The Ultrafine-Grain Yttria-Stabilized Zirconia Reinforced β-Titanium Matrix Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- Milling time-48 h
- Ball-to-powder ratio (BPR)-10:1
- Atmosphere-argon.
2.2. Materials Characterization
- (1)
- Mechanically alloyed powders:
- Ti0.9Mo0.1—ref. code 04-018-6034
- Ti0.33Mo0.67—ref. code 04-002-9769
- Ti0.67Mo0.33—ref. code 04-017-8941
- (2)
- Sintered specimens
- Ti(α)—ref. code 04-008-4973
- Zr0.72Y0.28O1.862—ref. code 01-077-2114
- Ti0.9Mo0.1—ref. code 04-018-6034
- ➢
- Ti23Mo
- Ti0.67Mo0.33—ref. code 04-017-8941
- Ti(β)—ref. code 04-003-7297
- Ti0.857Mo0.143—ref. code 01-086-2611
- ➢
- Ti27Mo
- Ti0.67Mo0.33—ref. code 04-017-8941
- Ti(β)—ref. code 04-003-7297
- Ti0.857Mo0.143—ref. code 01-086-2611
- Y2O3—ref. code 04-002-2584
- ➢
- Ti35Mo
- Ti0.6Mo0.4—ref. code 04-020-8692
- Ti0.93Zr0.07—ref. code 04-019-4073
2.3. Nanoindentation Test and Microhardness
- Load: 300 g,
- load operating time: 10 s.
2.4. Wetting and Free Surfaces Energy Analysis
2.5. Corrosion Resistance Analysis
3. Results and Discussion
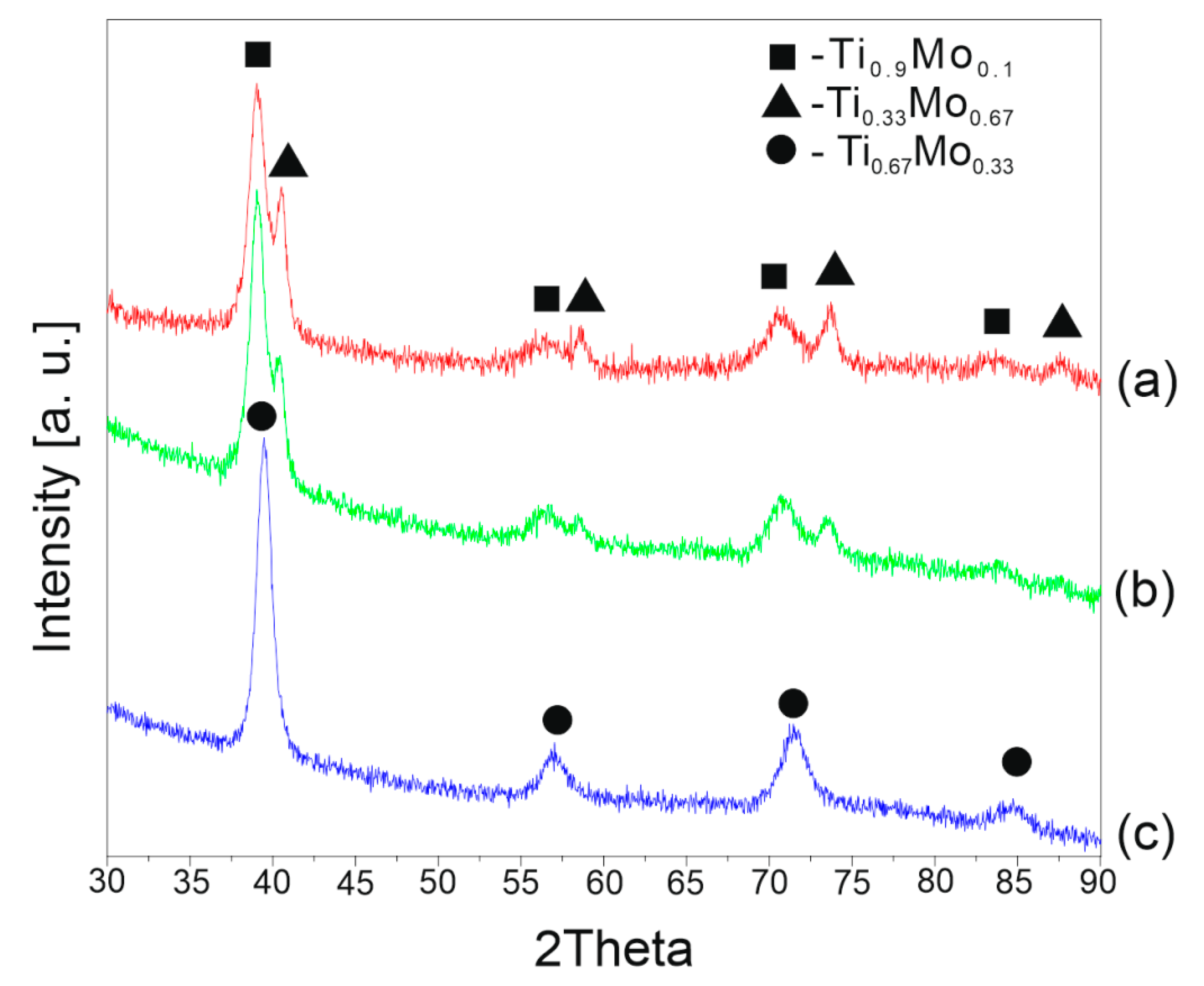
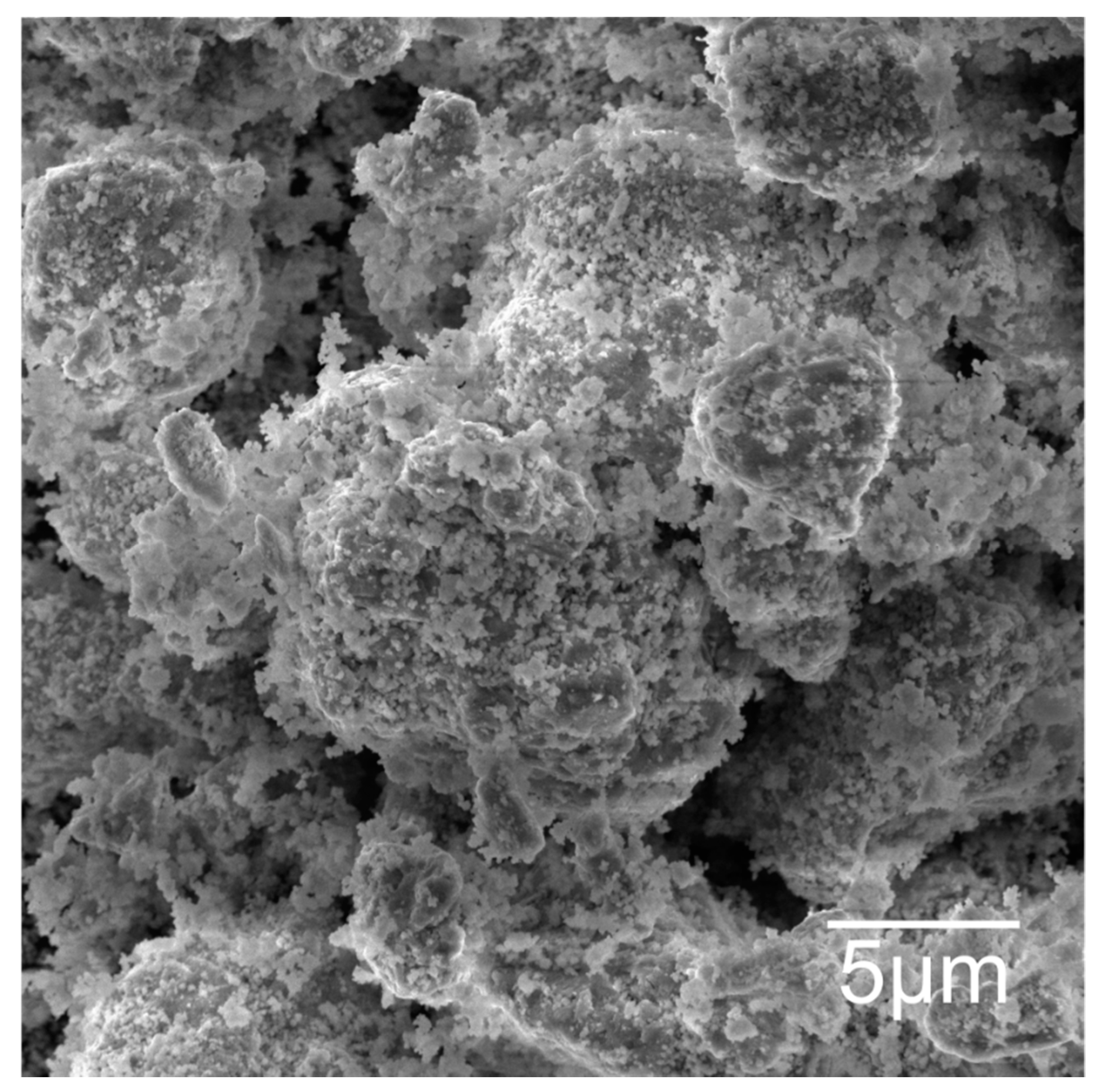
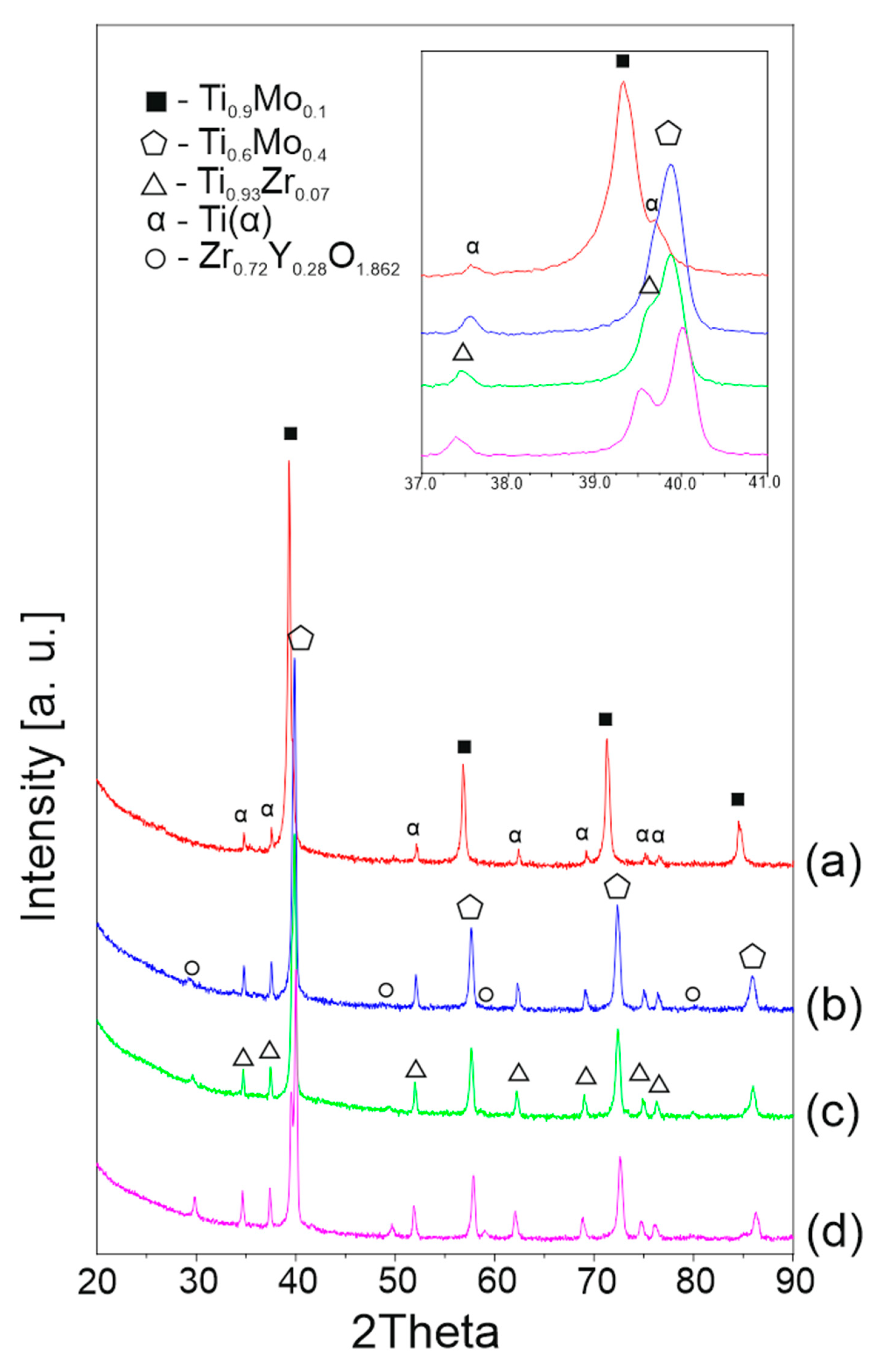
3.1. Structural and Morphological Powder Analysis
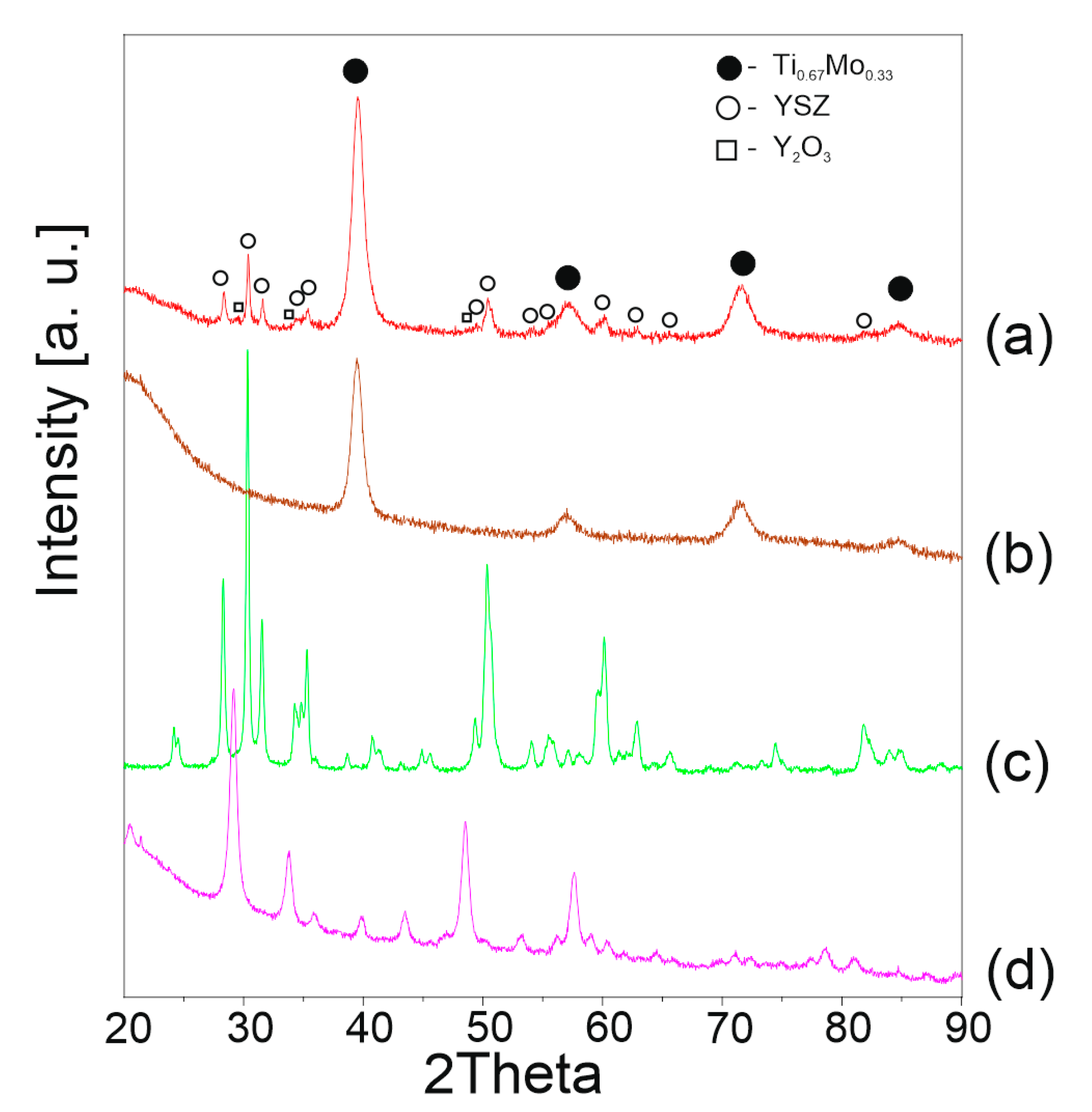
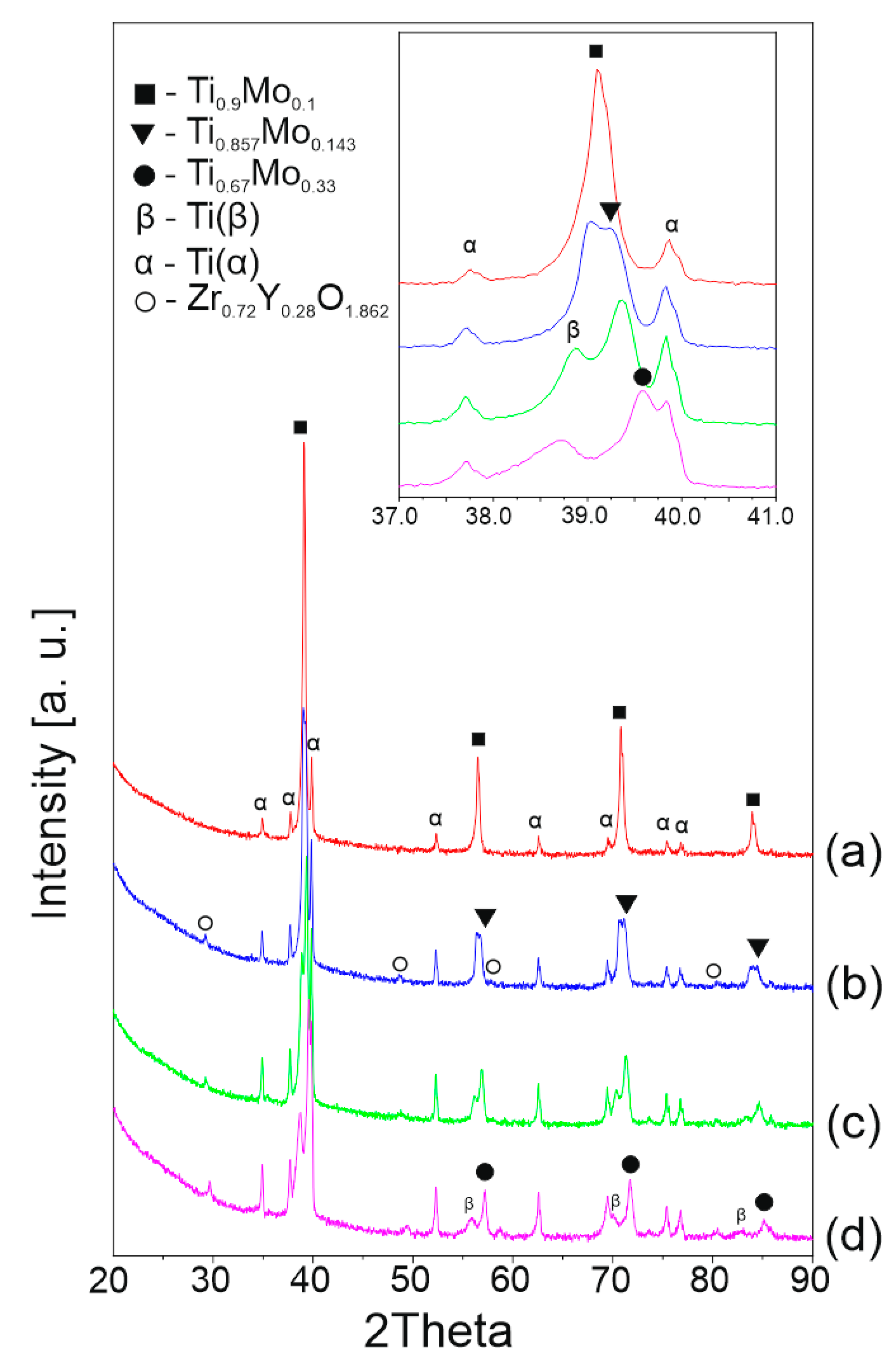
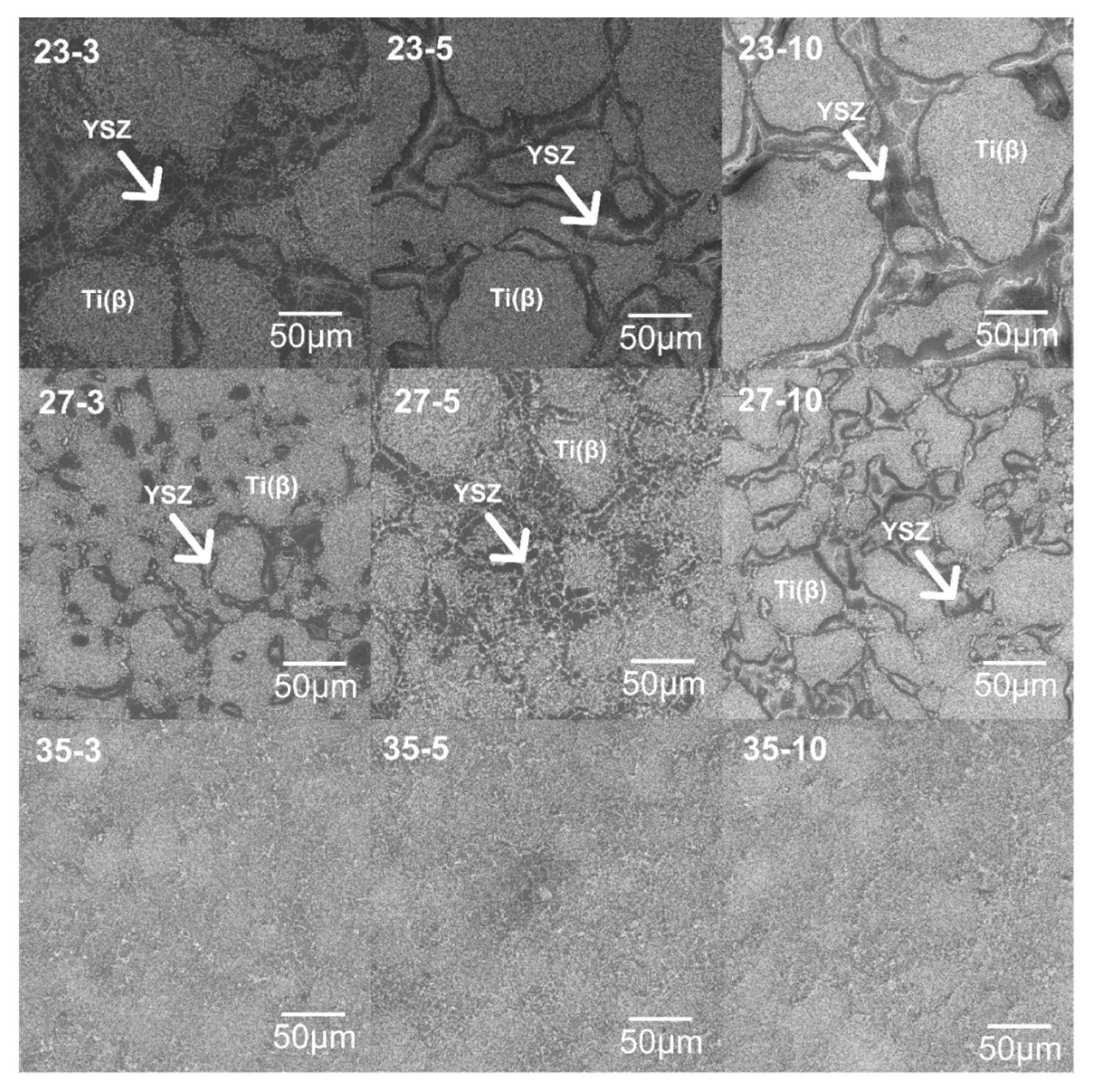
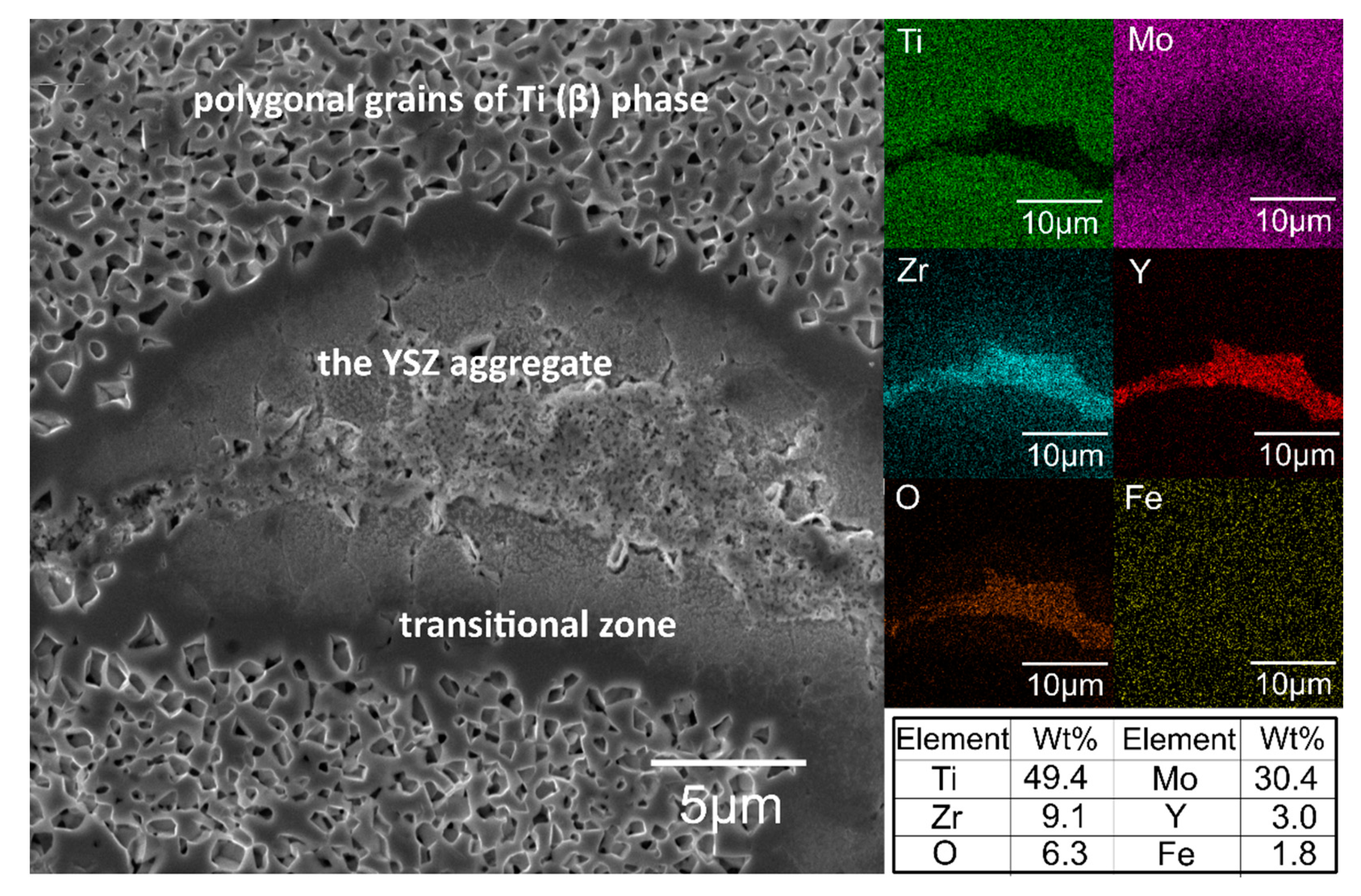
3.2. Structural and Microstructural Analysis of Bulk Composites
3.3. Nanoindentation Test and Microhardness
3.4. Surface Wettability Analysis
3.5. Surface Corrosion Resistance Analysis
4. Conclusions
- -
- the increase in the content of the YSZ reinforcement contributes to the stabilization of the Ti(α) phase for the fabrication path analyzed in this work;
- -
- the YSZ reinforcement aggregates and distribute in the areas of the grain boundaries of the mechanically alloyed particle of the matrix;
- -
