Microbial Processing of Waste Shredded PCBs for Copper Extraction Cum Separation—Comparing the Efficacy of Bacterial and Fungal Leaching Kinetics and Yields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
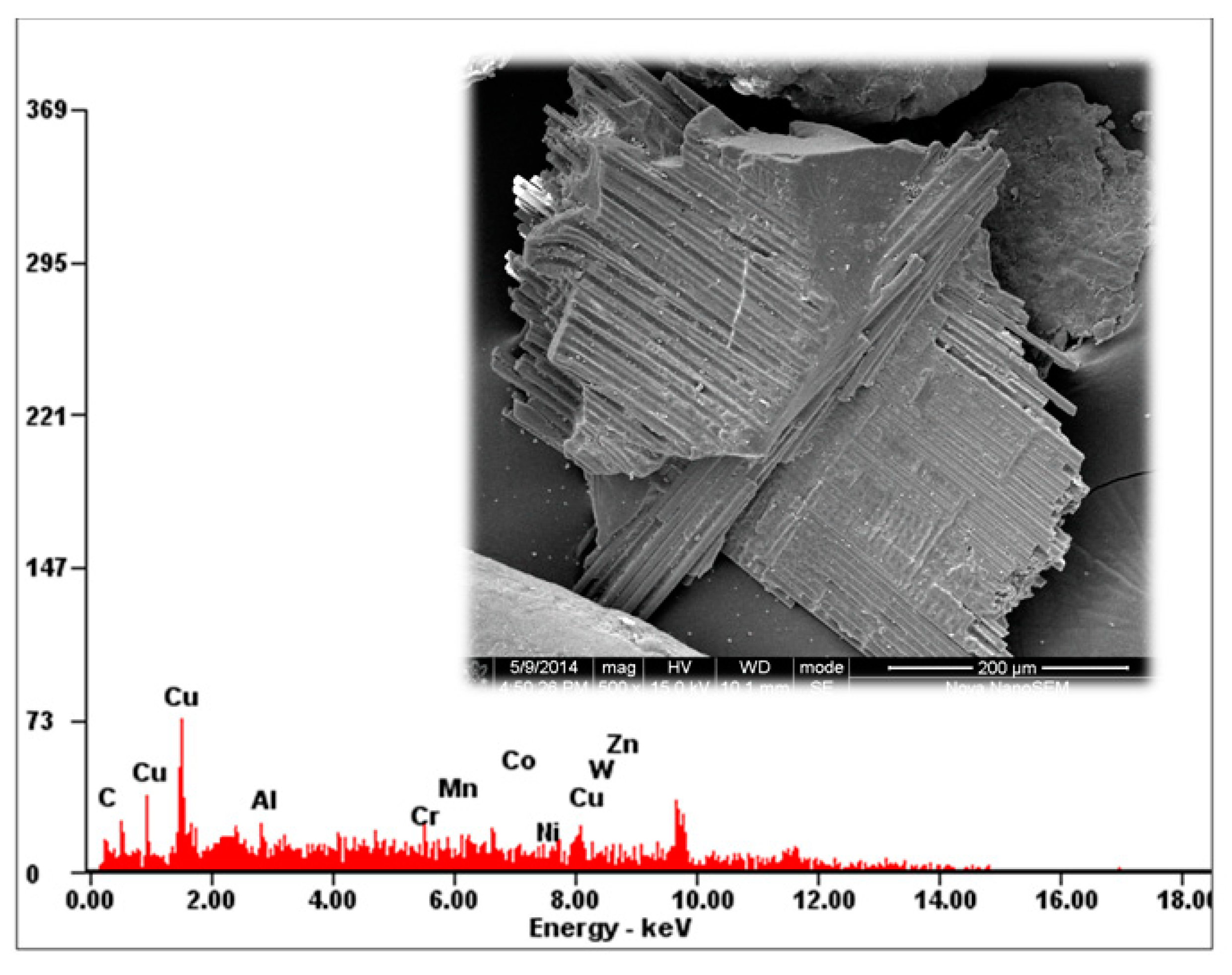
2.2. Printed Circuit Board (PCB) Sample
2.3. Bioleaching Experiments
2.4. Solvent Extraction Studies
3. Results and Discussion
3.1. Role of Microorganisms in Metal Solubilization
