Structure-Phase Transformations in the Course of Solid-State Mechanical Alloying of High-Nitrogen Chromium-Manganese Steels
Abstract
:1. Introduction
2. Experimental
2.1. Sample Compositions and Their Treatment
2.2. Mössbauer and TEM Analysis of MA Samples
3. Mechanical Alloying in the Course of the Ball Milling and Thermal Annealing
3.1. Mössbauer Analysis of Mechanical Alloying in the Cases of A and B Series
3.2. Mössbauer Analysis of MA Samples after Their Annealing
3.3. TEM Data on the Results of the MA and Subsequent Annealing
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rashev, T.V. High Nitrogen Steels. Metallurgy under Pressure; Bulgarien Academy of Science: Sofia, Bulgaria, 1995. [Google Scholar]
- Simmons, J.W. Overview: High-nitrogen alloying of stainless steels. Mater. Sci. Eng. A 1996, 207, 159–169. [Google Scholar] [CrossRef]
- Yang, K.; Ren, Y. Nickel-free austenitic stainless steels for medical applications. Sci. Technol. Adv. Mater. 2010, 11, 014105. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.; Mancha, H.; Mendoza, G.; Escalante, J.I.; Cisneros, M.M. Structure of a Fe-Cr-Mn-Mo-N alloy processed by mechanical alloying. Metall. Mater. Trans. A 2002, 33, 3273–3278. [Google Scholar] [CrossRef]
- Fukutsuka, T.; Anzai, T.; Kaneda, M.; Matsuo, Y.; Sugie, Y.; Fukaura, K. Preparation of High Nitrogen Containing Stainless Steels by Mechanical Alloying Method and Their Localized Corrosion Behavior. J. Soc. Mater. Sci. Jpn. 2004, 53, 1175–1179. [Google Scholar] [CrossRef] [Green Version]
- Cisneros, M.M.; López, H.F.; Mancha, H.; Rincón, E.; Vázquez, D.; Pérez, M.J.; de la Torre, S.D. Processing of nanostructured high nitrogen stainless steel by mechanical alloying. Metall. Mater. Trans. A 2005, 36, 1309–1316. [Google Scholar]
- Guan, L.; Qu, X.; Wang, S. Preparation of stainless steel powder containing nitrogen by mechanical alloying technique. J. Univ. Sci. Technol. Beijing 2005, 27, 692–694. [Google Scholar]
- Dawei, C.; Xuanhui, Q.; Ping, G.; Li, K. Preparation of nearly spherical nickel-free highnitrogen austenitic stainless steel powders by mechanical alloying. Powder Metall. Technol. 2008, 26, 265–268. [Google Scholar]
- Amini, R.; Hadianfard, M.J.; Salahinejad, E.; Marasi, M.; Sritharan, T. Microstructural phase evaluation of high-nitrogen Fe–Cr–Mn alloy powders synthesized by the mechanical alloying process. J. Mater. Sci. 2009, 44, 136–148. [Google Scholar] [CrossRef]
- Haghir, T.; Abbasi, M.H.; Golozar, M.A.; Panjepour, M. Investigation of α to γ transformation in the production of a nanostructured high-nitrogen austenitic stainless steel powder via mechanical alloying. Mater. Sci. Eng. A 2009, 507, 144–148. [Google Scholar] [CrossRef]
- Salahinejad, E.; Amini, R.; Hadianfard, M.J. Contribution of nitrogen concentration to compressive elastic modulus of 18Cr–12Mn–XN austenitic stain less steels developed by powder metallurgy. Mater. Des. 2010, 31, 2241–2244. [Google Scholar] [CrossRef]
- Salahinejad, E.; Amini, R.; Askari, B.E.; Hadianfard, M.J. Microstructural and hardness evolution of mechanically alloyed Fe–Cr–Mn–N powders. J. Alloys Compd. 2010, 497, 369–372. [Google Scholar] [CrossRef]
- Salahinejad, E.; Amini, R.; Hadianfard, M.J. Structural evolution during mechanical alloying of stainless steels under nitrogen. Powder Technol. 2012, 2015–2016, 247–253. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Uchida, H.; Kataoka, K.; Takaki, S. Fabrication of Fine-grained High Nitrogen Austenitic Steels through Mechanical Alloying Treatment. ISIJ Int. 2002, 42, 1438–1443. [Google Scholar] [CrossRef] [Green Version]
- Shabashov, V.A.; Borisov, S.V.; Zamatovsky, A.E.; Vildanova, N.F.; Mukoseev, A.G.; Litvinov, A.V.; Shepatkovsky, O.P. Deformation-induced transformations in nitride layers formed in bcc iron. Mater. Sci. Eng. A 2007, 452–453, 575–583. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Korshunov, L.G.; Sagaradze, V.V.; Kataeva, N.V.; Zamatovsky, A.E.; Litvinov, A.V.; Lyashkov, K.A. Mössbauer analysis of deformation dissolution of the products of cellular decomposition in high-nitrogen chromium manganese austenite steel. Philos. Mag. 2014, 94, 668–682. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Kozlov, K.A.; Lyashkov, K.A.; Litvinov, A.V.; Dorofeev, G.A.; Titova, S.G. Solid-Phase Mechanical Alloying of BCC Iron Alloys by Nitrogen in Ball Mills. Defect Diffus. Forum 2012, 330, 25–37. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Kozlov, K.; Lyashkov, K.A.; Kataeva, N.V.; Litvinov, A.V.; Sagaradze, V.V.; Zamatovskii, A.E. Solid-state mechanical synthesis of austenitic Fe-Ni-Cr-N alloys. Phys. Met. Metallograph. 2014, 115, 392–402. [Google Scholar] [CrossRef]