- the size of the powder after synthesis has a significant influence on the dispersion of the reinforcement phase in the sintered stage and further properties characteristics;
- -
- in the boundary areas to the oxide phase, Mo diffuses towards the matrix and contribute to the formation of cubic phases with different contents of Ti and Mo;
- -
- the addition of 1 wt% Y2O3 contributes to improving the stability of the zirconium oxide during sintering;
- -
- the microstructure of the obtained composites shows an ultrafine-grain range;
- -
- the starting powder size decreases with the increase of Mo content which yields the best dispersion for composites based on Ti35Mo;
- -
- in the case of Ti35Mo composites, both the matrix and the YSZ additive remain in the ultra-fine grained range;
- -
- the YSZ addition causes Young’s modulus and hardness to increase;
- -
- all of the specimens possess a hydrophilic surface characteristic;
- -
- in the cases of Ti27Mo and Ti35Mo sets (except 35-5), the addition of an oxide reduces the corrosion current in comparison to base Ti-xMo (x = 27 wt% and 35 wt%) alloys
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new β type titanium alloys for implant materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Piechowiak, D.; Miklaszewski, A.; Jurczyk, M. Low-temperature hydrothermal treatment surface functionalization of the ultrafine-grained timo alloys for medical applications. Materials 2020, 13, 5763. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.L.; Tao, S.C.; Bao, L.Z.; Luo, J.M.; Zheng, Y.F. Effects of Mo contents on the microstructure, properties and cytocompatibility of the microwave sintered porous Ti-Mo alloys. Mater. Sci. Eng. C 2018, 97, 156–165. [Google Scholar] [CrossRef]