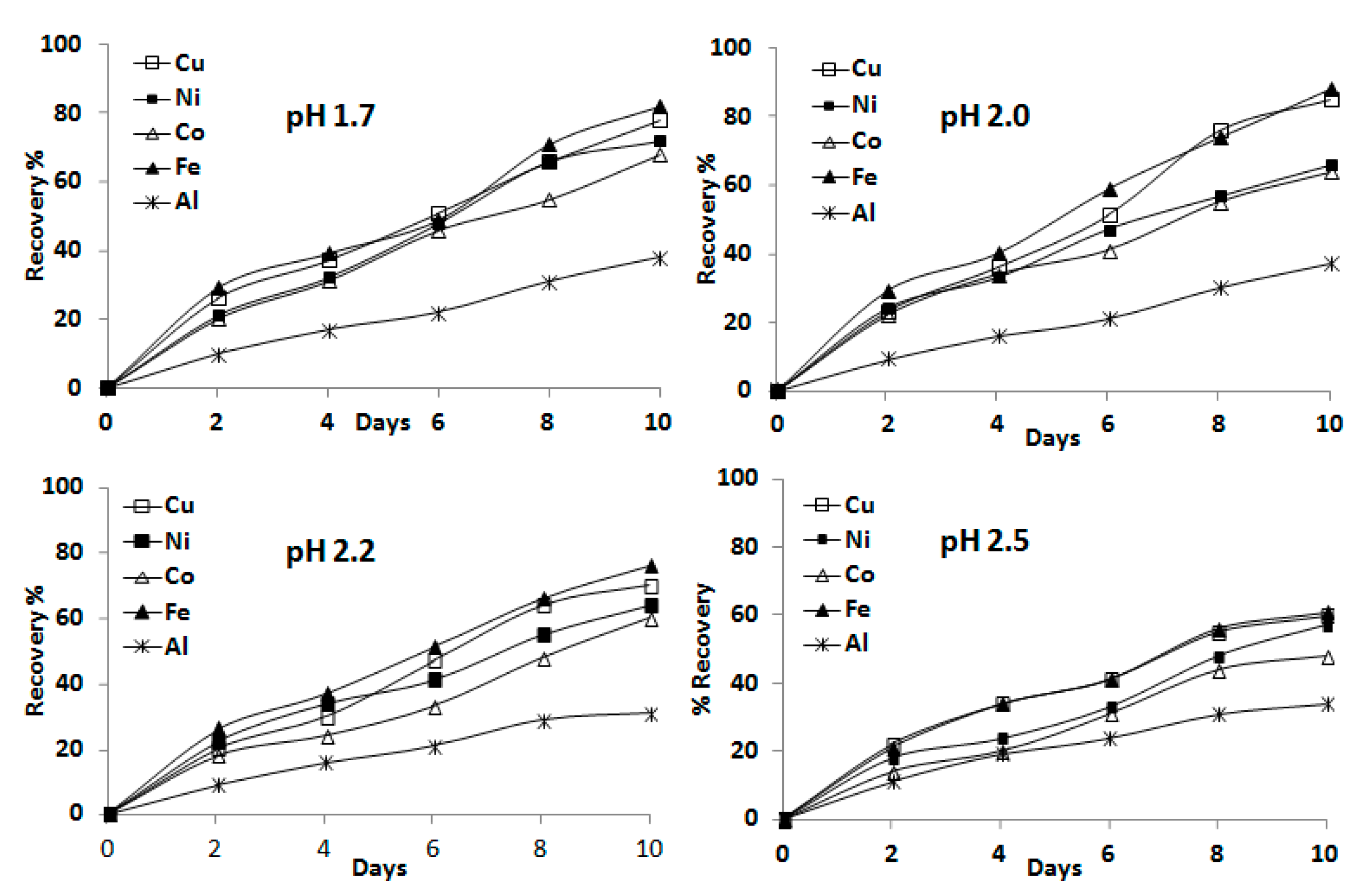
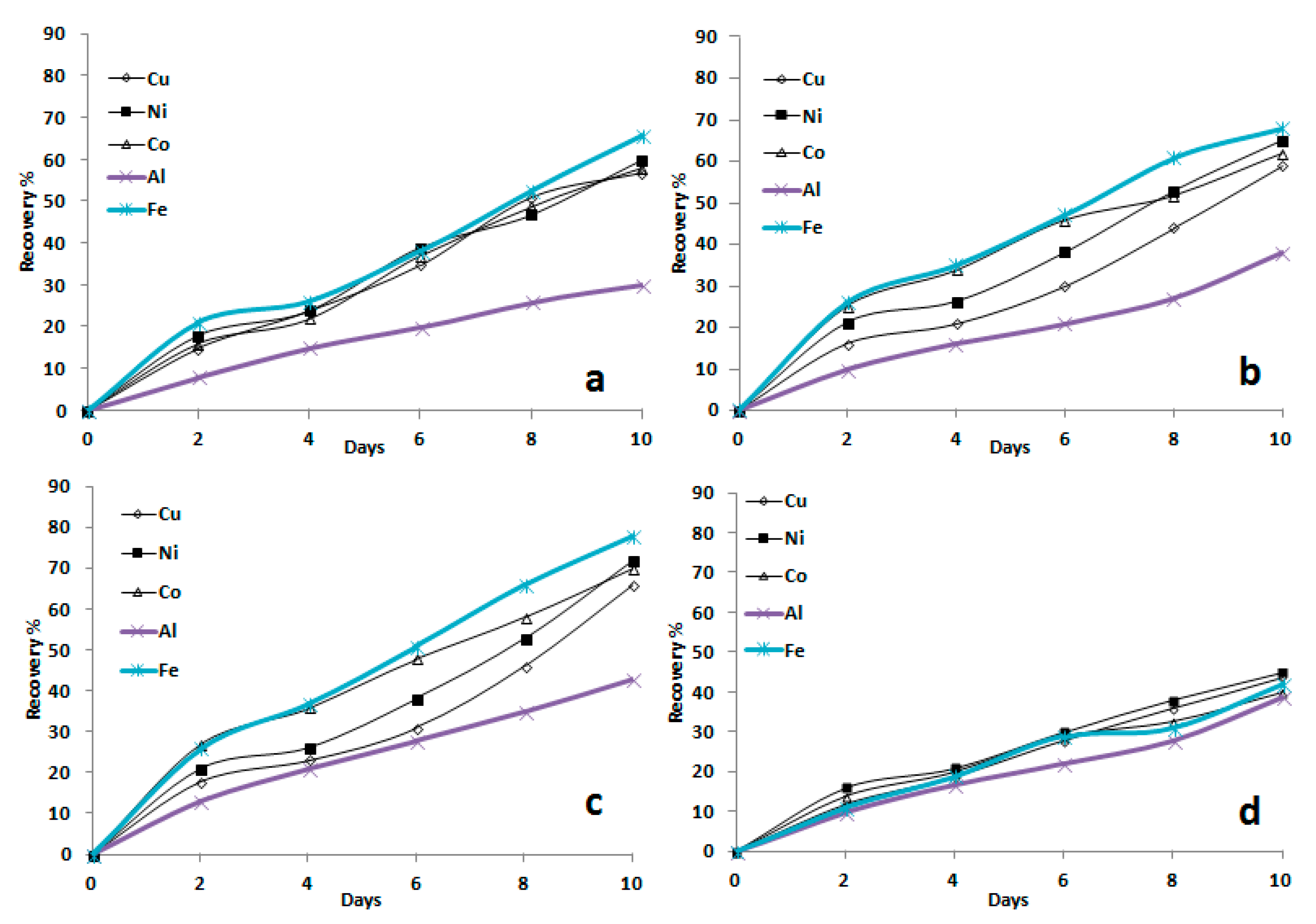
3.1.1. Influence of pH on Bioleaching
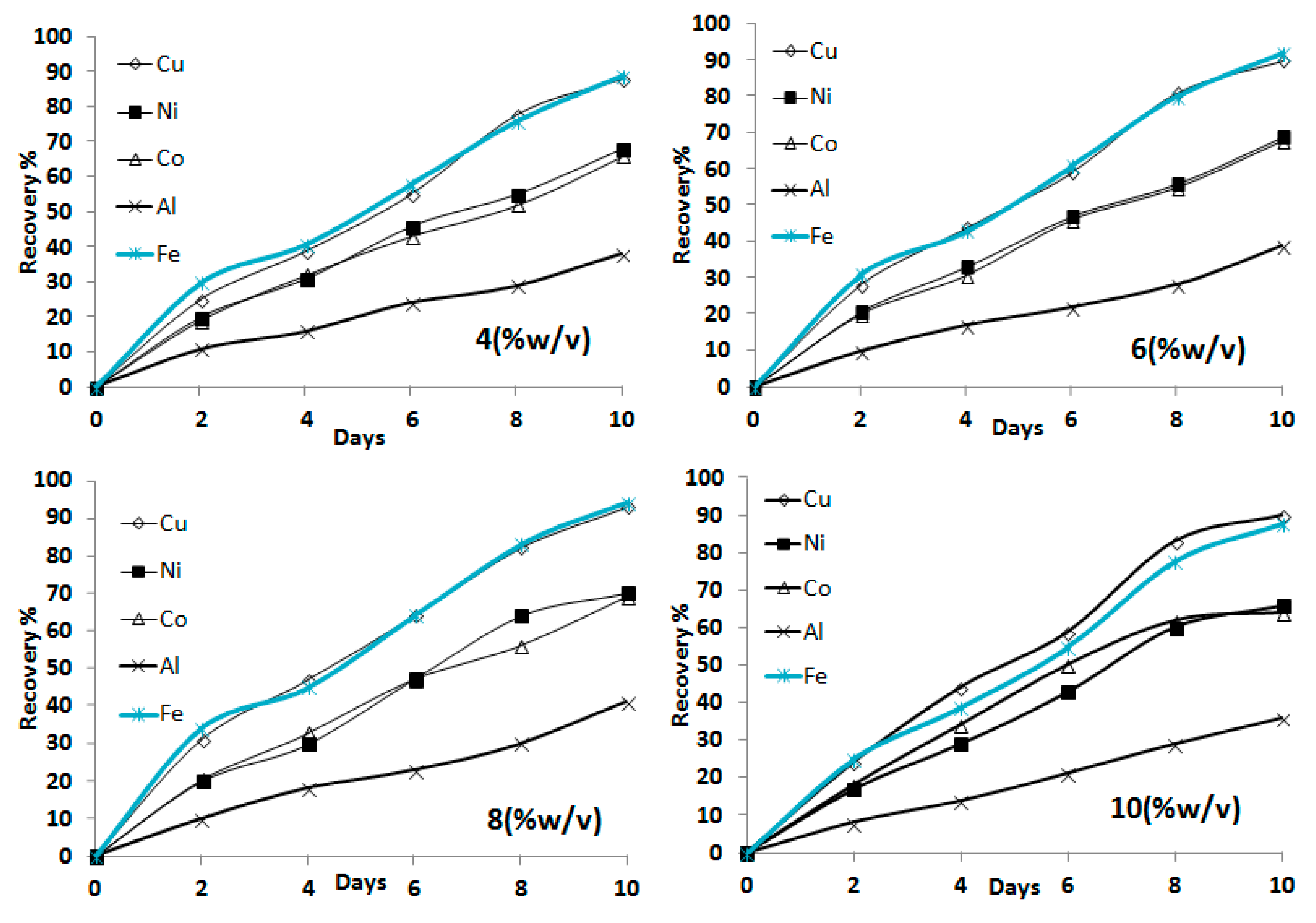
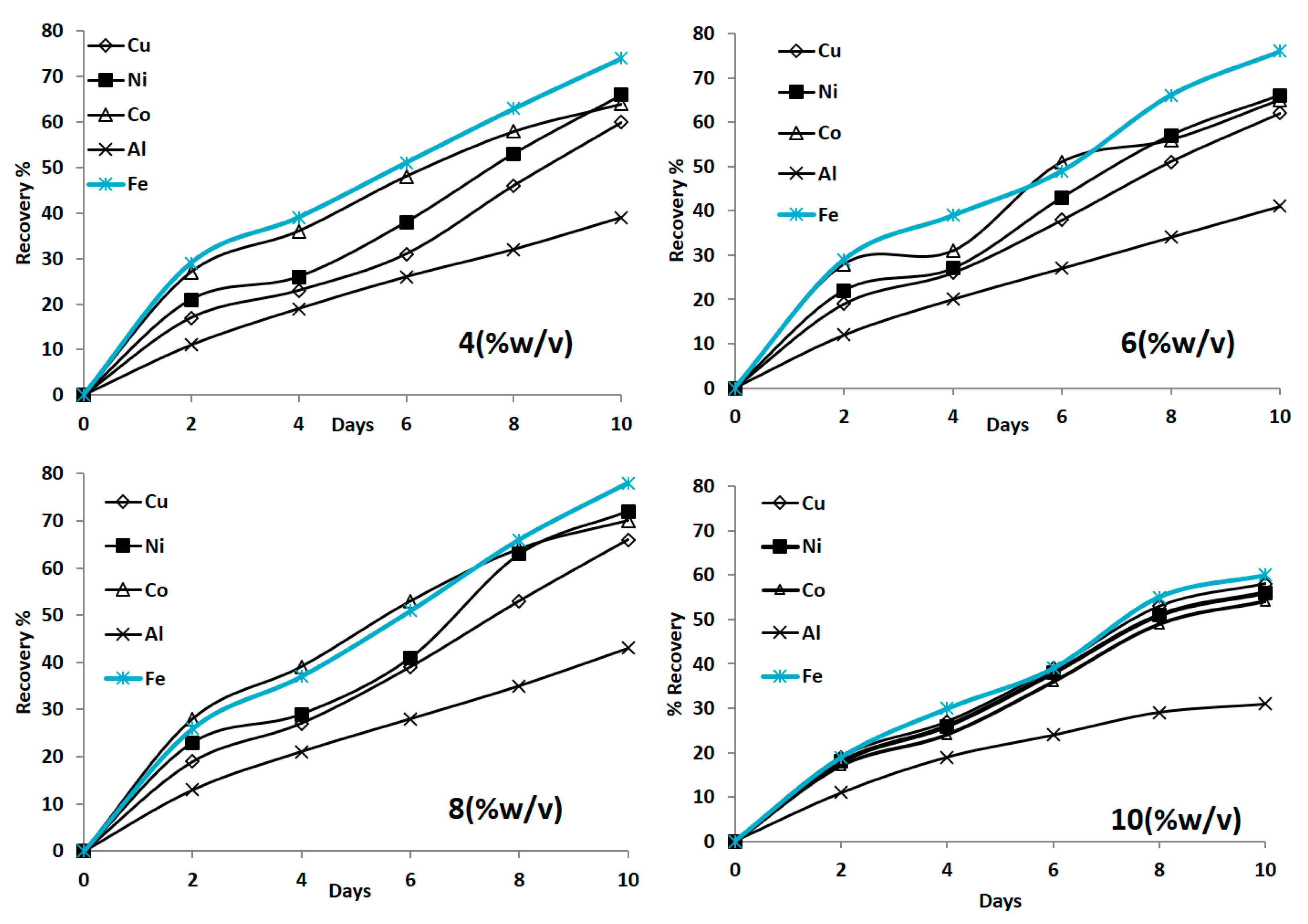
3.1.2. Influence of Pulp Density on Bioleaching
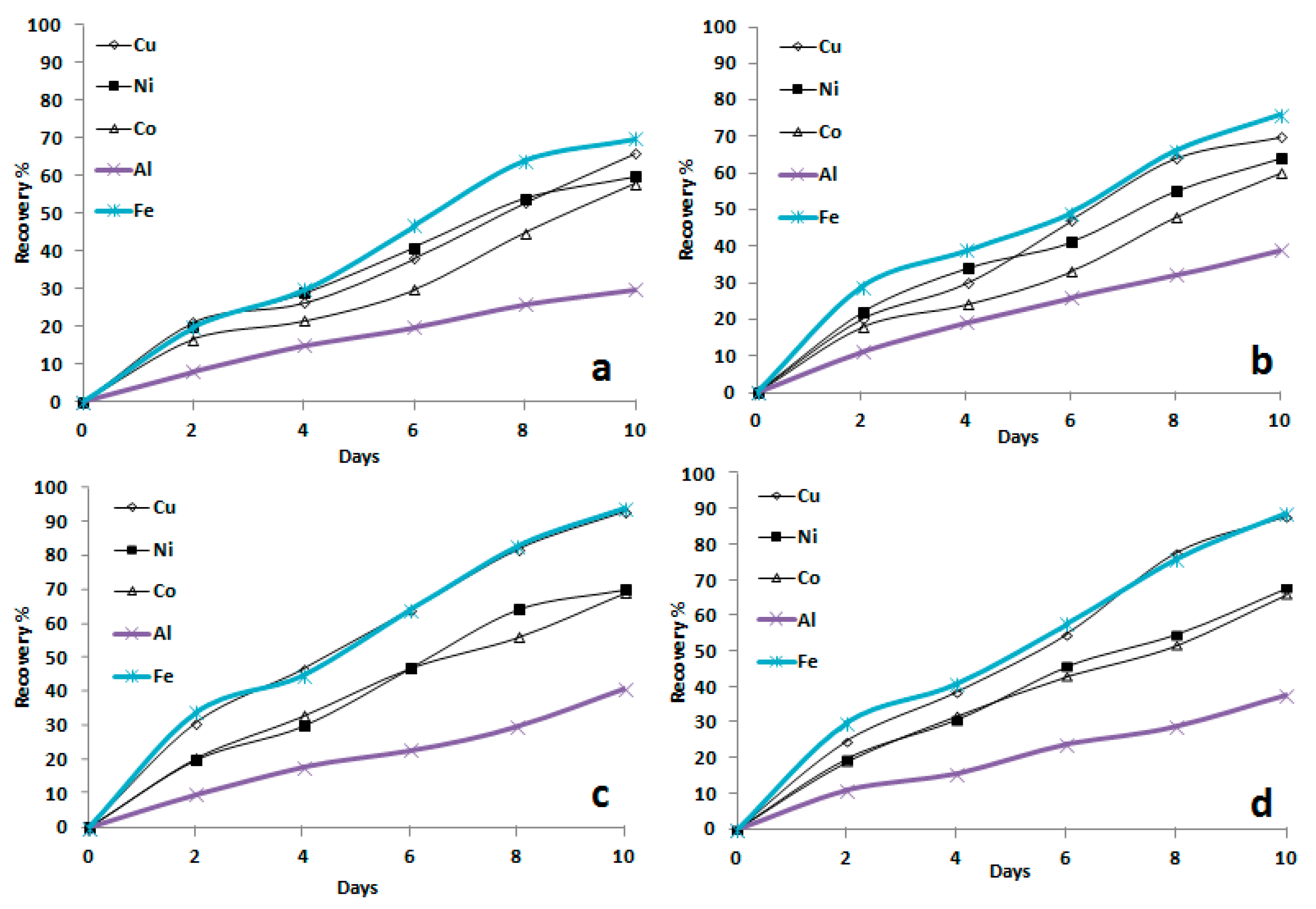
3.1.3. Influence of Temperature on Bioleaching
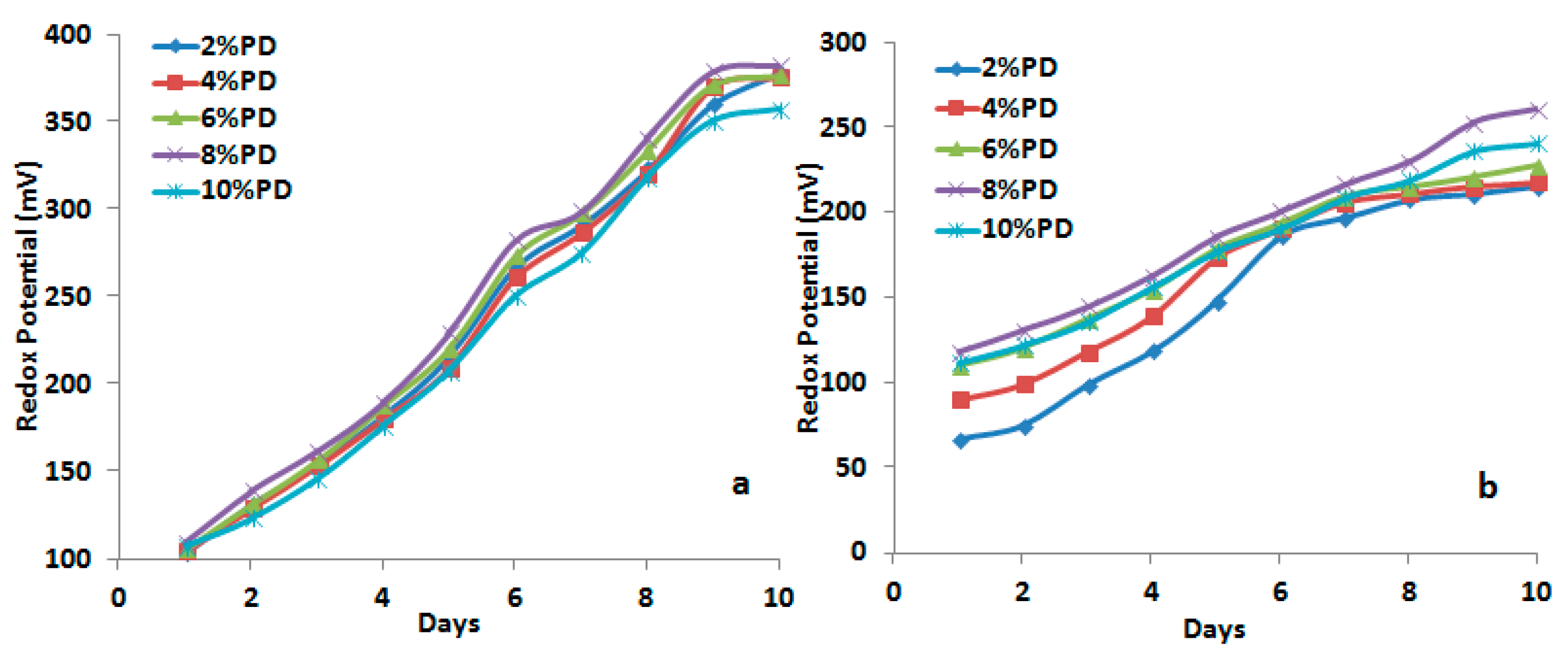
