenini, F. Stress corrosion crack initiation in machined type 316L austenitic stainless steel in simulated pressurized water reactor primary water. Corros. Sci. 2018, 138, 54–65. [Google Scholar] [CrossRef]
- Chang, L.; Volpe, L.; Wang, Y.L.; Burke, M.G.; Maurotto, A.; Tice, D.; Lozano-Perez, S.; Scenini, F. Effect of machining on stress corrosion crack initiation in warm-forged type 304L stainless steel in high temperature water. Acta Mater. 2019, 165, 203–214. [Google Scholar] [CrossRef]
- Abe, H.; Watanabe, Y.; Miyazaki, T. Characteristics of Work Hardened Surface Layer on Austenitic Stainless Steels and Its Relation to EAC Susceptibility in High Temperature Water. In Proceedings of the 16th International Symposium on Environmental Degradation of Materials in Nuclear Power System-Water Reactors, Boston, MA, USA, 18–22 August 2019. [Google Scholar]
- Chopra, O.K.; Rao, A.S. A Review of Irradiation Effects on LWR Core Internal Materials-IASCC Susceptibility and Crack Growth Rates of Austenitic Stainless Steels. J. Nucl. Mater. 2011, 409, 235–256. [Google Scholar] [CrossRef]
- Scott, P.M.; Combrade, P. On the Mechanism of Stress Corrosion Crack Initiation and Growth in Alloy 600 Exposed to PWR Primary Water. In Proceedings of the 11th International Symposium on Environmental Degradation of Materials in Nuclear Power System-Water Reactors, Stevenson, WA, USA, 10–14 August 2003. [Google Scholar]
- Couvant, T.; Legras, L.; Pokor, C.; Vaillant, F.; Brechet, Y.; Boursier, J.M.; Moulart, P. Investigations on The Mechanisms Of PWSCC Of Strain Hardened Austenitic Stainless Steels. In Proceedings of the 13th International Conference on Environmental Degradation of Materials in Nuclear Power Systems, Whistler, BC, Canada, 19–23 August 2007. [Google Scholar]
- Ford, F.P. Mechanisms of environmentally-assisted cracking. Int. J. Press. Vessels Pip. 1989, 40, 343–362. [Google Scholar] [CrossRef]
- Parkins, R.N. Metallography of environment sensitive fracture. Mater. Charact. 1991, 26, 303–323. [Google Scholar] [CrossRef]
- Magnin, T.; Chieragatti, R.; Oltra, R. Mechanism of brittle fracture in a ductile 316 alloy during stress corrosion. Acta Metall. Mater. 1990, 38, 1313–1319. [Google Scholar] [CrossRef]
- Shoji, T. Progress in the Mechanistic Understanding of BWR EAC and Its Implication to the Prediction of EAC Growth Behavior in Plants. In Proceedings of the 11th International Conference on Environmental Degradation of Materials in Nuclear Systems, Stevenson, WA, USA, 10–14 August 2003. [Google Scholar]
- Shoji, T.; Lu, Z.; Murakami, H. Formulating stress corrosion cracking growth rates by combination of crack tip mechanics and crack tip oxidation kinetics. Corros. Sci. 2010, 52, 769–779. [Google Scholar] [CrossRef]
- Lynch, S.P. Environmental assisted cracking: Overview of evidence for an adsorption-induced localised-slip process. Acta Metall. 1988, 36, 2639–2661. [Google Scholar] [CrossRef]
- Lynch, S.P. Chap.1: Mechanistic and Fractographic Aspects of Stress-Corrosion Cracking. In Stress Corrosion Cracking: Theory and Practice; Woodhead Publishing Limited: Cambridge, UK, 2011. [Google Scholar]
- Staehle, R.W. Introduction to initiation. In Proceedings of the Workshop on Detection, Avoidance, Mechanisms, Modelling, and Prediction of SCC Initiation in Water-Cooled Nuclear Reactor Plants, Beaune, France, 7–12 September 2008. [Google Scholar]
- Zhai, Z.; Toloczko, M.B.; Olszta, M.J.; Bruemmer, S.M. Stress corrosion crack initiation of alloy 600 in PWR primary water. Corros. Sci. 2017, 123, 76–87. [Google Scholar] [CrossRef]
- Seifert, H.P.; Hickling, J.; Lister, D. Corrosion and Environmentally-Assisted Cracking of Carbon and Low-Alloy Steels. In Comprehensive Nuclear Materials, Konings, R.J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; p. 110. ISBN 978-0-08-056033-5. [Google Scholar]