- Niinomi, M. Recent Research and Development in Titanium Alloys for Biomedical Applications and Healthcare Goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Eisenbarth, E.; Velten, D.; Muller, M. Biocompatibility of beta-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.R.N.; Vicente, F.B.; Araújo, R.O.; Lourenço, M.L.; Kuroda, P.A.B.; Buzalaf, M.A.R.; Grandini, C.R. Effect of the substitutional elements on the microstructure of the Ti-15Mo-Zr and Ti-15Zr-Mo systems alloys. J. Mater. Res. Technol. 2015, 4, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.D.; Liu, Y.; Wu, H.; Song, M.; Zhang, T.Y.; Lan, X.D.; Yao, T.H. Materials Characterization Elastic modulus of phases in Ti—Mo alloys. Mater. Charact. 2015, 106, 302–307. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, K.; He, S.; Wang, B.; Zhu, W.; Ren, F. Materials Science & Engineering C Fabrication of high strength, antibacterial and biocompatible Ti-5Mo-5Ag alloy for medical and surgical implant applications. Mater. Sci. Eng. C 2020, 106, 110165. [Google Scholar] [CrossRef]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M. The influence of mo content on phase transformation in Ti-Mo alloys. Arch. Metall. Mater. 2017, 62, 2051–2056. [Google Scholar] [CrossRef] [Green Version]
- Sochacka, P.; Miklaszewski, A.; Jurczyk, M. Development of b-type Ti-x at. % Mo alloys by mechanical alloying and powder metallurgy: Phase evolution and mechanical properties. J. Alloys Compd. 2019, 776, 370–378. [Google Scholar] [CrossRef]
- Hu, H.Y.; Zhang, L.; He, Z.Y.; Jiang, Y.H.; Tan, J. Microstructure evolution, mechanical properties, and enhanced bioactivity of Ti-13Nb-13Zr based calcium pyrophosphate composites for biomedical applications. Mater. Sci. Eng. C 2019, 98, 279–287. [Google Scholar] [CrossRef]
- Kumar, A.M.; Adesina, A.Y.; Hussein, M.A.; Ramakrishna, S.; Al-Aqeeli, N.; Akhtar, S.; Saravanan, S. PEDOT/FHA nanocomposite coatings on newly developed Ti-Nb-Zr implants: Biocompatibility and surface protection against corrosion and bacterial infections. Mater. Sci. Eng. C 2019, 98, 482–495. [Google Scholar] [CrossRef]
