Application of Active-Screen Plasma Nitriding to an Austenitic Stainless Steel Small-Diameter Thin Pipe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
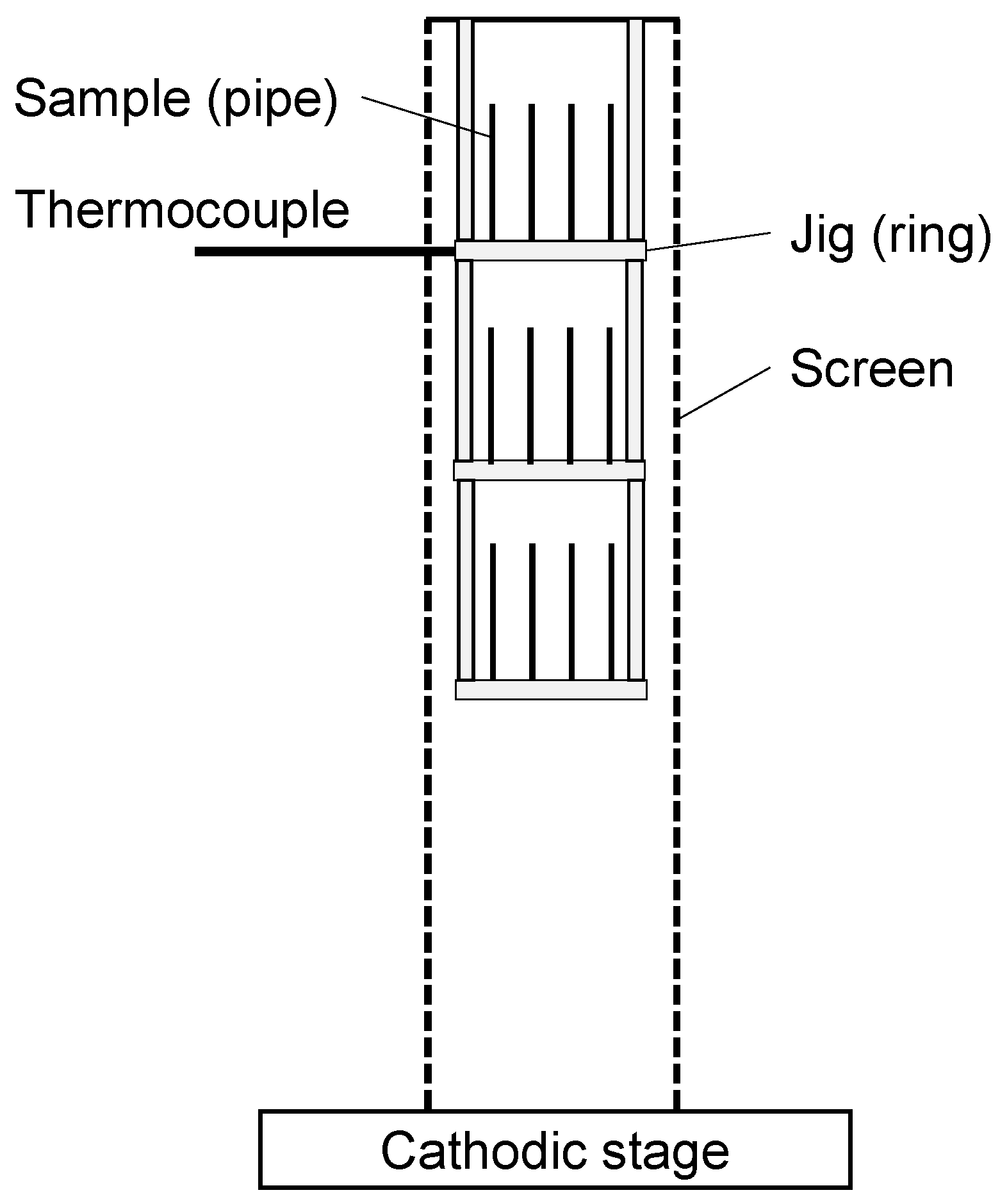
2.2. Plasma Nitriding
2.3. Characterization