- Arioka, K. Role of Cavity Formation on Long-Term Stress Corrosion Cracking Initiation: A Review. Corrosion 2020, 76, 142–175. [Google Scholar] [CrossRef]
- Seifert, H.P.; Ritter, S.; Hickling, J. Environmentally-Assisted Cracking of Low-Alloy RPV and Piping Steels under LWR Conditions. In Proceedings of the 11th Int. Conf. Environmental Degradation of Materials in Nuclear Systems, Stevenson, WA, USA, 10–14 August 2003. [Google Scholar]
- Seifert, H.P.; Ritter, S. Stress corrosion cracking of low-alloy reactor pressure vessel steels under boiling water reactor conditions. J. Nucl. Mater. 2008, 372, 114–131. [Google Scholar] [CrossRef]
- Seifert, H.P.; Ritter, S. Strain-induced corrosion cracking behaviour of low-alloy steels under boiling water reactor conditions. J. Nucl. Mater. 2008, 378, 312–326. [Google Scholar] [CrossRef]
- Roth, A.; Devrient, B.; Gómez-Briceño, D.; Lapeña, J.; Ernestová, M.; Žamboch, M.; Ehrnstén, U.; Föhl, J.; Weißenberg, T.; Seifert, H.-P.; et al. The Effect of Transients on the Crack Growth Behavior of Low Alloy Steels for Pressure Boundary Components Under Light Water Reactor Operating Conditions-CASTOC. In Proceedings of the 12th International Conference on Environmental Degradation of Materials in Nuclear Power System-Water Reactors, Salt Lake City, UT, USA, 14–18 August 2005. [Google Scholar]
- Arioka, K.; Miyamoto, T.; Yamada, T.; Terachi, T. Formation of Cavities Prior to Crack Initiation and Growth on Cold-Worked Carbon Steel in High-Temperature Water. Corrosion 2010, 66, 015008–015008-14. [Google Scholar] [CrossRef]
- Arioka, K.; Miyamoto, T.; Yamada, T.; Aoki, M. Role of Cavity Formation in Crack Initiation of Cold-Worked Carbon Steel in High-Temperature Water. Corrosion 2013, 69, 487–496. [Google Scholar] [CrossRef]
- Arioka, K. 2014 W.R. Whitney Award Lecture: Change in Bonding Strength at Grain Boundaries Before Long-Term SCC Initiation. Corrosion 2015, 71, 403–419. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Lo, Y.-C.; Neeraj, T.; Srinivasan, R.; Ding, X.; Sun, J.; Qi, L.; Gumbsch, P.; Li, J. The interaction of dislocations and hydrogen-vacancy complexes and its importance for deformation-induced proto nano-voids formation in α-Fe. Int. J. Plast. 2015, 74, 175–191. [Google Scholar] [CrossRef]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-enhanced localized plasticity-a mechanism for hydrogen-related fracture. Mater. Sci. Eng. 1994, A176, 191–202. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Huang, M.; Fan, H. Study on interactions of an edge dislocation with vacancy-H complex by atomistic modelling. Int. J. Plast. 2017, 92, 31–44. [Google Scholar] [CrossRef]
- Magnin, T. The corrosion enhanced plasticity model for SCC in ductile fcc alloys. In Advances in Corrosion-Deformation Interactions (Material Science Forum); Trans Tech Publications Ltd.: Zurich, Switzerland, 1996; pp. 113–117. [Google Scholar]
- Terachi, T.; Fujii, K.; Arioka, K. Microstructural characterization of SCC crack tip and oxide film for SUS 316 stainless steel in simulated PWR primary water at 320 °C. J. Nucl. Sci. Tech. 2005, 42, 225–232. [Google Scholar] [CrossRef]
- Terachi, T.; Yamada, T.; Miyamoto, T.; Arioka, K.; Fukuya, K. Corrosion Behavior of Stainless Steels in Simulated PWR Primary Water—Effect of Chromium Content in Alloys and Dissolved Hydrogen. J. Nucl. Sci. Technol. 2008, 45, 975–984. [Google Scholar] [CrossRef]
- Cissé, S.; Laffont, L.; Tanguy, B.; Lafont, M.-C.; Andrieu, E. Effect of surface preparation on the corrosion of austenitic stainless steel 304L in high temperature steam and simulated PWR primary water. Corros. Sci. 2012, 56, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Matthews, R.P.; Knusten, R.D.; Westraadt, J.E.; Couvant, T. Intergranular Oxidation of 316L Stainless Steel in the PWR Primary Water Environment. Corros. Sci. 2017, 125, 175–183. [Google Scholar] [CrossRef]