- Mareci, D.; Solcan, C.; Mircea, F.; Carmen, L.; Fern, L. New Ti-6Al-2Nb-2Ta-1Mo alloy as implant biomaterial: In vitro corrosion and in vivo osseointegration evaluations. Mater. Chem. Phys. 2020, 240, 1–10. [Google Scholar] [CrossRef]
- Cardoso, F.F.; Ferrandini, P.L.; Lopes, E.S.N.; Cremasco, A.; Caram, R. Ti-Mo alloys employed as biomaterials: Effects of composition and aging heat treatment on microstructure and mechanical behavior. J. Mech. Behav. Biomed. Mater. 2014, 32, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Selective laser melting of in situ titanium-titanium boride composites: Processing, microstructure and mechanical properties. Acta Mater 2014, 76, 13–22. [Google Scholar] [CrossRef]
- Gu, D.; Hagedorn, Y.C.; Meiners, W.; Wissenbach, K.; Poprawe, R. Nanocrystalline TiC reinforced Ti matrix bulk-form nanocomposites by Selective Laser Melting (SLM): Densification, growth mechanism and wear behavior. Compos. Sci. Technol. 2011, 71, 1612–1620. [Google Scholar] [CrossRef]
- Zhang, L.C.; Attar, H. Selective Laser Melting of Titanium Alloys and Titanium Matrix Composites for Biomedical Applications: A Review. Adv. Eng. Mater. 2016, 18, 463–475. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Zhu, X. Osteoblast cell response to nanoscale SiO2/ZrO2 particulate-reinforced titanium composites and scaffolds by powder metallurgy. J. Mater. Sci. 2012, 47, 4410–4414. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. International Journal of Adhesion & Adhesives Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Ning, C.Q.; Zhou, Y. On the microstructure of biocomposites sintered from Ti, HA and bioactive glass. Biomaterials 2004, 25, 3379–3387. [Google Scholar] [CrossRef]
- Jurczyk, K.; Jurczyk, M.U.; Niespodziana, K.; Jakubowicz, J.; Jurczyk, M. Titanium—10 wt % 45S5 Bioglass nanocomposite for biomedical applications. Mater. Chem. Phys. 2011, 131, 540–546. [Google Scholar] [CrossRef]