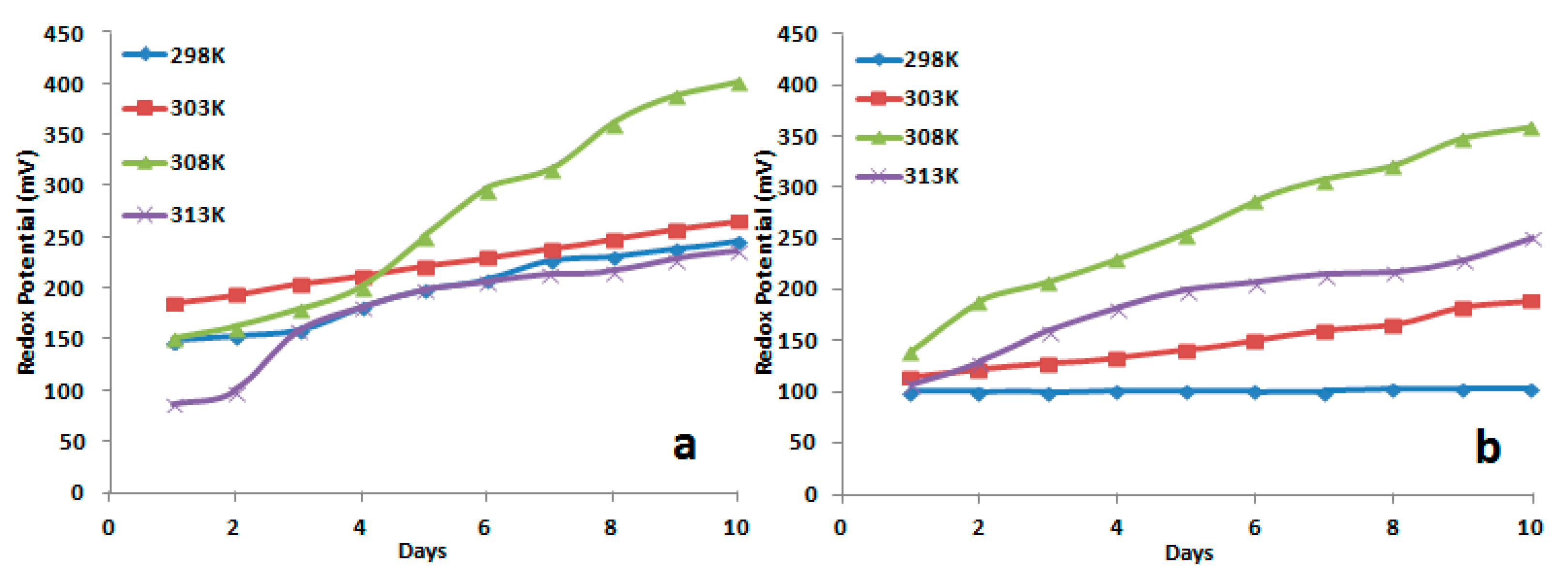
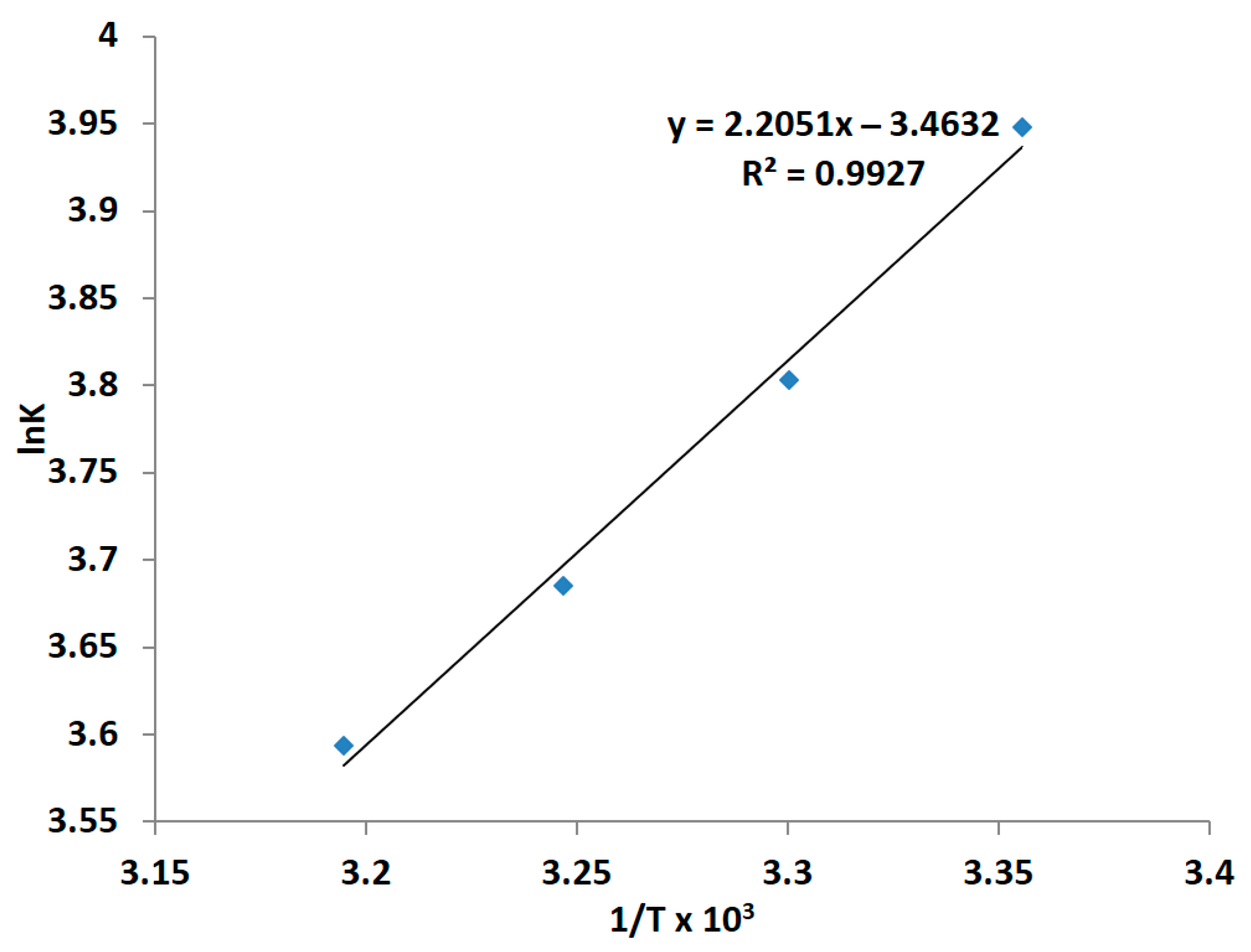
3.1.4. Kinetics and Mechanism of Bacterial Leaching
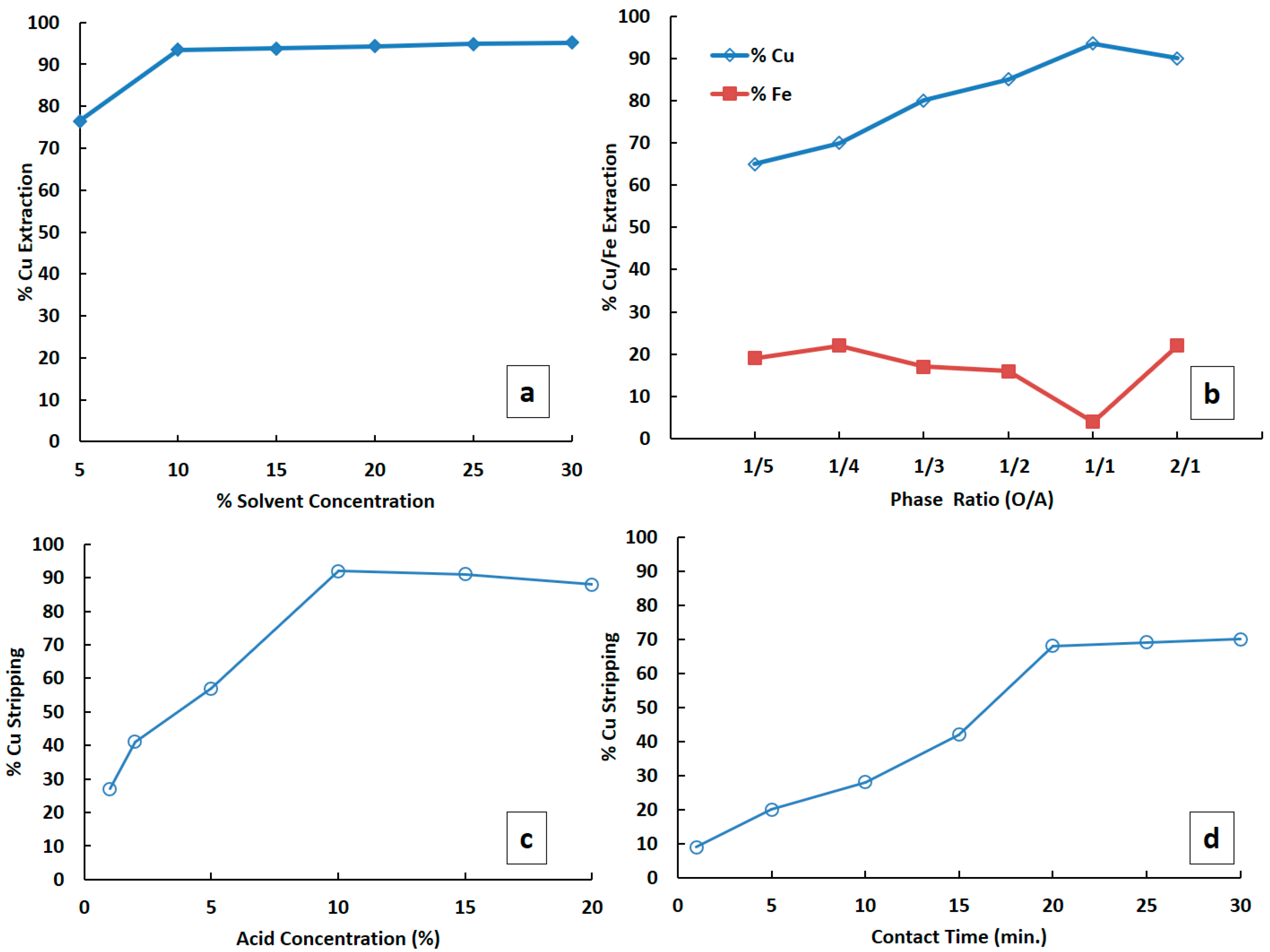
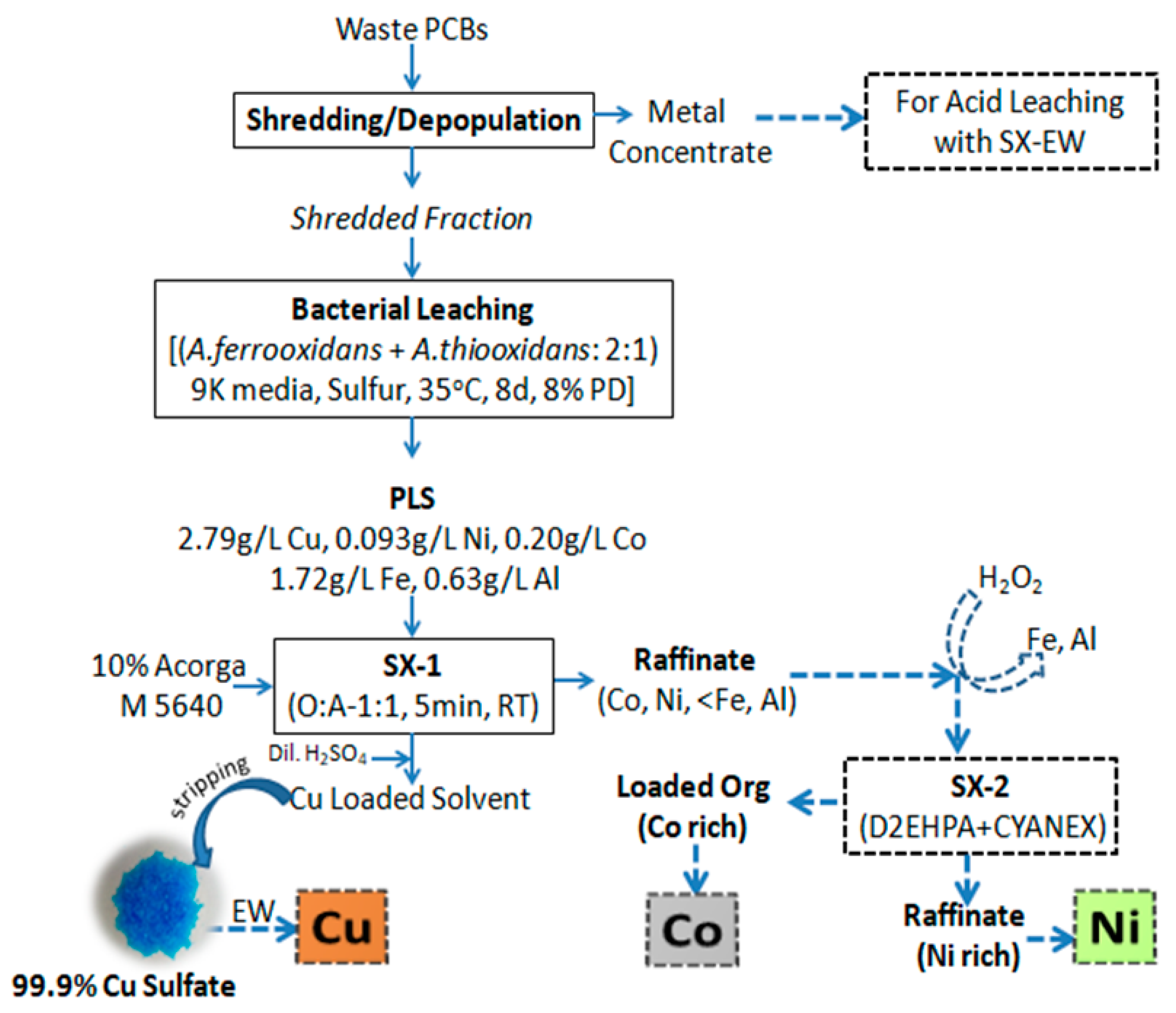
3.2. Solvent Extraction of Pregnant Leach Liquor (PLS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaccari, M.; Vinti, G.; Cesaro, A.; Belgiorno, V.; Salhofer, S.; Dias, M.I.; Jandric, A. WEEE treatment in developing countries: Environmental pollution and health consequences—An overview. Int. J. Environ. Res. Public Health 2019, 16, 1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesaro, A.; Belgiorno, V.; Vaccari, M.; Jandric, A.; Chung, T.D.; Dias, M.I.; Hursthouse, A.; Salhofer, S. A device-specific prioritization strategy based on the potential for harm to human health in informal WEEE recycling. Environ. Sci. Pollut. Res. 2018, 25, 683–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, B.H. E-Waste: An Assessment of Global Production and Environmental Impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef]
- World Copper Supply. 2020. Available online: https://www.brgm.eu/project/review-of-world-copper-supply-2019 (accessed on 23 October 2020).
- Turaga, R.M.R.; Bhaskar, K.; Sinha, S.; Hinchliffe, D.; Hemkhaus, M.; Arora, R.; Chatterjee, S.; Khetriwal, D.S.; Radulovic, V.; Singhal, P.; et al. E-Waste Management in India: Issues and Strategies. Vikalpa 2019, 44, 127–162. [Google Scholar] [CrossRef] [Green Version]
- India Voluntary National Review (VNR). 2020. Available online: https://sustainabledevelopment.un.org/content/documents/26281VNR_2020_India_Report.pdf (accessed on 8 October 2020).