- Dorofeev, G.A.; Sapegina, I.V.; Lad’Yanov, V.I.; Pushkarev, B.E.; Pechina, E.A.; Prokhorov, D.V. Mechanical alloying and severe plastic deformation of nanocrystalline high-nitrogen stainless steels. Phys. Met. Metallograph. 2012, 113, 963–973. [Google Scholar] [CrossRef]
- Dorofeev, G.A.; Lubnin, A.N.; Ulyanov, A.L.; Mukhgalin, V.V. Accelerated mechanosynthesis of high-nitrogen stainless steel: Mössbauer and X-ray diffraction studies. Bull. Russ. Acad. Sci. Phys. 2017, 81, 803–806. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Berns, H. High Nitrogen Steel: Structure, Properties, Manufacture, Applications; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Kirchner, G.; Uhrenins, B. Experimental study of the ferrite/austenite equilibrium in the Fe-Cr-Mn system and the optimization of thermodynamic parameters by means of a general mathematic method. Acta Metall. 1974, 22, 523–532. [Google Scholar] [CrossRef]
- Dorofeev, G.A.; Karev, V.; Goncharov, O.; Kuzminykh, E.; Sapegina, I.; Lubnin, A.; Mokrushina, M.; Lad’Yanov, V. Aluminothermic Reduction Process Under Nitrogen Gas Pressure for Preparing High Nitrogen Austenitic Steels. Met. Mater. Trans. B 2019, 50, 632–640. [Google Scholar] [CrossRef]
- Konygin, G.N.; Stevulova, N.; Dorofeev, G.A.; Elsukov, E.P. The effect of wear of grinding tools on the results of mechanical alloying of Fe and Si(C). Chem. Sustain. Dev. 2002, 10, 73–80. [Google Scholar]
- Rusakov, V.S.; Kadyrzhanov, K.K. Mössbauer Spectroscopy of Locally Inhomogeneous Systems. Hyperfine Interact. 2005, 164, 87–97. [Google Scholar] [CrossRef]
- Kang, S.G.; Onodera, H.; Jamamoto, H.; Watanabe, H. Mössbauer effect study of Fe-Mn alloys. J. Phys. Soc. Japan 1974, 36, 975–979. [Google Scholar] [CrossRef]
- Vincze, I.; Campbell, I.A. Mössbauer measurements in iron based alloys with transition metals. J. Phys. F Met. Phys. 1973, 3, 647–663. [Google Scholar] [CrossRef]
- van der Woude, F.; Sawatzky, G.A. Mössbauer effect in iron and dilute iron based alloys. Phys. Rep. 1974, 12, 335–374. [Google Scholar] [CrossRef]
- Pepperhoff, W.; Acet, M. Constitution and Magnetism of Iron and its Alloys; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Oda, K.; Umezu, K.; Ino, H. Interaction and arrangement of nitrogen atoms in FCC γ-iron. J. Phys. Condens. Matter 1990, 2, 10147–10158. [Google Scholar] [CrossRef]
- Ino, H.; Umezu, K.; Kajiwara, S.; Uehara, S. Interstitial solute atom configuration in Fe-N and Fe-C based austenite and relation to the abnormal tetragonality of fresh martensite. In Proceedings of the International Conference on Martensitic Transformations (ICOMAT-86), Nara, Japan, 26–30 August 1986; The Japan Institute of Metals: Sendai, Japan, 1986; pp. 313–318. [Google Scholar]
- Endoh, Y.; Ishikawa, Y. Antiferromagnetism of γ–iron–manganes alloys. J. Phys. Soc. Jpn. 1973, 30, 1614–1627. [Google Scholar] [CrossRef]
- Amigud, G.G.; Bogachev, I.N.; Dorofeev, G.A.; Karakishev, S.D.; Litvinov, V.S. Mössbauer spectroscopy study of iron-manganese austenite alloys. Fiz. Met. Met. 1973, 36, 666–668. [Google Scholar]
- Renot, R.C.; Swartzendruber, L.J. Origin of Mössbauer linewidth in stainless steel. AIP Conf. Proc. 1973, 10, 1350–1353. [Google Scholar]
- Srivastava, B.P.; Sarma, H.N.K.; Bhattacharya, D.L. Quadrupole splitting in deformed stainless steel. Phys. Status Solidi A 1972, 10, K117–K118. [Google Scholar] [CrossRef]
- Dubiel, S.M.; Żukrowski, J. Distribution of Cr atoms in a strained and strain-relaxed Fe 89.15 Cr 10.75 alloy: A Mössbauer effect study. Philos. Mag. Lett. 2017, 97, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Mirkin, L.I. X-ray Control of Engineering Materials; Mashinostroenie: Moscow, Russia, 1979.