- Berbecaru, C.; Alexandru, H.V.; Ianculescu, A.; Popescu, A.; Socol, G.; Sima, F.; Mihailescu, I. Bioglass thin films for biomimetic implants. Appl. Surf. Sci. 2009, 255, 5476–5479. [Google Scholar] [CrossRef]
- Conrad, H.J.; Seong, W. Current ceramic materials and systems with clinical recommendations: A systematic review. J. Prothetic Dent. 2007, 98, 389–404. [Google Scholar] [CrossRef]
- Graeve, O.A. Zirconia. In Ceramic and Glass Materials: Structure, Properties and Processing; Springer: Boston, MA, USA, 2008. [Google Scholar]
- Nakonieczny, D.S.; Ziębowicz, A.; Paszenda, Z.K.; Krawczyk, C. Trends and perspectives in modification of zirconium oxide for a dental prosthetic applications—A review. Biocybern. Biomed. Eng. 2017, 37, 229–245. [Google Scholar] [CrossRef]
- del Monte, F.; Larsen, W.; Mackenzie, J.D. Chemical Interactions Promoting the ZrO2 Tetragonal Stabilization in ZrO2-SiO2 Binary Oxides. J. Am. Ceram. Soc. 2004, 83, 1506–1512. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.-W.; Penner-Hahn, J.E. Effect of Dopants on Zirconia Stabilization—An X-ray Absorption Study: II, Tetravalent Dopants. J. Am. Ceram. Soc. 1994, 77, 1281–1288. [Google Scholar] [CrossRef]
- Thammachart, M.; Meeyoo, V.; Risksomboon, T.; Osuwan, S. Catalytic activity of CeO2-ZrO2 mixed oxide catalysts prepared via sol-gel technique: CO oxidation. Catal. Today 2001, 68, 53–61. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.-W.; Penner-Hahn, J.E. Effect of Dopants on Zirconia Stabilization—An X-ray Absorption Study: I, Trivalent Dopants. J. Am. Ceram. Soc. 1994, 77, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Ostanin, S.A.; Salamatov, E.I. Microscopic mechanism of stability in yttria-doped zirconia. J. Exp. Theor. Phys. Lett. 2002, 74, 552–555. [Google Scholar] [CrossRef]