- Arioka, K.; Iijima, Y.; Miyamoto, M. Acceleration of nickel diffusion by high tensile stress in cold-worked type 316 stainless steel at 450 °C. Phil. Mag. 2018, 98, 2609–2617. [Google Scholar] [CrossRef]
- Kruska, K.; Lozano-Perez, S.; Saxey, D.W.; Terachi, T.; Yamada, T.; Smith, G.D.W. Nanoscale characterisation of grain boundary oxidation in cold-worked stainless steels. Corros. Sci. 2012, 63, 225–233. [Google Scholar] [CrossRef]
- Takazaki, D.; Tsuchiyama, T.; Komoda, R.; Dadfarnia, M.; Somerday, B.P.; Sofronis, P.; Kubota, M. Effect of Hydrogen on Creep Properties of SUS304 Austenitic Stainless Steel. Corrosion 2021, 177, 3678. [Google Scholar]
- Devrient, B.; Kilian, R.; Kuster, K.; Widera, M. Influence of Bulk and Surface Cold Work on Crack Initiation and Crack Growth of Austenitic Stainless Steels under Simulated BWR Environment. In Proceedings of the 15th Int. Conf. on Environmental Degradation of Materials in Nuclear Power System, Colorado Springs, CO, USA, 19–23 August 2011. [Google Scholar]
- Couvant, T.; Legras, L.; Herbelin, A.; Musienko, A.; Ilevbare, G.; Delafosse, D.; Cailletaud, G.; Hickling, J. Development of Understanding of the Interaction between Localized Deformation and EAC of Austenitic Stainless Steels Exposed to Primary PWR Environment. In Proceedings of the 14th International Conference on Environmental Degradation of Materials in Nuclear Power Systems, Virginia Beach, VA, USA, 23–27 August 2009. [Google Scholar]
- Huang, Q.; Charles, Y.; Duhamel, C.; Gaspérini, M.; Crépin, J. Influence of the Combination of Microstructure and Mechanical Fields on Stress Corrosion Cracking Initiation of Cold-Worked Austenitic Stainless Steels. In Proceedings of the 19th International Conference on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, Boston, MA, USA, 18–22 August 2019. [Google Scholar]
- Hojná, A.; Halodová, P.; Janoušek, J.; Zimina, M. Accelerated Environmentally assisted cracking initiation of Type 316L steel in high temperature water and hydrogenated steam vapor environments. Corrosion 2020, 76, 1177–1193. [Google Scholar] [CrossRef]
- Karlsen, W.; Diego, G.; Devrient, B. Localized deformation as a key precursor to initiation of intergranular stress corrosion cracking of austenitic stainless steels employed in nuclear power plants. J. Nucl. Mater. 2010, 406, 138–151. [Google Scholar] [CrossRef]
- Couvant, T.; Burger, E.; Thaury, C.; Rainasse, C. Simulating the Susceptibility to IGEAC of Cold Work 316 Austenitic Stainless Steel Exposed to Primary Water. In Proceedings of the 19th International Conference on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, Boston, MA, USA, 18–22 August 2019. [Google Scholar]
- Panter, J.; Viguier, B.; Cloué, J.M.; Foucault, M.; Combrade, P.; Andrieu, E. Influence of oxide films on primary water stress corrosion cracking initiation of alloy 600. J. Nucl. Mater. 2006, 348, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Kuang, W.; Song, M.; Wang, P.; Was, G.S. The oxidation of alloy 690 in simulated pressurized water reactor primary water. Corros. Sci. 2017, 126, 227–237. [Google Scholar] [CrossRef]
- Peng, Q.; Hou, J.; Sakaguchi, K.; Takeda, Y.; Shoji, T. Effect of dissolved hydrogen on corrosion of Inconel Alloy 600 in high temperature hydrogenated water. Electrochim. Acta 2011, 56, 8375–8386. [Google Scholar]
- Fournier, L.; Calonne, O.; Combrade, P.; Scott, P.; Chou, P.; Pathania, R. Grain boundary oxidation and embrittlement prior to crack initiation in Alloy 600 in PWR primary water. In Proceedings of the 15th International Conference on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, Colorado Springs, CO, USA, 7–11 August 2011. [Google Scholar]