- Dorofeev, G.A.; Elsukov, E.P. Thermodynamic Modeling of Mechanical Alloying in the Fe-Sn System. Inorg. Mater. 2000, 36, 1228–1234. [Google Scholar] [CrossRef]
- Sagaradze, V.V.; Kataeva, N.V.; Zavalishin, V.A.; Kozlov, K.A.; Makarov, V.V.; Kuznetsov, A.R.; Rogozhkin, S.V.; Ustyugov, Y.M. Formation of low-temperature deformation-induced segregations of nickel in Fe–Ni-based austenitic alloys. Philos. Mag. 2020, 100, 1868–1879. [Google Scholar] [CrossRef]
- Leslie, W.C.; Mieler, R.Z. The stabilization of austenite by closely spaced boundaries. Trans. ASM 1964, 57, 972–979. [Google Scholar]
- Gruzin, P.L.; Rodionov, U.L.; Mikhailova, L.K.; Isphandiarov, G.G. Low-temperature isothermal alpha yields gamma transformations in ordered iron-nickel alloys. Phys. Met. Metallograph. 1977, 44, 178–179. [Google Scholar]
- Hornbogen, E.; Meyer, W. Martensitische Umwadlung von mischkristallen mit kohӓrenten teilchen. Acta Metall. 1967, 15, 584–588. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Sagaradze, V.V.; Yurchikov, E.E.; Savel’eva, A.V. Mössbauer spectroscopy and electron microscopy studies of α→γ transformation and stabilization of iron-nickel austenite. Fiz. Met. Met. 1977, 44, 1060–1070. [Google Scholar]