- Sari, A.; Keddam, M.; Guittoum, A. Effect of iron impurity on structural development in ball-milled ZrO2-3 mol% Y2O3. Ceram. Int. 2015, 41, 1121–1128. [Google Scholar] [CrossRef]
- Fabris, S.; Paxton, A.T.; Finnis, M.W. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002, 50, 5171–5178. [Google Scholar] [CrossRef] [Green Version]
- Ranti, S.; Eso, O.; Moses, A.; Tshephe, T.S.; Apata, P. Effect of ZrO2 addition on densification and properties of Spark plasma sintered Ti6Al4V-Ni. Mater. Today Proc. 2019, 18, 2454–2460. [Google Scholar] [CrossRef]
- Li, Y.; Liang, H.; Xie, D.; Shen, L.; Tian, Z.; Ahsan, N.; Xiao, M. Study on properties and biocompatibility of ZrO2/Ti composites prepared by selective laser melting Yize. In Proceedings of the 2020 17th International Bhurban Conference on Applied Sciences and Technology, Islamabad, Pakistan, 14–18 January 2020; pp. 5–11. [Google Scholar]
- Yehia, H.M.; El-Tantawy, A.; Ghayad, I.M.; Eldesoky, A.S.; El-kady, O. Effect of zirconia content and sintering temperature on the density, microstructure, corrosion, and biocompatibility of the Ti–12Mo matrix for dental applications. J. Mater. Res. Technol. 2020, 9, 8820–8833. [Google Scholar] [CrossRef]
- Prepared, C.; Powder, B.Y.; Method, M. Microstructure evolution of Ti/ZrO2 and Ti/Al2O3 composites prepared by powder metallurgy method. Arch. Metall. Mater. 2019, 64, 443–450. [Google Scholar] [CrossRef]
- Alloy, T.; Irimescu, R.; Raducanu, D.; Galbinasu, B.M. Improving the Mechanical Properties of a β-type Ti-Nb-Zr-Fe-O Alloy. Metals 2020, 10, 1491. [Google Scholar]
- Gentleman, M.M.; Gentleman, E. The role of surface free energy in osteoblast-biomaterial interactions. Int. Mater. Rev. 2014, 59, 417–429. [Google Scholar] [CrossRef]
- Marczewski, M.; Jurczyk, M.; Pecyna, P.; Ratajczak, M.; Gajecka, M.; Jurczyk, M.U. The Effect of 45S5 Bioglass and Ag, Cu, or Zn Addition on the Crystal Structure, Properties, and Antibacterial Effect of Bulk Ti23Zr25Nb Biocomposites. Metals 2020, 10, 1115. [Google Scholar] [CrossRef]