3. Results and Discussion
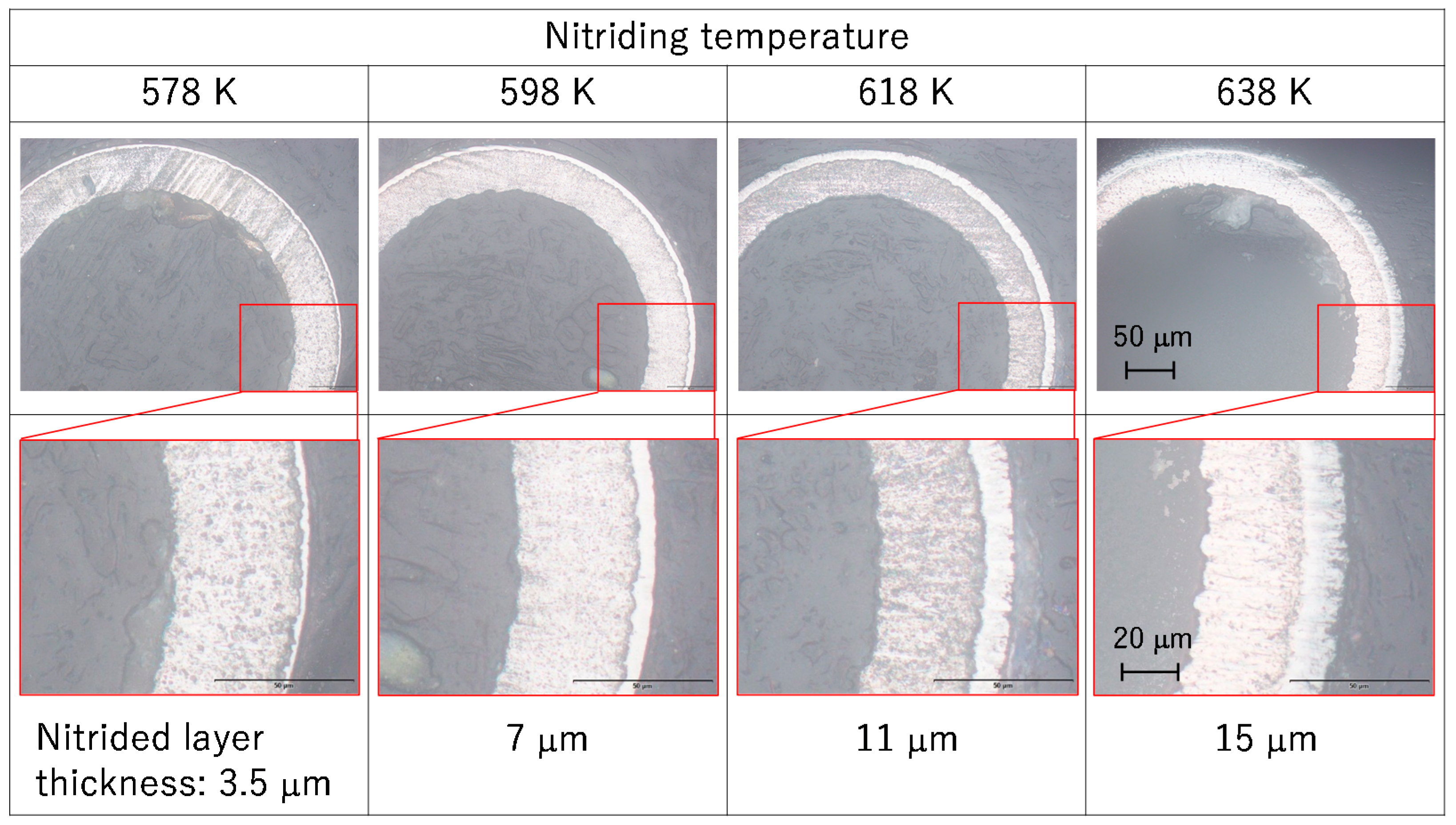
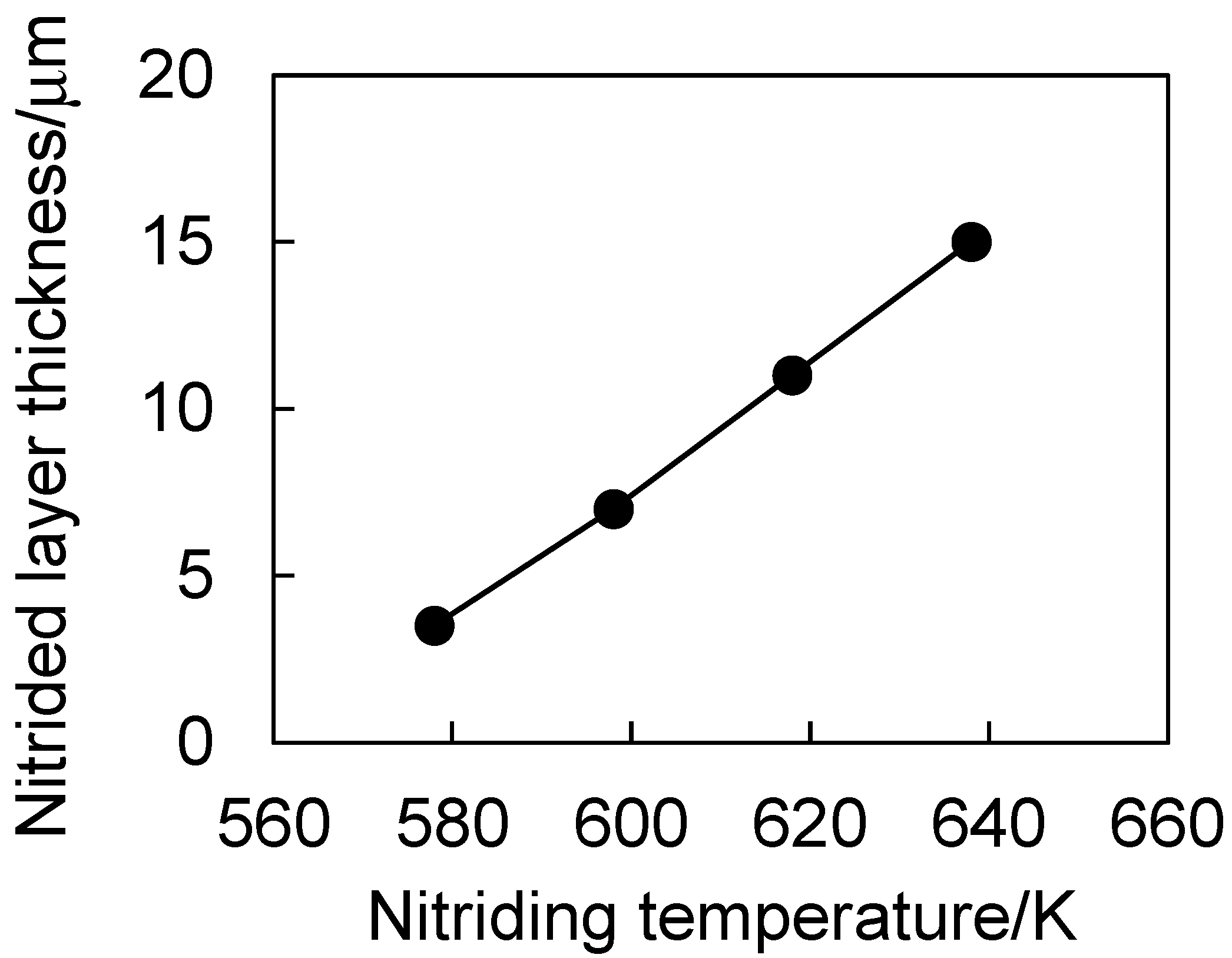
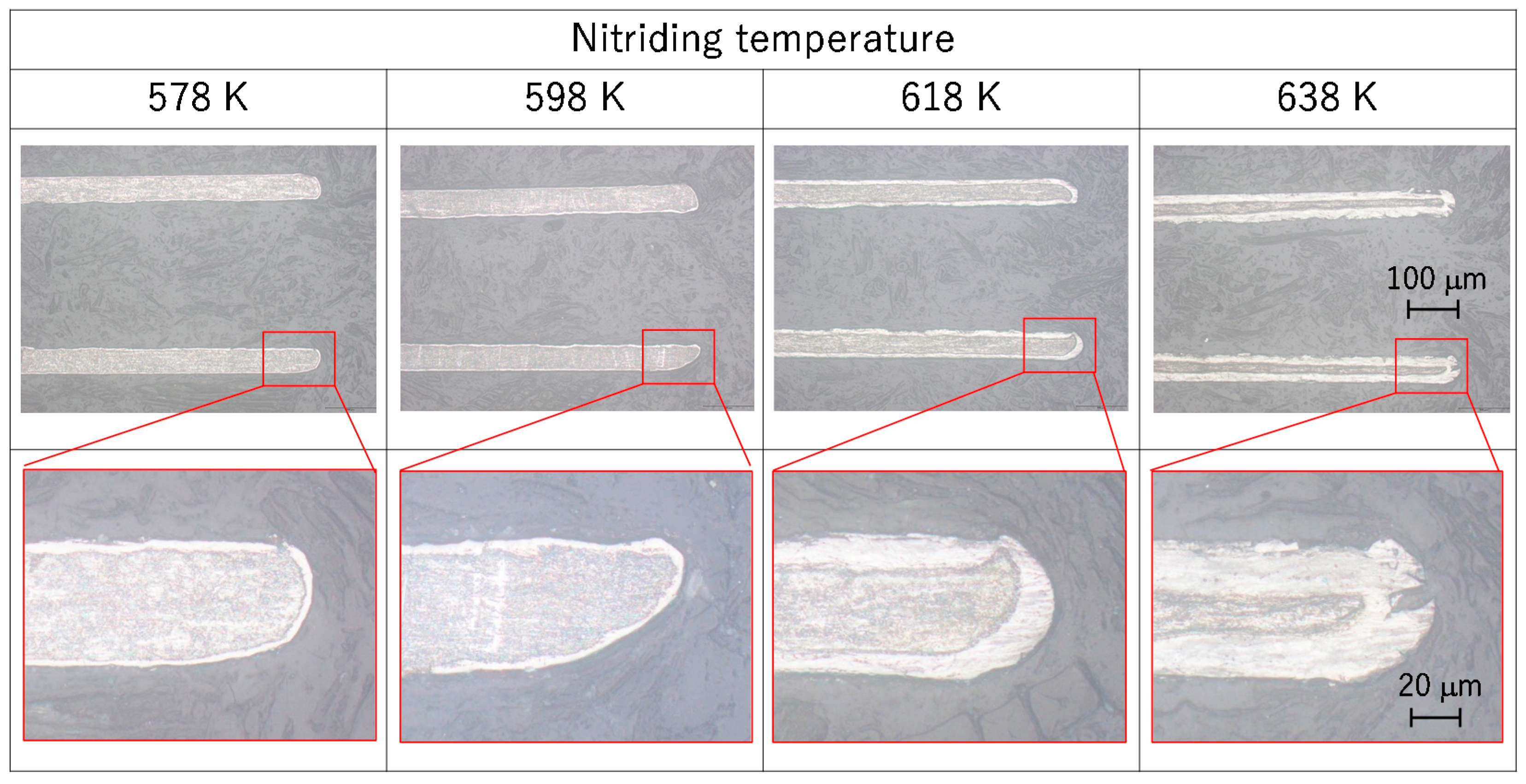
3.1. Nitrided Layer Thickness and Phase Structure
3.2. Mechanical Properties