- Shabashov, V.A.; Kozlov, K.A.; Zamatovskii, A.E.; Lyashkov, K.A.; Sagaradze, V.V.; Danilov, S.E. Short-Range Atomic Ordering Accelerated by Severe Plastic Deformation in FCC Invar Fe-Ni Alloys. Phys. Met. Metallograph. 2019, 120, 686–693. [Google Scholar] [CrossRef]
- Kozlov, K.; Shabashov, V.A.; Zamatovskii, A.; Novikov, E.; Ustyugov, Y. Inversion of the Sign of the Short-Range Order as a Function of the Composition of Fe–Cr Alloys at Warm Severe Plastic Deformation and Electron Irradiation. Metals 2020, 10, 659. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Sagaradze, V.V.; Kozlov, K.A.; Ustyugov, Y.M. Atomic Order and Submicrostructure in Iron Alloys at Megaplastic Deformation. Metals 2018, 8, 995. [Google Scholar] [CrossRef] [Green Version]
- Shabashov, V.A.; Kozlov, K.; Ustyugov, Y.; Zamatovskii, A.; Tolmachev, T.; Novikov, E. Mössbauer Analysis of Deformation–Induced Acceleration of Short-Range Concentration Separation in Fe-Cr Alloys—Effect of the Substitution Impurity: Sb and Au. Metals 2020, 10, 725. [Google Scholar] [CrossRef]