| Sample | Symbol |
|---|---|
| Ti23Mo | 23 |
| Ti23Mo + 3% YSZ + 1% Y2O3 | 23–3 |
| Ti23Mo + 5% YSZ + 1% Y2O3 | 23–5 |
| Ti23Mo + 10% YSZ + 1% Y2O3 | 23–10 |
| Ti27Mo | 27 |
| Ti27Mo + 3% YSZ + 1% Y2O3 | 27–3 |
| Ti27Mo + 5% YSZ + 1% Y2O3 | 27–5 |
| Ti27Mo + 10% YSZ + 1% Y2O3 | 27–10 |
| Ti35Mo | 35 |
| Ti35Mo + 3% YSZ + 1% Y2O3 | 35–3 |
| Ti35Mo + 5% YSZ + 1% Y2O3 | 35–5 |
| Ti35Mo + 10% YSZ + 1% Y2O3 | 35–10 |
| Liquids | γL (mN/m) | γLd (mN/m) | γLp (mN/m) |
|---|---|---|---|
| Distilled water | 72.8 | 21.8 | 51.0 |
| Glycerol | 62.7 | 21.2 | 41.5 |
| Sample | EIT ± σ (GPa) | HV ± σ | HV0.3 ± σ |
|---|---|---|---|
| Ti | 123.3 ± 14.3 | 170 ± 19 | 174 ± 13 |
| 23 | 126.5 ± 3.2 | 503 ± 11 | 447 ± 8 |
| 23–3 | 136.3 ± 9.2 | 497 ± 59 | 545 ± 44 |
| 23–5 | 140.7 ± 6.9 | 542 ± 87 | 552 ± 21 |
| 23–10 | 144.3 ± 14.6 | 525 ± 114 | 678 ± 54 |
| 27 | 134.4 ± 4.9 | 332 ± 26 | 463 ± 18 |
| 27–3 | 146.4 ± 2.5 | 530 ± 39 | 541 ± 20 |
| 27–5 | 143.6 ± 12.0 | 588 ± 40 | 619 ± 22 |
| 27–10 | 163.2 ± 25.6 | 537 ± 176 | 683 ± 57 |
| 35 | 148.5 ± 7.8 | 446 ± 39 | 507 ± 11 |
| 35–3 | 147.2 ± 8.7 | 578 ± 25 | 622 ± 10 |
| 35–5 | 149.4 ± 4.8 | 612 ± 39 | 645 ± 18 |
| 35–10 | 143.0 ± 4.9 | 522 ± 41 | 705 ± 25 |
| Sample | Water CA (°) | Glycerol CA (°) | Surface Free Energy (mN/m) | Disperse (mN/m) | Polar (mN/m) |
|---|---|---|---|---|---|
| 23 | 36.59 (±0.16) | 50.51 (±3.74) | 68.11 ± 8.20 | 2.91 ± 2.00 | 65.20 ± 6.20 |
| 23–3 | 33.87 (±0.11) | 42.62 (±3.09) | 64.95 ± 6.46 | 6.78 ± 2.22 | 58.17 ± 4.25 |
| 23–5 | 32.01 (±0.79) | 44.02 (±2.85) | 68.30 ± 6.23 | 5.02 ± 1.83 | 63.28 ± 4.39 |
| 23–10 | 39.11 (±0.20) | 45.78 (±2.97) | 60.47 ± 6.46 | 7.18 ± 2.32 | 53.29 ± 4.14 |
| 27 | 34.19 (±0.19) | 49.48 (±4.26) | 70.67 ± 9.26 | 2.66 ± 2.15 | 68.01 ± 7.11 |