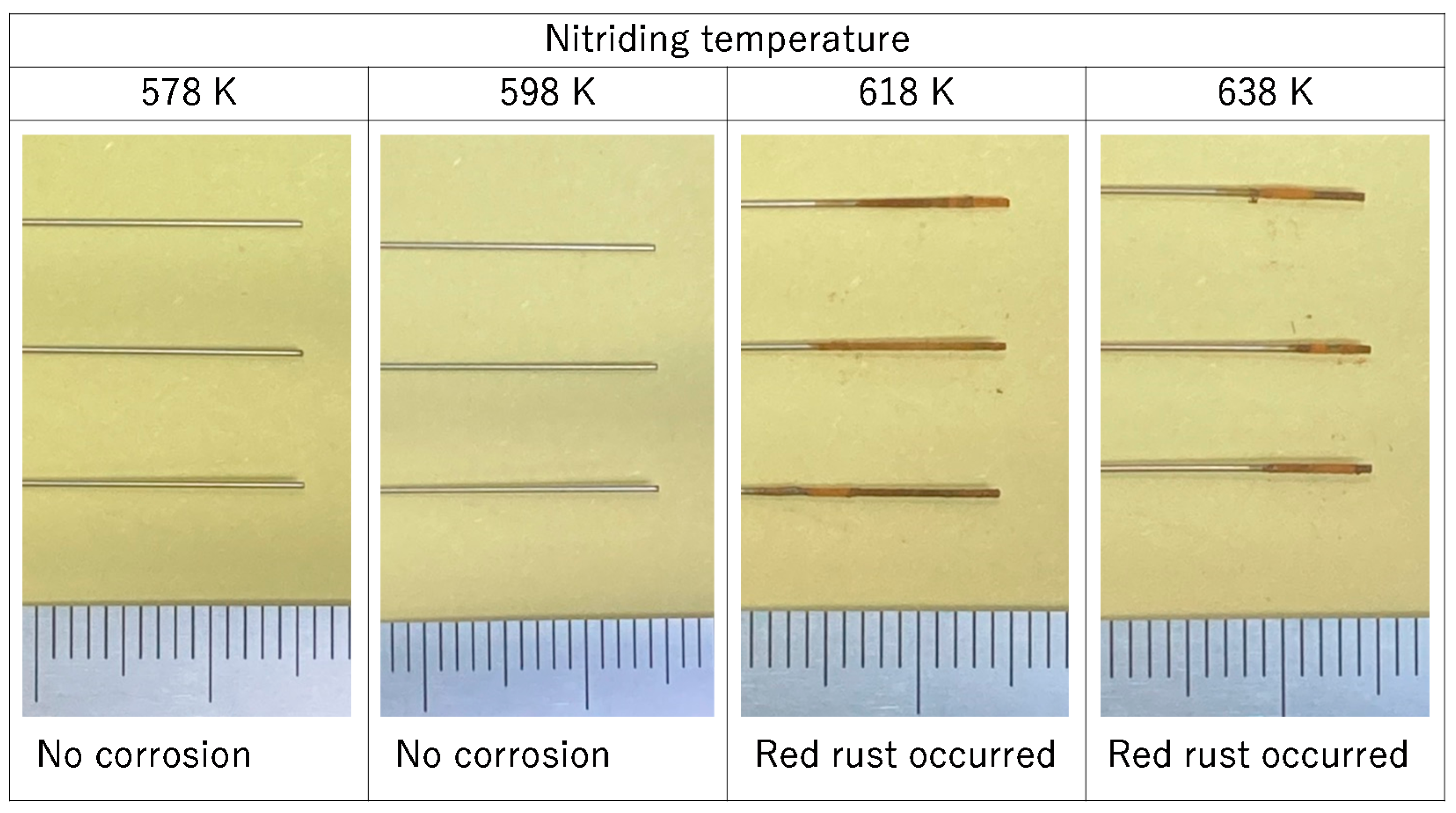
3.3. Corrosion Resistance
4. Conclusions
- An austenitic stainless-steel small-diameter thin pipe was successfully nitrided using low-temperature ASPN.
- The nitriding layer thickness increased monotonically with the nitriding temperature from 578 to 638 K. The nitriding layer thickness at 638 K was approximately 15 μm.
- The existence of expanded austenite (S phase) was revealed using the XRD pattern. At high nitriding temperatures of 618 and 638 K, peaks related to the formation of CrN were also observed.
- The bending load increased with the nitriding temperature because the nitriding layer became thicker and the surface hardness increased.