- Goldschmidt, H.J. Intersitial Alloys; Butterworth-Heinemann: London, UK, 1967. [Google Scholar]
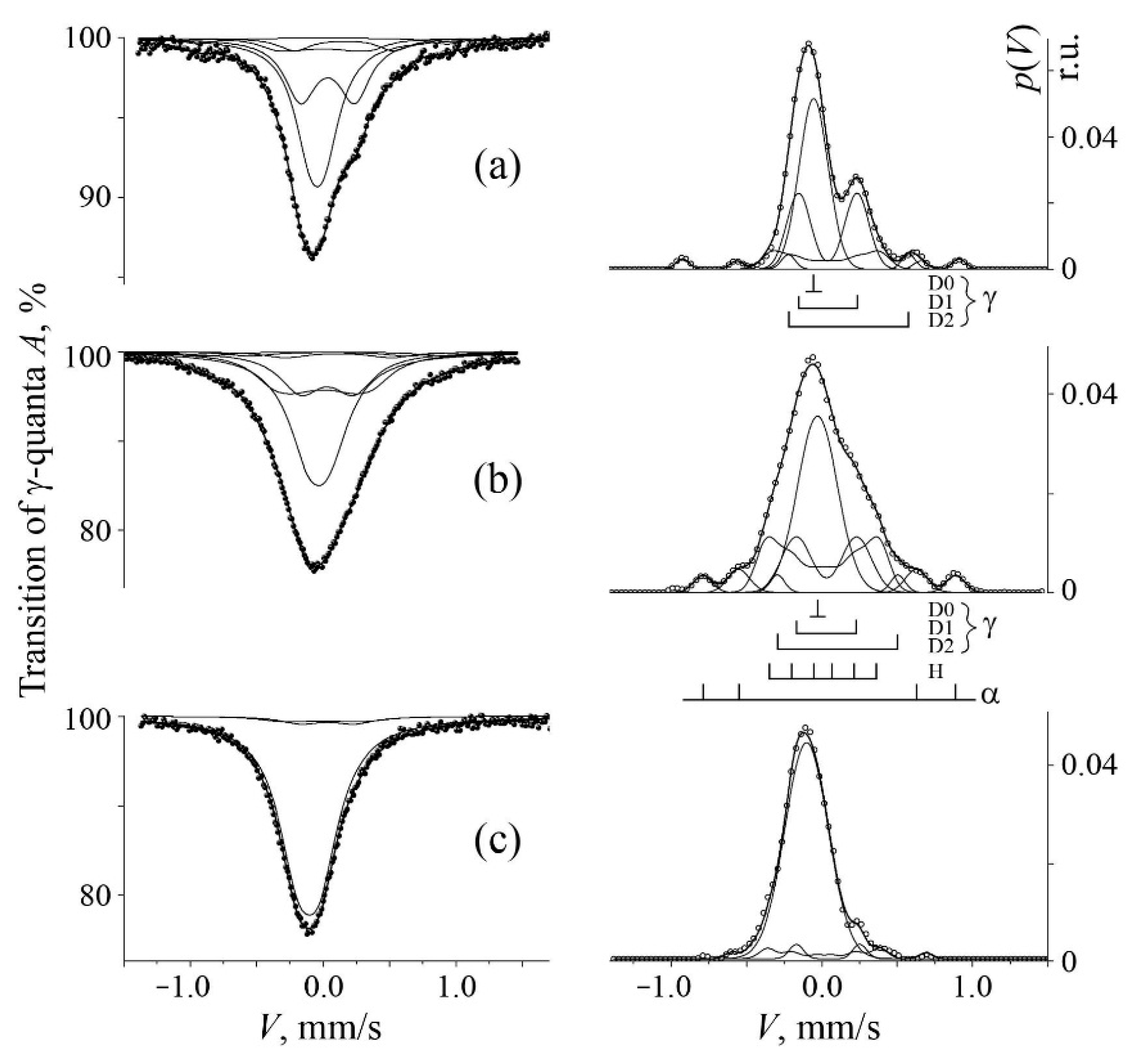

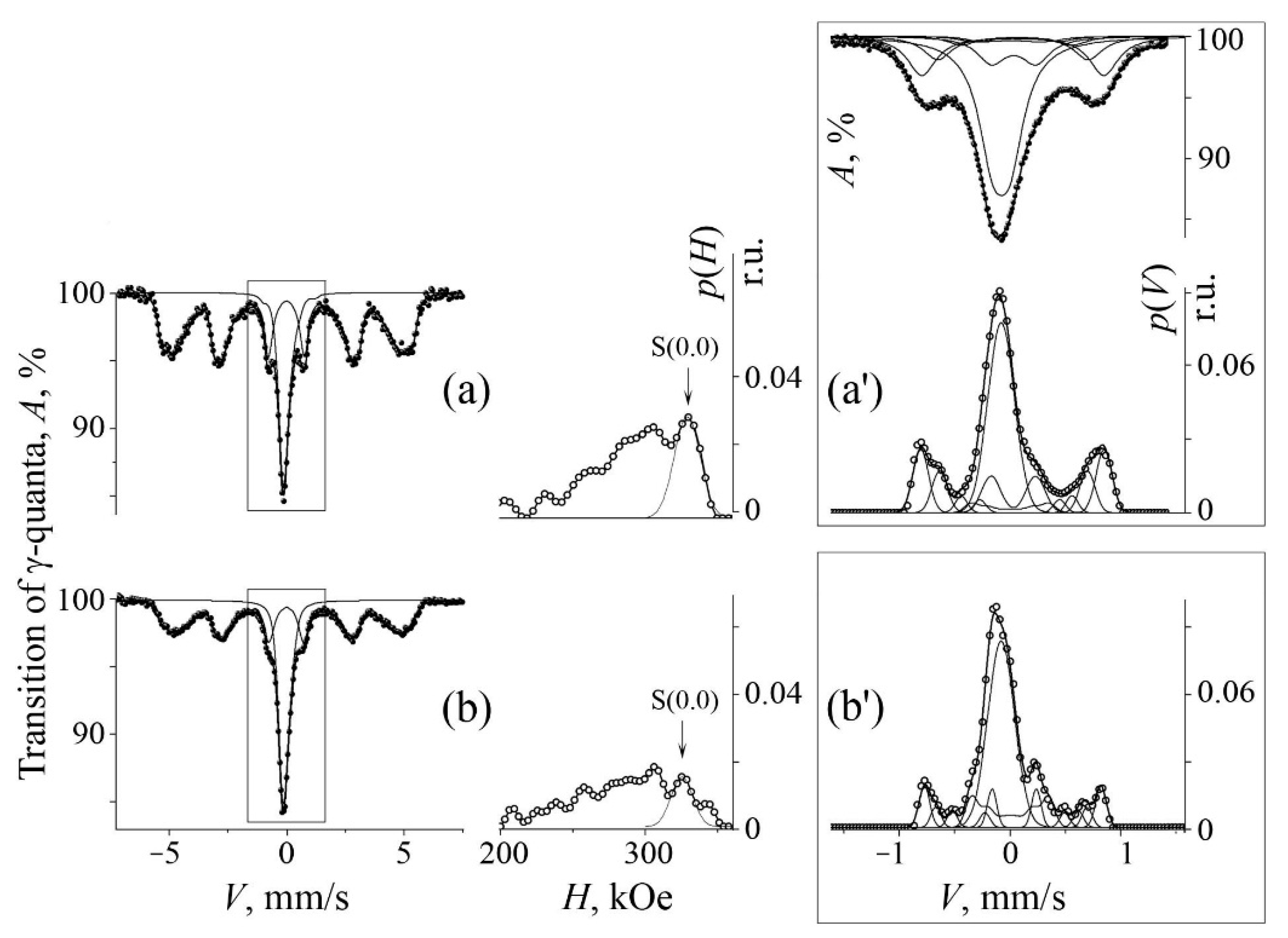
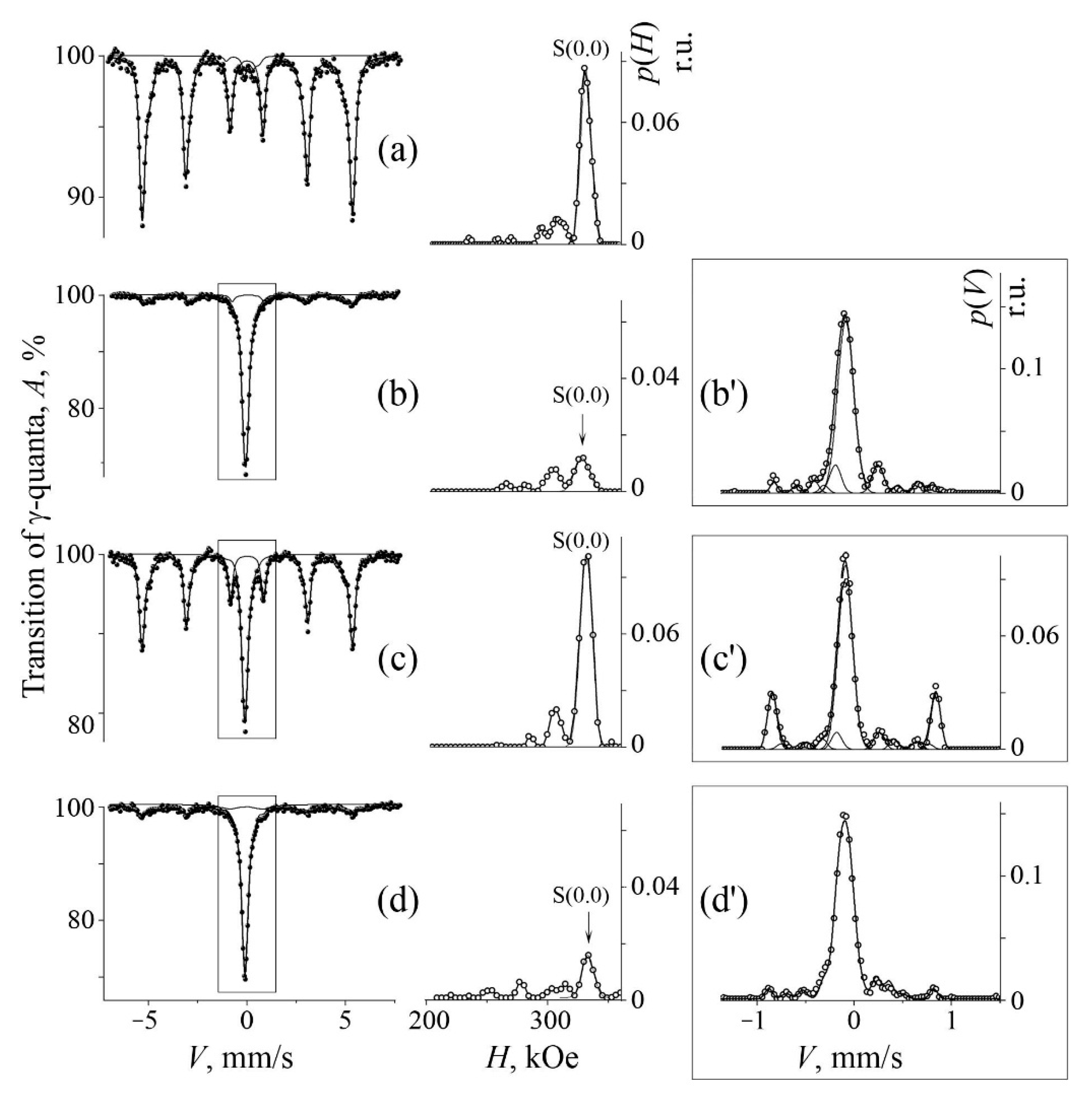
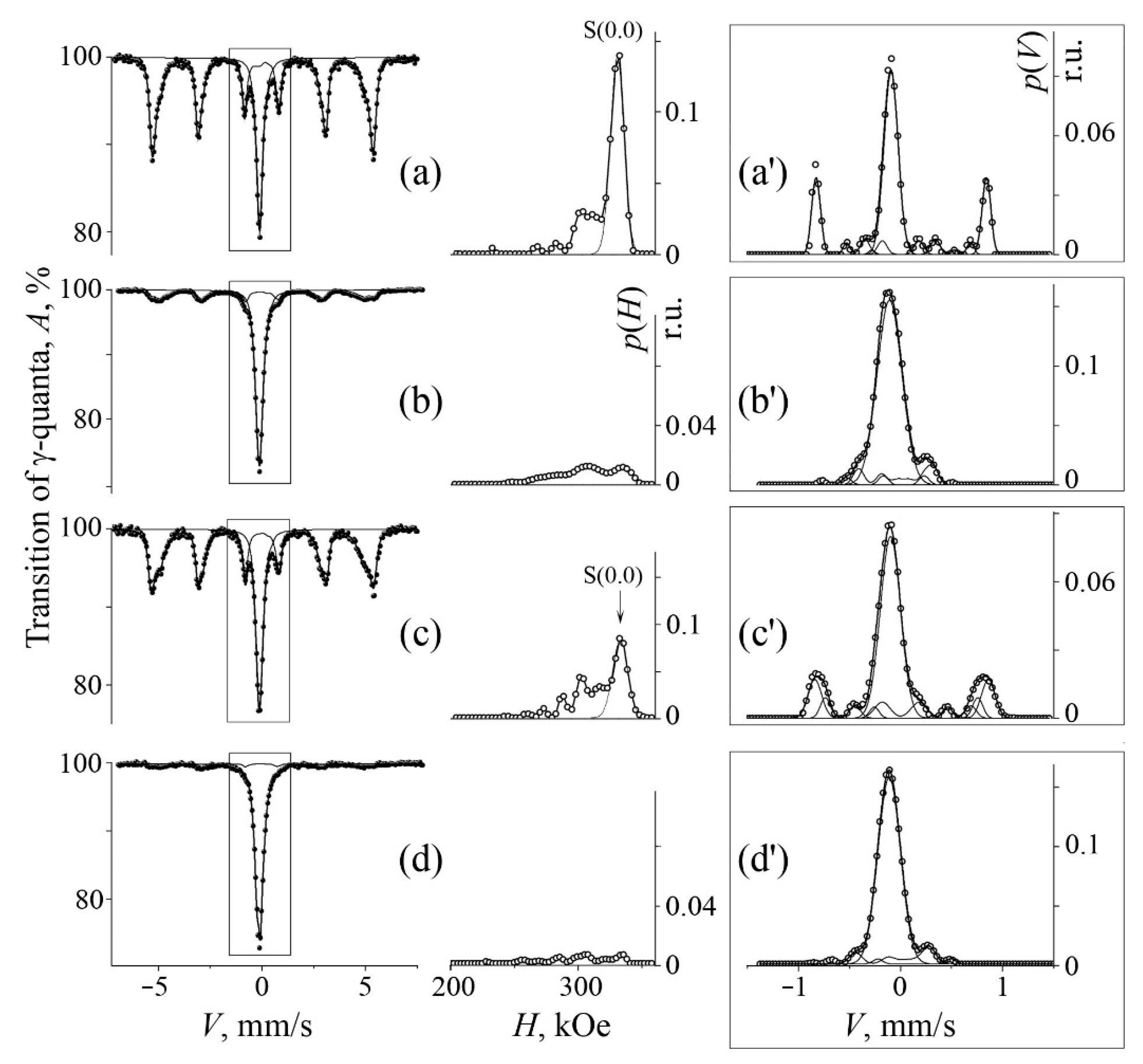
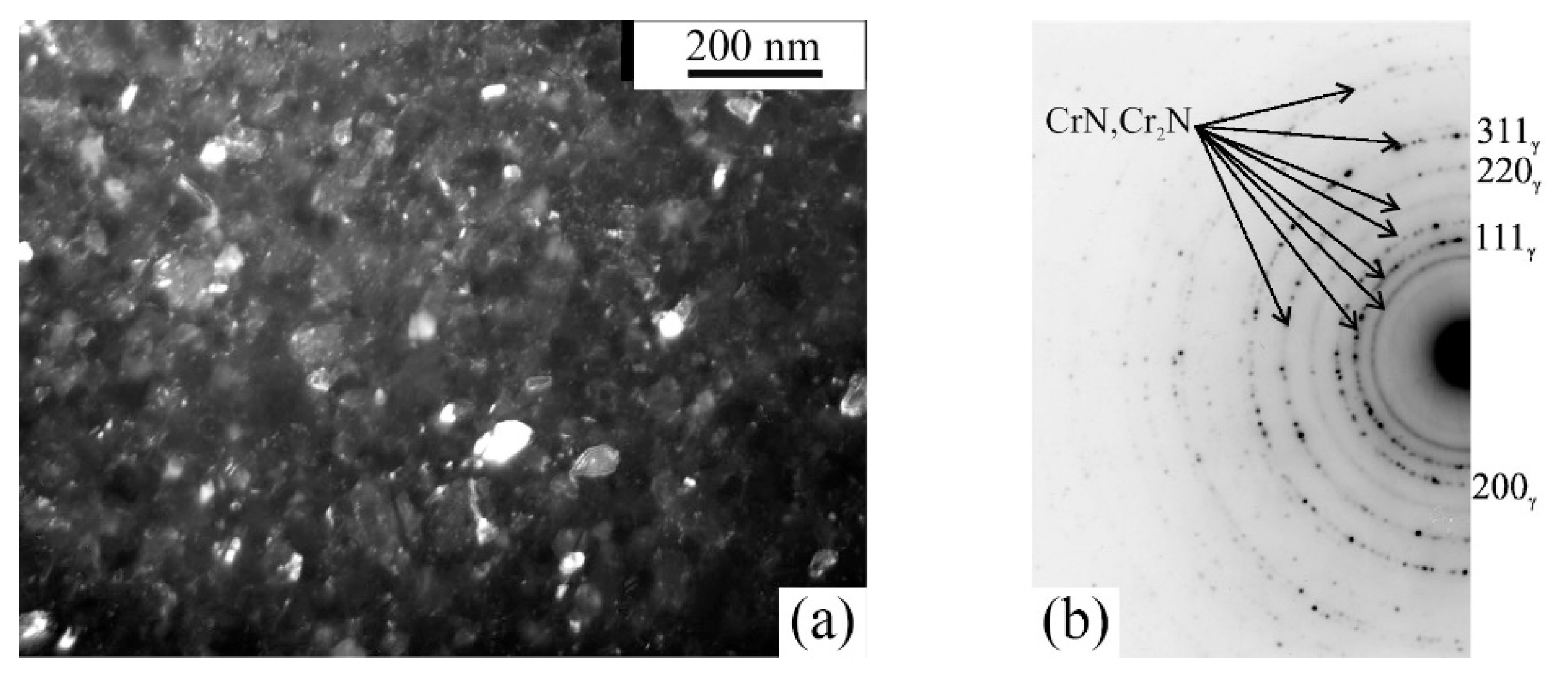
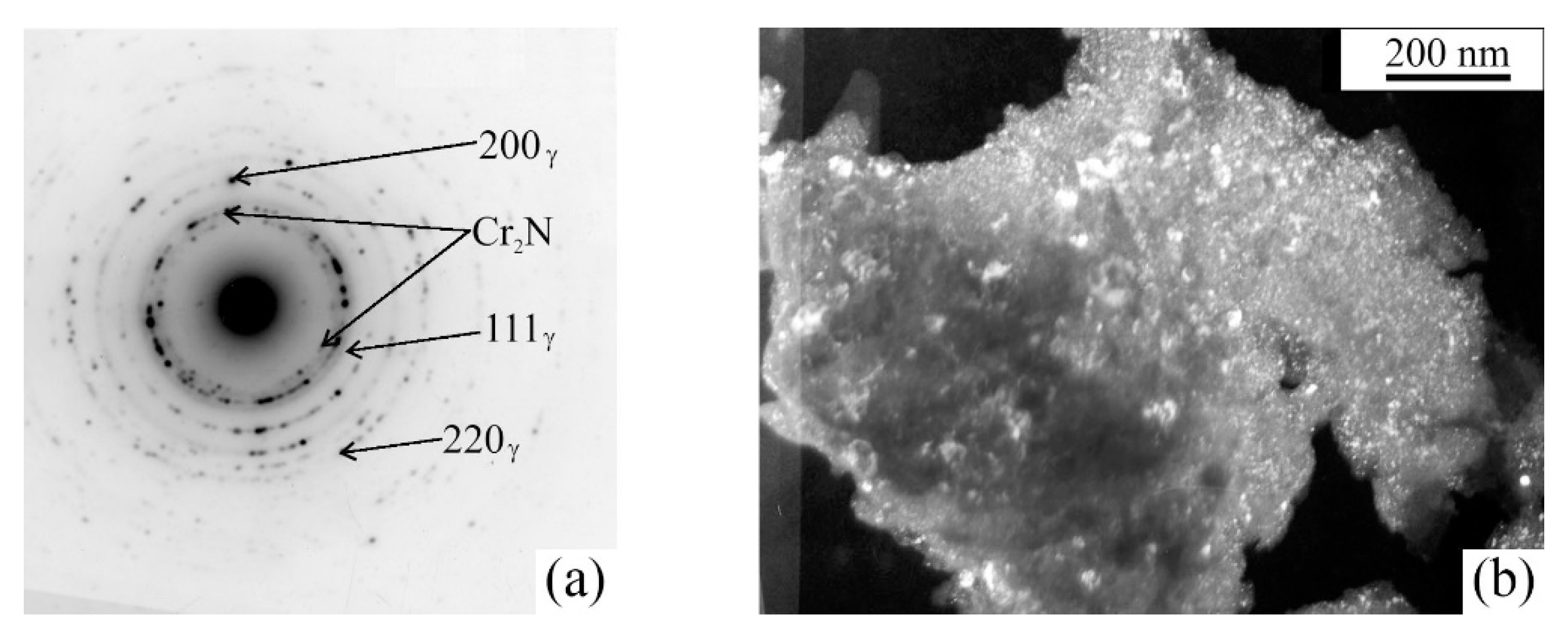
| No. | Formula of Composition | Ball Milling for 10 h | Annealing after BM at 700 °C (a) and 800 °C (b) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FeXMn + 20CrN (X in wt%) | Quantity of Austenite γ, vol.% | Content of Nitrogen in Austenite γCN, wt% | Contents of Cr and Mn in the α Phase αCMn+Cr, wt% | Quantity of Austenite γ, vol.% | Content of Nitrogen in Austenite γCN, wt% | Contents of Cr and Mn in the α Phase αCMn+Cr, wt% | ||||
| a | b | a | b | a | b | |||||
| 1 | X = 0.0 | 6 | 3.7 | 4.0 | ||||||
| 2 | X = 4.0 | 7 | 1.4 | 5.2 | 0.0 | 5.0 | - | - | 2.3 | 5.0 |
| 3 | X = 6.7 | 10 | 1.1 | 7.0 | 3.0 | 75.0 | - | 0.5 | 2.2 | 4.6 |