- Brandl, H.; Bosshard, R.; Wegmann, M. Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sethurajan, M.; van Hullebusch, E.D.; Nancharaiah, Y.V. Biotechnology in the management and resource recovery from metal bearing solid wastes: Recent advances. J. Environ. Manag. 2018, 211, 138–153. [Google Scholar] [CrossRef]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR)—Co-hosted SCYCLE Programme: Bonn, Germany; International Telecommunication Union (ITU): Geneva, Switzerland; International Solid Waste Association (ISWA): Rotterdam, The Netherlands, 2020. [Google Scholar]
- Ehrlich, H.L. Beginnings of Rational Bioleaching and Highlights in the Development of Biohydrometallurgy: A Brief History. Eur. J. Miner. Process Environ. Prot. 2004, 4, 102–112. [Google Scholar]
- Akcil, A.; Deveci, H. Mineral Biotechnology of Sulphides. In Geomicrobiology, 1st ed.; Jain, S., Khan, A., Rai, M.K., Eds.; Science Publishers: Enfield, NH, USA, 2010; pp. 101–137. [Google Scholar]
- Arya, S.; Kumar, S. Bioleaching: Urban mining option to curb the menace of E-waste challenge. Bioengineered 2020, 11, 640–660. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Li, S.; Chen, M.; Chen, H.; Liu, B. Bioleaching waste printed circuit boards by Acidithiobacillus ferrooxidans and its kinetics aspect. J. Biotechnol. 2014, 173, 24–30. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Xu, J.; Liang, B. Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. J. Hazard. Mater. 2009, 172, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Anwar, M.A.; Niazi, S.B.; Ghauri, M.A. Bioleaching of metals from electronic scrap by moderately thermophilic acidophilic bacteria. Hydrometallurgy 2007, 88, 80–188. [Google Scholar] [CrossRef]
- Chi, T.C.; Lee, J.C.; Pandey, B.D.; Yoo, K.; Jeong, J. Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Miner. Eng. 2011, 24, 1219–1222. [Google Scholar] [CrossRef]
- Olson, G.J.; Brierley, J.A.; Brierley, C.L. Bioleaching Review. Part B. Progress in bioleaching: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2003, 63, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Abhilash; Pandey, B.D. Microbially assisted leaching of uranium—A review. Miner. Process. Extr. Metall. Rev. 2013, 34, 81–113. [Google Scholar] [CrossRef]
- Keekan, K.; Jalondhara, J.; Abhilash. Extraction of Ce and Th from monazite using REE tolerant Aspergillus niger. Miner. Process. Extr. Metall. Rev. 2017, 38, 312–320. [Google Scholar] [CrossRef]
- Choi, M.; Cho, K.; Kim, D.; Kim, D. Microbial recovery of copper from printed wire boards of waste computer by Acidithiobacillus ferrooxidans. J. Environ. Sci. Health A 2004, 39, 2973–2982. [Google Scholar] [CrossRef]
- Yamane, L.H.; Espinosa, D.C.R.; Tenorio, J.A.S. Bioleaching of copper from electronic scrap. Rem Rev. Esc. Minas 2011, 64, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Bas, A.D.; Deveci, H.; Yazici, E.Y. Bioleaching of copper from low grade scrap TV circuit boards using mesophilic bacteria. Hydrometallurgy 2013, 138, 65–70. [Google Scholar] [CrossRef]
- Karwowska, E.; Andrzejewska-Morzuch, D.; Łebkowska, M.; Tabernacka, A.; Wojtkowska, M.; Telepko, A.; Konarzewska, A. Bioleaching of metals from printed circuit boards supported with surfactant-producing bacteria. J. Hazard. Mater. 2014, 264, 203–210. [Google Scholar] [CrossRef]
- Adhapure, N.N.; Dhakephalkar, P.K.; Dhakephalkar, A.P.; Tembhurkar, V.R.; Rajgure, A.V.; Deshmukh, A.M. Use of large pieces of printed circuit boards for bioleaching to avoid ‘precipitate contamination problem’ and to simplify overall metal recovery. MethodsX 2014, 1, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Bai, J.; Dai, J.; Zhang, C.; Yuan, W.; Wang, J.; Wang, P.; Zhao, X. Characterization of extreme acidophile bacteria (Acidithiobacillus ferrooxidans) bioleaching copper from flexible PCB by ICP-AES. J. Spectrosc. 2014, 2014, 269351. [Google Scholar] [CrossRef] [Green Version]
- Arshadi, M.; Mousavi, S.M. Simultaneous recovery of Ni and Cu from computer-printed circuit boards using bioleaching: Statistical evaluation and optimization. Bioresour. Technol. 2014, 174, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Bryan, C.G.; Watkin, E.L.; McCredden, T.J.; Wong, Z.R.; Harrison, S.T.L.; Kaksonen, A.H. The use of pyrite as a source of lixiviant in the bioleaching of electronic waste. Hydrometallurgy 2015, 152, 33–43. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Y.; Liu, C.; Dong, F.; Liu, B. Column bioleaching copper and its kinetics of waste printed circuit boards (WPCBs) by Acidithiobacillus ferrooxidans. Chemosphere 2015, 141, 162–168. [Google Scholar] [CrossRef]
- Nie, H.; Yang, C.; Zhu, N.; Wu, P.; Zhang, T.; Zhang, Y.; Xing, Y. Isolation of Acidithiobacillus ferrooxidans strain Z1 and its mechanism of bioleaching copper from waste printed circuit boards. J. Chem. Technol. Biotechnol. 2015, 90, 714–721. [Google Scholar] [CrossRef]
- Jadhav, U.; Su, C.; Hocheng, H. Leaching of metals from printed circuit board powder by an Aspergillus niger culture supernatant and hydrogen peroxide. RSC Adv. 2016, 6, 43442–43452. [Google Scholar] [CrossRef]
- Xia, M.; Bao, P.; Liu, A.; Wang, M.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. Bioleaching of low-grade waste printed circuit boards by mixed fungal culture and its community structure analysis. Resour. Conserv. Recycl. 2018, 136, 267–275. [Google Scholar] [CrossRef]
- Annamalai, M.; Gurumurthy, K. Enhanced bioleaching of copper from circuit boards of computer waste by Acidithiobacillus ferrooxidans. Environ. Chem. Lett. 2019, 17, 1873–1879. [Google Scholar] [CrossRef]
- Benzal, E.; Cano, A.; Solé, M.; Lao‑Luque, C.; Gamisans, X.; Dorado, A.D. Copper recovery from PCBs by Acidithiobacillus ferrooxidans: Toxicity of bioleached metals on biological activity. Waste Biomass Valoriz. 2020, 11, 5483–5492. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Zhang, X.; Zhu, M.; Tan, W. Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron–sulfur-oxidizing bacteria. Bioresour. Bioprocess. 2018, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Erust, C.; Akcil, A.; Tuncuk, A.; Panda, S. Intensified acidophilic bioleaching of multi-metals from waste printed circuit boards (WPCBs) of spent mobile phones. J. Chem. Technol. Biotechnol. 2020, 95, 2272–2285. [Google Scholar] [CrossRef]
- Hubau, A.; Minier, M.; Chagnes, A.; Joulian, C.; Silvente, C.; Guezennec, A.G. Recovery of metals in a double-stage continuous bioreactor for acidic bioleaching of printed circuit boards (PCBs). Sep. Purif. Technol. 2020, 238, 116481. [Google Scholar] [CrossRef]