- At low nitriding temperatures of 578 and 598 K, the nitrided samples did not corrode in the corrosion test. At high nitriding temperatures of 618 and 638 K, corrosion was only observed near the tip of the pipe; no corrosion was observed near the center of the pipe.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, L. The use of stainless steel in structures. Prog. Struct. Eng. Mater. 2005, 7, 45–55. [Google Scholar] [CrossRef]
- Baddoo, N.R. Stainless steel in construction: A review of research, applications, challenges and opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Wang, H.T.; Wang, G.Z.; Xuan, F.Z.; Tu, S.T. Fracture mechanism of a dissimilar metal welded joint in nuclear power plant. Eng. Fail. Anal. 2013, 28, 134–148. [Google Scholar] [CrossRef]
- Liang, W.; Bin, X.; Zhiwei, Y.; Yaqin, S. The wear and corrosion properties of stainless steel nitrided by low-pressure plasma-arc source ion nitriding at low temperatures. Surf. Coat. Technol. 2000, 130, 304–308. [Google Scholar] [CrossRef]
- Dos Santos, J.F.; Garzón, C.M.; Tschiptschin, A.P. Improvement of the cavitation erosion resistance of an AISI 304L austenitic stainless steel by high temperature gas nitriding. Mater. Sci. Eng. A 2004, 382, 378–386. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. Low temperature gaseous nitriding and carburising of stainless steel. Surf. Eng. 2005, 21, 445–455. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Fujii, Y.; Terazawa, Y.; Nakashima, K.; Ando, T.; Takaki, S. Factors inducing intergranular fracture in nickel-free high nitrogen austenitic stainless steel produced by solution nitriding. ISIJ Int. 2008, 48, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Dossett, J.; Totten, G.E. Introduction to surface hardening of steels. ASM Handb. 2013, 4, 389–398. [Google Scholar]
- Zhang, X.; Wang, J.; Fan, H.; Pan, D. Erosion–corrosion resistance properties of 316L austenitic stainless steels after low-temperature liquid nitriding. Appl. Surf. Sci. 2018, 440, 755–762. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kang, J.; Hashimoto, F. Metastable austenitic stainless steel tool for magnetic abrasive finishing. CIRP Ann. 2011, 60, 339–342. [Google Scholar] [CrossRef]
- Le, H.M.; Cao, L.; Do, T.N.; Phee, S.J. Design and modelling of a variable stiffness manipulator for surgical robots. Mechatronics 2018, 53, 109–123. [Google Scholar] [CrossRef]
- Duerig, T.; Pelton, A.; Stöckel, D.J.M.S. An overview of nitinol medical applications. Mater. Sci. Eng. A 1999, 273, 149–160. [Google Scholar] [CrossRef]
- Ichii, K.; Fujimura, K.; Takase, T. Structure of the ion-nitrided layer of 18-8 stainless steel. Technol. Rep. Kansai Univ. 1986, 27, 135–144. [Google Scholar]
- Zhang, Z.L.; Bell, T. Structure and corrosion resistance of plasma nitrided stainless steel. Surf. Eng. 1985, 1, 131–136. [Google Scholar] [CrossRef]
- Li, X.Y. Low temperature plasma nitriding of 316 stainless steel—Nature of S phase and its thermal stability. Surf. Eng. 2001, 17, 147–152. [Google Scholar] [CrossRef]
- Dong, H. S-phase surface engineering of Fe-Cr, Co-Cr and Ni-Cr alloys. Int. Mater. Rev. 2010, 55, 6598. [Google Scholar] [CrossRef]
- Li, C.X.; Bell, T. Corrosion properties of active screen plasma nitrided 316 austenitic stainless steel. Corros. Sci. 2004, 46, 1527–1547. [Google Scholar] [CrossRef]
- Alves, C., Jr.; de Araüjo, F.O.; Ribeiro, K.J.B.; da Costa, J.A.P.; Sousa, R.R.M.; de Sousa, R.S. Use of cathodic cage in plasma nitriding. Surf. Coat. Technol. 2006, 201, 2450–2454. [Google Scholar] [CrossRef]
- Nishimoto, A.; Bell, T.E.; Bell, T. Feasibility study of active screen plasma nitriding of titanium alloy. Surf. Eng. 2010, 26, 79–84. [Google Scholar] [CrossRef]
- Nishimoto, A.; Nii, H.; Narita, R.; Akamatsu, K. Simultaneous duplex process of TiN coating and nitriding by active screen plasma nitriding. Surf. Coat. Technol. 2013, 228, S558–S562. [Google Scholar] [CrossRef]