| 4 | X = 8.9 | 36 | 1.1 | 10.0 | 30.4 | 75.0 | 0.5 | 0.1 | 1.7 | 5.2 |
| No. | Formula of Composition | Ball Milling for 10 h | Annealing after BM at 700 °C (a) and 800 °C (b) | |||||||
| FeYCr + 20Mn2N (Y in wt%) | Quantity of Austenite γ, vol.% | Content of Nitrogen in Austenite γCN, wt% | Contents of Cr and Mn in the α Phase αCMn+Cr, wt% | Quantity of Austenite γ, vol.% | Content of Nitrogen in Austenite γCN, wt% | Contents of Cr and Mn in the α Phase αCMn+Cr, wt% | ||||
| a | b | a | b | a | b | |||||
| 1 | Y = 0.0 | 90 | 1.6 | 11.0 | 95 | 100 | - | 0.3 | - | - |
| 2 | Y = 14.2 | 95 | 2.6 | - | 100 | 100 | - | - | - | - |
| No. | FeYCr + 10Mn2N (Y in wt%) | Quantity of Austenite γ, vol.% | Content of Nitrogen in Austenite γCN, wt% | Contents of Cr and Mn in the α Phase αCMn+Cr, wt% | Quantity of Austenite γ, vol.% | Content of Nitrogen in Austenite γCN, wt% | Contents of Cr and Mn in the α Phase αCMn+Cr, wt% | |||
| a | b | a | b | a | b | |||||
| 3 | Y = 0.0 | 10 | 1.1 | 4.0 | 28 | 10 | 0.4 | 0.4 | 2.0 | 4.0 |
| 4 | Y = 4.7 | 24 | 1.0 | 8.7 | 24 | 80 | 0.1 | 0.1 | 2.5 | 5.5 |