- Kim, T.; Choi, K.J.; Yoo, S.C.; Kim, J.H. Effects of dissolved hydrogen on the crack-initiation and oxidation behavior of nickel-based alloys in high-temperature water. Corros. Sci. 2016, 106, 260–270. [Google Scholar] [CrossRef]
- Bertali, G.; Scenini, F.; Burke, M.G.; Huin, N. The effect of temperature on the preferential intergranular oxidation susceptibility of alloy 600. Metall. Mater. Trans. A 2018, 49, 1879–1894. [Google Scholar] [CrossRef] [Green Version]
- Capell, B.M.; Was, G.S. Selective Internal Oxidation as a Mechanism of Intergranular Stress Corrosion cracking of Ni-Cr-Fe alloys. Metall. Mater. Trans. A 2007, 38, 1244–1259. [Google Scholar] [CrossRef]
- Lim, Y.S.; Kim, S.W.; Hwang, S.S.; Kim, H.P.; Jang, C. Intergranular oxidation of Ni-based Alloy 600 in a simulated PWR primary water environment. Corros. Sci. 2016, 108, 125–133. [Google Scholar] [CrossRef]
- Etien, R.A.; Richey, E.; Morton, D.S.; Eager, J. SCC Initiation Testing of Alloy 600 in High Temperature Water. In Proceedings of the 15th International Conference on Environmental Degradation of Materials in Nuclear Power System–Water Reactors, Colorado Springs, CO, USA, 19–23 August 2011. [Google Scholar]
- Attanasio, S.A.; Morton, D.S. Measurement of the Nickel/Nickel Oxide Transition in Ni-Cr-Fe Alloys and Updated Data and Correlations to Quantify the Effect of Aqueous Hydrogen on Primary Water SCC. In Proceedings of the 11th International Conference on Environmental Degradation on Materials in Nuclear Power Systems-Water Reactors, Stevenson, WA, USA, 10–14 August 2003. [Google Scholar]
- Bai, J.; Ritter, S.; Seifert, H.-P.; Virtanen, S. Stress corrosion cracking initiation and short crack growth behaviour in Alloy 182 weld metal under simulated boiling water reactor hydrogen water chemistry conditions. Corros. Sci. 2018, 131, 208–222. [Google Scholar] [CrossRef]
- Arioka, K.; Staehle, R.W.; Yamada, T.; Miyamoto, T.; Terachi, T. Degradation of Alloy 690 After Relatively Short Times. Corrosion 2016, 72, 1252–1268. [Google Scholar] [CrossRef]
- Zhai, Z.; Olszta, M.J.; Toloczko, M.B.; Bruemmer, S.M. Crack Initiation Behavior of Cold-Worked Alloy 690 in Simulated PWR Primary Water—Role of Starting Microstructure, Applied Stress and Cold Work on Precursor Damage Evolution. In Proceedings of the 16th International Symposium on Environmental Degradation of Materials in Nuclear Power System-Water Reactors, Boston, MA, USA, 18–22 August 2019. [Google Scholar]
- Zhai, Z.; Toloczko, M.; Kruska, K.; Bruemmer, S.M. Precursor Evolution and Stress Corrosion Cracking Initiation of Cold-Worked Alloy 690 in Simulated Pressurized Water Reactor Primary Water. Corrosion 2017, 73, 1224–1236. [Google Scholar] [CrossRef]
- Kuang, W.; Was, G.S. A high-resolution characterization of the initiation of stress corrosion crack in Alloy 690 in simulated pressurized water reactor primary water. Corros. Sci. 2020, 163, 108243. [Google Scholar] [CrossRef]
- Moss, T.; Kuang, W.; Was, G.S. Stress corrosion crack initiation in Alloy 690 in high temperature water. Curr. Opin. Solid State Mater. Sci. 2018, 22, 16–25. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hojná, A. Environmentally Assisted Cracking Initiation in High-Temperature Water. Metals 2021, 11, 199. https://doi.org/10.3390/met11020199
Hojná A. Environmentally Assisted Cracking Initiation in High-Temperature Water. Metals. 2021; 11(2):199. https://doi.org/10.3390/met11020199
Chicago/Turabian StyleHojná, Anna. 2021. "Environmentally Assisted Cracking Initiation in High-Temperature Water" Metals 11, no. 2: 199. https://doi.org/10.3390/met11020199
APA StyleHojná, A. (2021). Environmentally Assisted Cracking Initiation in High-Temperature Water. Metals, 11(2), 199. https://doi.org/10.3390/met11020199