- Nagamatsu, H.; Ichiki, R.; Yasumatsu, Y.; Inoue, T.; Yoshida, M.; Akamine, S.; Kanazawa, S. Steel nitriding by atmospheric-pressure plasma jet using N2/H2 mixture gas. Surf. Coat. Technol. 2013, 225, 26–33. [Google Scholar] [CrossRef]
- Naeem, M.; Waqas, M.; Jan, I.; Zaka-ul-Islam, M.; Díaz-Guillén, J.C.; Rehman, N.U.; Shafiq, M.; Zakaullah, M. Influence of pulsed power supply parameters on active screen plasma nitriding. Surf. Coat. Technol. 2016, 300, 67–77. [Google Scholar] [CrossRef]
- Nishimoto, A.; Fukube, T.; Tanaka, T. Effect of surface deposits on nitriding layer formation of active screen plasma nitriding. Mater. Trans. 2016, 57, 1811–1815. [Google Scholar] [CrossRef] [Green Version]
- Hoshiyama, Y.; Mizobata, R.; Miyake, H. Mechanical properties of austenitic stainless steel treated by active screen plasma nitriding. Surf. Coat. Technol. 2016, 307, 1041–1044. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Xiu, J.J.; Wang, W.; Zhu, Y.J.; Hu, B. Wear and corrosion properties of AISI 420 martensitic stainless steel treated by active screen plasma nitriding. Surf. Coat. Technol. 2017, 329, 184–192. [Google Scholar] [CrossRef]
- Dalke, A.; Burlacov, I.; Hamann, S.; Puth, A.; Böcker, J.; Spies, H.-J.; Röpcke, J.; Biermann, H. Solid carbon active screen plasma nitrocarburizing of AISI 316L stainless steel: Influence of N2-H2 gas composition on structure and properties of expanded austenite. Surf. Coat. Technol. 2019, 357, 1060–1068. [Google Scholar] [CrossRef]
- Miyamoto, J.; Abraha, P. The effect of plasma nitriding treatment time on the tribological properties of the AISI H13 tool steel. Surf. Coat. Technol. 2019, 375, 15–21. [Google Scholar] [CrossRef]
- Ichimura, S.; Takashima, S.; Tsuru, I.; Ohkubo, D.; Matsuo, H.; Goto, M. Application and evaluation of nitriding treatment using active screen plasma. Surf. Coat. Technol. 2019, 374, 210–221. [Google Scholar] [CrossRef]
- Naeem, M.; Raza, H.A.; Shafiq, M.; Shabbir, F.; Iqbal, J.; Diaz-Guillen, J.C.; Lopez-Badillo, C.M. Improved nitriding capability of nonalloyed steels assisted with active screen plasma treatment. Surf. Rev. Lett. 2020, 27, 1950118. [Google Scholar] [CrossRef]
- Sousa, F.A.; da Costa, J.A.P.; de Sousa, R.R.M.; Barbosa, J.C.P.; de Araújo, F.O. Internal coating of pipes using the cathodic cage plasma nitriding technique. Surf. Interfaces 2020, 21, 100691. [Google Scholar] [CrossRef]
- Toshioka, N.; Nishimoto, A. Surface-modified layer formed by plasma nitriding using chromium screen. Mater. Trans. 2020, 61, 1115–1121. [Google Scholar] [CrossRef]
- Morell-Pacheco, A.; Kim, H.; Wang, T.; Shiau, C.H.; Balerio, R.; Gabriel, A.; Shao, L. Ni coating on 316L stainless steel using cage plasma treatment: Feasibility and swelling studies. J. Nucl. Mater. 2020, 540, 152385. [Google Scholar] [CrossRef]




| C | Si | Mn | P | S | Ni | Cr |
|---|---|---|---|---|---|---|
| ≤0.08 | ≤1.00 | ≤2.00 | ≤0.045 | ≤0.030 | 8.00–10.50 | 18.00–20.00 |
| Nitriding Time/h | Nitriding Temperature/K | Gas Pressure/Pa | Gas Flow Ratio (N2:H2) | Discharge Voltage/V | Pulse Width/μs |
|---|---|---|---|---|---|
| 4 | 578, 598, 618, 638 | 200 | 1:1 | −500 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumiya, K.; Tokuyama, S.; Nishimoto, A.; Fukui, J.; Nishiyama, A. Application of Active-Screen Plasma Nitriding to an Austenitic Stainless Steel Small-Diameter Thin Pipe. Metals 2021, 11, 366. https://doi.org/10.3390/met11020366
Sumiya K, Tokuyama S, Nishimoto A, Fukui J, Nishiyama A. Application of Active-Screen Plasma Nitriding to an Austenitic Stainless Steel Small-Diameter Thin Pipe. Metals. 2021; 11(2):366. https://doi.org/10.3390/met11020366
Chicago/Turabian StyleSumiya, Kenzo, Shinkichi Tokuyama, Akio Nishimoto, Junichi Fukui, and Atsushi Nishiyama. 2021. "Application of Active-Screen Plasma Nitriding to an Austenitic Stainless Steel Small-Diameter Thin Pipe" Metals 11, no. 2: 366. https://doi.org/10.3390/met11020366