- Liang, G.; Tang, J.; Liu, W.; Zhou, Q. Optimizing mixed culture of two acidophiles to improve copper recovery from printed circuit boards (PCBs). J. Hazard. Mater. 2013, 250, 238–245. [Google Scholar] [CrossRef]
- Becci, A.; Karaj, D.; Merli, G.; Beolchini, F. Biotechnology for metal recovery from end-of-life printed circuit boards with Aspergillus niger. Sustainability 2020, 12, 6482. [Google Scholar] [CrossRef]
- Arshadi, M.; Esmaeili, A.; Yaghmaei, S. Investigating critical parameters for bioremoval of heavy metals from computer printed circuit boards using the fungus Aspergillus niger. Hydrometallurgy 2020, 197, 105464. [Google Scholar] [CrossRef]
- Liu, Q.; Bai, J.F.; Gu, W.H.; Peng, S.J.; Wang, L.C.; Wang, J.W.; Li, H.X. Leaching of copper from waste printed circuit boards using Phanerochaete chrysosporium fungi. Hydrometallurgy 2020, 196, 105427. [Google Scholar] [CrossRef]
- Krebs, W.; Brombacher, C.; Bosshard, P.P.; Bachofen, R.; Brandl, H. Microbial recovery of metals from solids. FEMS Microbiol. Rev. 1997, 20, 605–617. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Sun, X.; Wang, L. Separation and recovery of copper from waste printed circuit boards leach solution using solvent extraction with Acorga M5640 as extractant. Sep. Sci. Technol. 2009, 54, 1302–1311. [Google Scholar] [CrossRef]
- Deep, A.; Kumar, P.; Carvalho, J.M.R. Recovery of copper from zinc leaching liquor using ACORGA M5640. Sep. Sci. Technol. 2010, 76, 21–25. [Google Scholar] [CrossRef]
- Reddy, B.R.; Venkateswara Rao, S.; Park, K.H. Solvent extraction separation and recovery of cobalt and nickel from sulphate medium using mixtures of TOPS 99 and TIBPS extractants. Miner. Eng. 2009, 22, 500–505. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Amato, A.; Becci, A.; Beolchini, F. Sustainable recovery of Cu, Fe and Zn from end-of-life printed circuit boards. Resourc. Conserv. Recycl. 2020, 158, 104792. [Google Scholar] [CrossRef]




| Type of PCB | Composition | Microbes Used | Additive | pH | Particle Size | Pulp Density | Temp (K) | Duration (h) | % Cu Extraction | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| PCB powder | 759 ppm Cu | At. ferrooxidans | 1 g/L citric acid | - | <1410 µm | 1% | 308 K | 144 h | 87% | [22] |
| Printed Wire Boards | 3.38% Cu, 0.016% Ni | At. ferrooxidans, At. thiooxidans and mixture | - | 2.5 | 0.5–1.0 mm | 0.78% | 308 K | 216 h | 99% (At. ferrooxidans) 74.9% (At. thiooxidans) 99.9% (mix) | [16] |
| Computer printed circuit boards | 1.88% Cu, 0.25% Ni, 1.25% Al, 6.97% Fe | At. ferrooxidans | - | 1.8–2.0 | <2 mm | 1.5% | 303 K | 720 h | 56% | [23] |
| Scrap TV circuit boards | 11.2% Cu, 0.02% Ni, 0.04% Co, 0.0043% Fe, 0.30% Al, 91 ppm Cr | At. ferrooxidans, L. ferrooxidans, At. thiooxidans | 8% pyrite | 1.7 | <250 μm | 1% | 308 K | 115 h | 89% | [24] |
| Crumbled fine PCB fines | 4.1% Cu, 1.3% Ni, 72.4 ppm Cr | Bacillus subtilis PCM 2021 and Bacillus cereus PCM 2019 | 1% sulphur | - | <2.0 mm | 0.5% | 310 K | 600 h | 53–90% | [25] |
| Printed circuit board assemblies (PCBA) | NA | Mixed microbial consortium of Acidiphilum spp., Leptospirillum spp., Thiobacillus ferroxidans, Thiobacillus caldus and Sulfobacillus | - | 2.38 | 12 cm × 6 cm | - | 303 K | 240 h | 920 ppm | [26] |
| Computer flexible PCB | 42.6% Cu | At. ferrooxidans | 30 g/L FeSO4 ·7H2O | 2.5 | 0.42~0.84 mm | 1% | 301 K | 120 h | 90% | [27] |
| Computer printed circuit boards | 3.92% Cu, 0.63% Ni, 1.98% Fe, 1.47% Al, 47 ppm Cr | At. ferrooxidans | 8.4 g/L Fe (III) | 3.0 | <95 µm | 2% | 308 K | 480 h | 100% | [28] |
| Ground electronic wastes (copper-rich) | 86.63% Cu, 0.25% Al, 0.063% Fe | At. thiooxidans | - | <1.0 | 40–104 µm | 1% | 308 K | 12 h | 90% | [29] |
| Waste computer motherboards | 255 ppm Cu, 4.3 ppm Ni, 63.2 ppm Al, 31.7 ppm Fe | At. ferrooxidans | - | - | 420 µm | 1.5% | 308 K | 72 h | 96.8% | [30] |
| Shredded, low-grade, waste PCBs | 3.38% Cu, 0.41% Ni, 16.1% Fe, 0.04% Cr | At. caldus BRGM3, L. ferriphilum BRGM1, Sb. benefaciens BRGM2, Fp. acidiphilum BRGM4 | 5% pyrite | - | <2 mm | 1% | 308 K | 96 h | 100 | [15] |
| Waste mixed PCBs | 18–23% Cu, 0.09–1.15% Ni, 0.2–5.2% Fe, 1–3.6% Al, 0.2–1 ppm Cr | At. ferrivorans and At. thiooxidans | - | 1.0–1.6 | - | 1% | 296 K | 168 h | 98.14% | [31] |
| Mixed PCBs | 24.8% Cu, 2.5% Al, 0.18% Fe | At. ferrooxidans | - | - | 4–10 mm | 5% | 303 K | 672 h | 98% | [9] |
| Waste PCBs powder | 63.37% Cu | At. ferrooxidans | 12 g/L Fe(II) | - | 420 µm | 1.2% | 303 K | 168 h | 96% | [32] |
| PCB powder | 80.25% Cu, 0.26% Ni, 0.0059% Co, 56.28% Al, 2.10% Fe | A. niger | 20 g/L citric acid + 3.18% H2O2 | 1.97 | 299.3 µm | 1% | 353 K | 12 h | 99% | [33] |
| Depopulated PCB | 2.49% Cu, 0.03% Fe, 0.88% Al | Purpureocillium lilacinum and Aspergillus niger | - | - | 150 μm–1 mm | 8% | 308 K | <528 h | 56.1% | [34] |
| Waste computer motherboards | 646 ppm, 11.79 ppm Ni, 1.69 ppm Fe | At. ferrooxidans | - | 2.0 | <1 mm | 1% | 303 K | 168 h | 32.44% | [35] |
| PCBs from end-of-life mobile phones | 3.98% Cu, 1.15% Ni, 0.014% Co, 0.19% Fe, 0.13% Al | At. ferrooxidans | - | - | 0.2–1.0 mm | 3% | 308 K | 144 h | 80% (3 cycles) | [36] |
| Composition of PCBs | Microbes Used | Additive | pH | Particle Size | Pulp Density | Temp (K) | Duration (h) | % Cu Extraction |
|---|---|---|---|---|---|---|---|---|
| 3.88% Cu, 0.169% Ni, 0.368% Co, 2.3% Fe, 1.92% Al, 0.021% W, 0.006% Cr. | At. ferrooxidans, At. thiooxidans | - | 2.0 | <150 µm | 8% | 308 K | 240 h | 93% |
| A. niger | - | 2.0 | <150 µm | 8% | 308 K | 240 h | 66% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abhilash; Tabassum, S.; Ghosh, A.; Meshram, P.; van Hullebusch, E.D. Microbial Processing of Waste Shredded PCBs for Copper Extraction Cum Separation—Comparing the Efficacy of Bacterial and Fungal Leaching Kinetics and Yields. Metals 2021, 11, 317. https://doi.org/10.3390/met11020317
Abhilash, Tabassum S, Ghosh A, Meshram P, van Hullebusch ED. Microbial Processing of Waste Shredded PCBs for Copper Extraction Cum Separation—Comparing the Efficacy of Bacterial and Fungal Leaching Kinetics and Yields. Metals. 2021; 11(2):317. https://doi.org/10.3390/met11020317
Chicago/Turabian StyleAbhilash, Shirin Tabassum, Anirban Ghosh, Pratima Meshram, and Eric D. van Hullebusch. 2021. "Microbial Processing of Waste Shredded PCBs for Copper Extraction Cum Separation—Comparing the Efficacy of Bacterial and Fungal Leaching Kinetics and Yields" Metals 11, no. 2: 317. https://doi.org/10.3390/met11020317