| 5 | Y = 8.6 | 36 | 0.7 | 11.5 | 31 | 100 | 0.3 | 0.3 | 4.6 | - |
| Formula of Composition | Doublet D(0) | Doublet D(1) | Doublet D(2) | Sextet SAFM | Comments and Refs | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IS, mm/s ±0.01 | QS, mm/s ±0.01 | IS, mm/s ±0.01 | QS, mm/s ±0.01 | IS, mm/s ±0.01 | QS, mm/s ±0.01 | IS, mm/s ±0.02 | QS, mm/s ±0.02 | H, kOe ±2 | ||
| Fe + 20% CrN | −0.07 | 0.00 | 0.03 | 0.19 | 0.18 | 0.41 | - | - | - | Series A, composition 1 |
| Fe-14.2Cr + 20% Mn2N | −0.05 | 0.14 | 0.03 | 0.20 | 0.16 | 0.36 | -- | - | - | Series B, composition 2 |
| Fe + 20% Mn2N | −0.05 | 0.06 | 0.03 | 0.20 | 0.16 | 0.37 | 0.00 | 0.00 | 22 | Series B, composition 1 |
| Fe-4.7Cr + 10% Mn2N | −0.11 | 0.00 | 0.03 | 0.20 | - | - | −0.01 | −0.04 | 24 | Series B, composition 4 annealing |
| Fe-9.0N | −0.01 | 0.00 | 0.08 | 0.20 | 0.20 | 0.36 | - | - | - | [30] |
| Fe-8.5N | −0.02 | 0.00 | 0.06 | 0.20 | 0.31 | 0.33 | - | - | - | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyashkov, K.; Shabashov, V.; Zamatovskii, A.; Kozlov, K.; Kataeva, N.; Novikov, E.; Ustyugov, Y. Structure-Phase