| 27–3 | 43.64 (±1.26) | 48.87 (±5.07) | 56.50 ± 11.81 | 7.40 ± 4.30 | 49.09 ± 7.51 |
| 27–5 | 43.31 (±0.36) | 44.44 (±4.28 | 55.01 ± 9.39 | 11.14 ± 4.08 | 43.87 ± 5.32 |
| 27–10 | 52.45 (±1.13) | 48.52 (±3.94) | 47.16 ± 9.68 | 14.99 ± 4.79 | 32.17 ± 4.89 |
| 35 | 39.74 (±0.13) | 49.10 (±2.85) | 61.99 ± 6.30 | 5.06 ± 1.97 | 56.93 ± 4.33 |
| 35–3 | 47.22 (±0.32) | 39.65 (±2.89) | 51.73 ± 6.17 | 19.84 ± 3.36 | 31.89 ± 2.82 |
| 35–5 | 36.24 (±2.85) | 40.46 (±3.17) | 61.10 ± 9.35 | 9.76 ± 3.15 | 51.34 ± 6.1 |
| 35–10 | 50.06 (±0.15) | 45.74 (±3.04) | 49.10 ± 6.87 | 15.79 ± 3.52 | 33.31 ± 3.35 |
| Sample | Ecorr (V) | Icorr (10−7 A·cm−2) |
|---|---|---|
| 23 | −0.47156 | 1.2978 |
| 23–3 | −0.41636 | 4.0679 |
| 23–5 | −0.44637 | 5.9423 |
| 23–10 | −0.54237 | 2.9754 |
| 27 | −0.43916 | 8.7883 |
| 27–3 | −0.40917 | 6.0742 |
| 27–5 | −0.52555 | 6.0629 |
| 27–10 | −0.48249 | 5.4389 |
| 35 | −0.46641 | 8.0063 |
| 35–3 | −0.51548 | 4.6376 |
| 35–5 | −0.50704 | 9.0438 |
| 35–10 | −0.4155 | 4.7374 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piechowiak, D.; Miklaszewski, A.; Makuch-Dziarska, N. The Ultrafine-Grain Yttria-Stabilized Zirconia Reinforced β-Titanium Matrix Composites. Metals 2021, 11, 240. https://doi.org/10.3390/met11020240
Piechowiak D, Miklaszewski A, Makuch-Dziarska N. The Ultrafine-Grain Yttria-Stabilized Zirconia Reinforced β-Titanium Matrix Composites. Metals. 2021; 11(2):240. https://doi.org/10.3390/met11020240
Chicago/Turabian StylePiechowiak, Daria, Andrzej Miklaszewski, and Natalia Makuch-Dziarska. 2021. "The Ultrafine-Grain Yttria-Stabilized Zirconia Reinforced β-Titanium Matrix Composites" Metals 11, no. 2: 240. https://doi.org/10.3390/met11020240
APA StylePiechowiak, D., Miklaszewski, A., & Makuch-Dziarska, N. (2021). The Ultrafine-Grain Yttria-Stabilized Zirconia Reinforced β-Titanium Matrix Composites. Metals, 11(2), 240. https://doi.org/10.3390/met11020240