Transformations in the Course of Solid-State Mechanical Alloying of High-Nitrogen Chromium-Manganese Steels. Metals 2021, 11, 301. https://doi.org/10.3390/met11020301
Lyashkov K, Shabashov V, Zamatovskii A, Kozlov K, Kataeva N, Novikov E, Ustyugov Y. Structure-Phase Transformations in the Course of Solid-State Mechanical Alloying of High-Nitrogen Chromium-Manganese Steels. Metals. 2021; 11(2):301. https://doi.org/10.3390/met11020301
Chicago/Turabian StyleLyashkov, Kirill, Valery Shabashov, Andrey Zamatovskii, Kirill Kozlov, Natalya Kataeva, Evgenii Novikov, and Yurii Ustyugov. 2021. "Structure-Phase Transformations in the Course of Solid-State Mechanical Alloying of High-Nitrogen Chromium-Manganese Steels" Metals 11, no. 2: 301. https://doi.org/10.3390/met11020301
APA StyleLyashkov, K., Shabashov, V., Zamatovskii, A., Kozlov, K., Kataeva, N., Novikov, E., & Ustyugov, Y. (2021). Structure-Phase Transformations in the Course of Solid-State Mechanical Alloying of High-Nitrogen Chromium-Manganese Steels. Metals, 11(2), 301. https://doi.org/10.3390/met11020301







