Effect of Lead and Zinc Impurities in Ironmaking and the Corresponding Removal Methods: A Review
Abstract
:1. Introduction
1.1. Ironmaking Process
1.2. Impurities in the Ironmaking Process
2. Effect of Pb and Zn Impurities on the Ironmaking Process
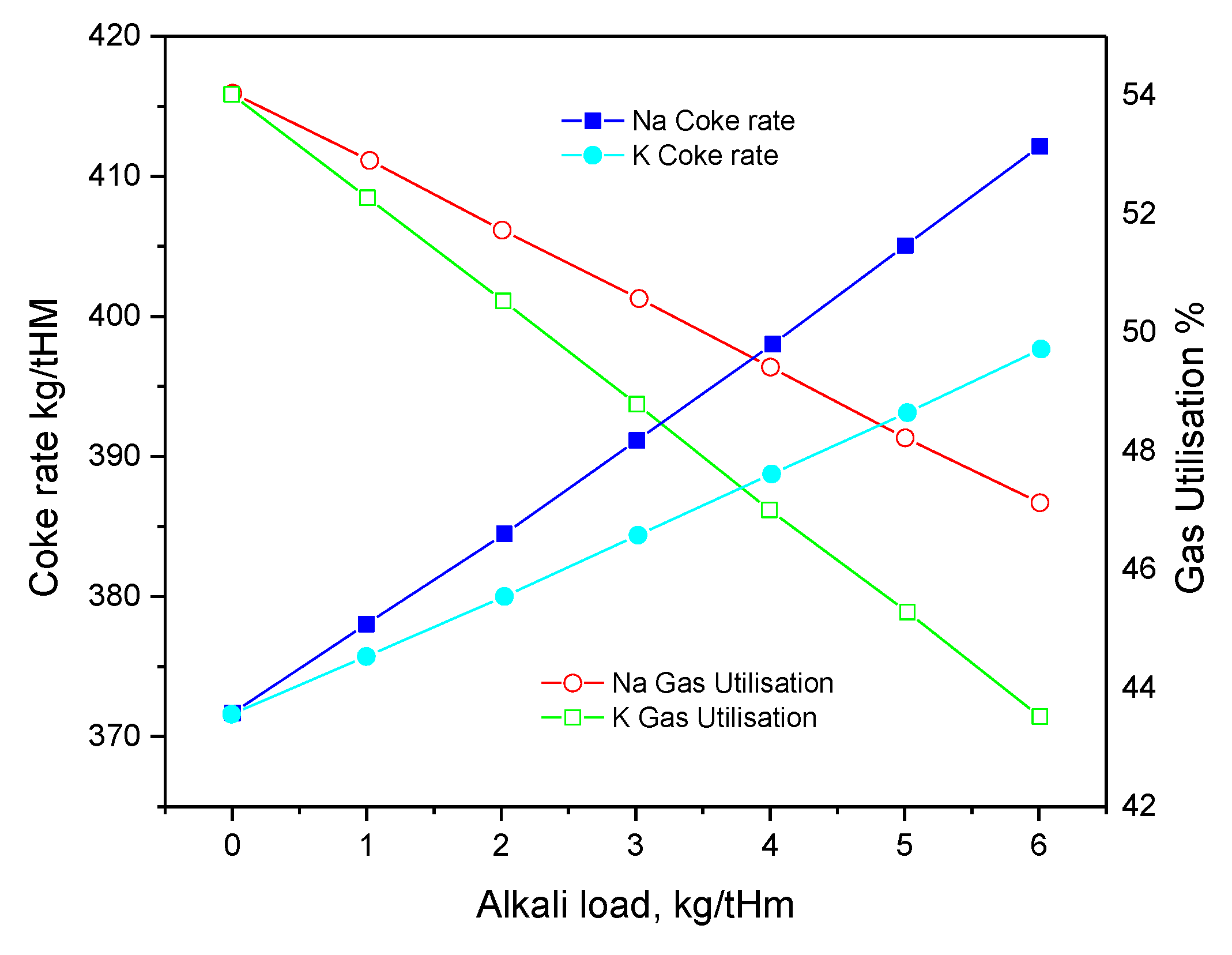
2.1. Effect of Pb on Ironmaking
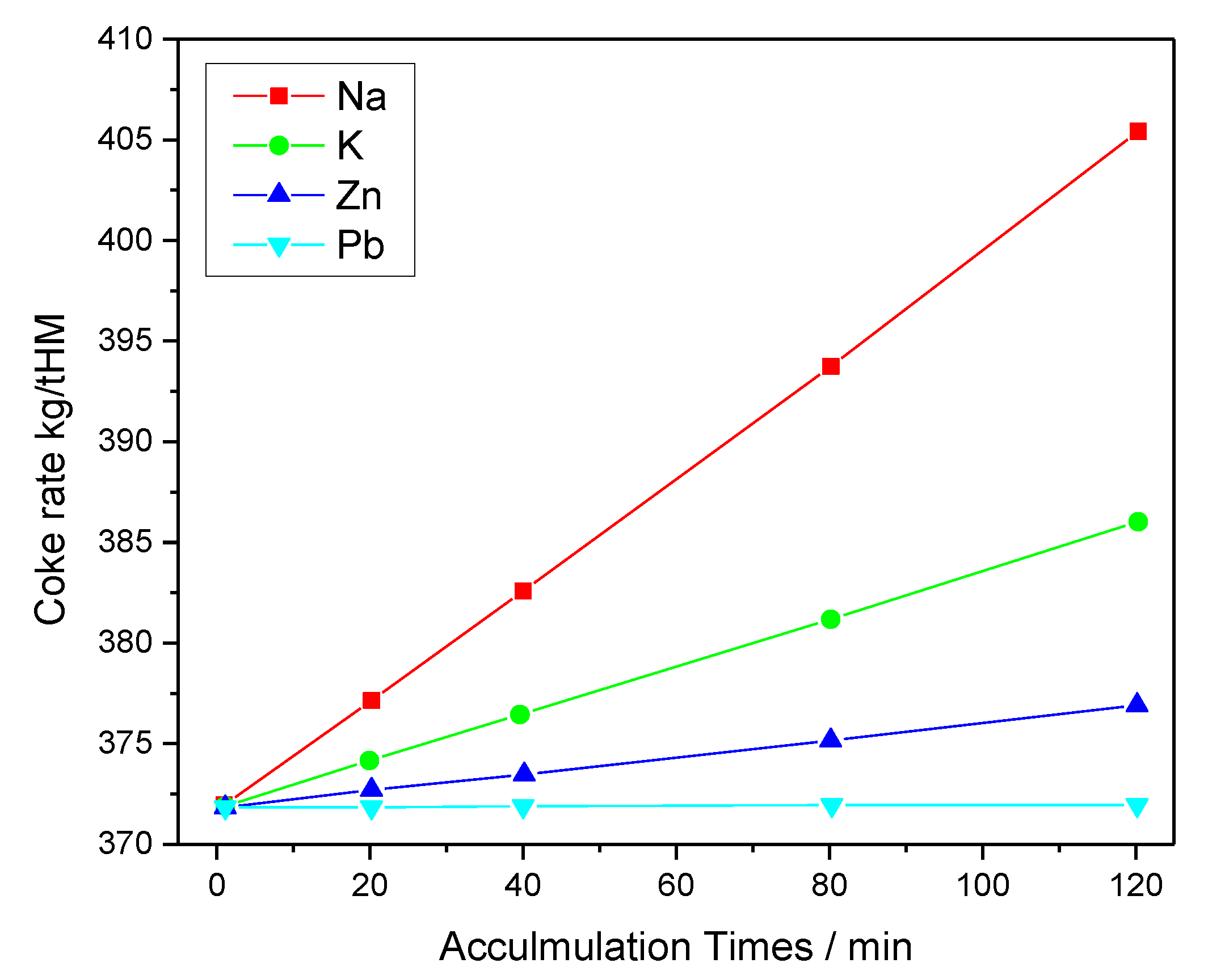
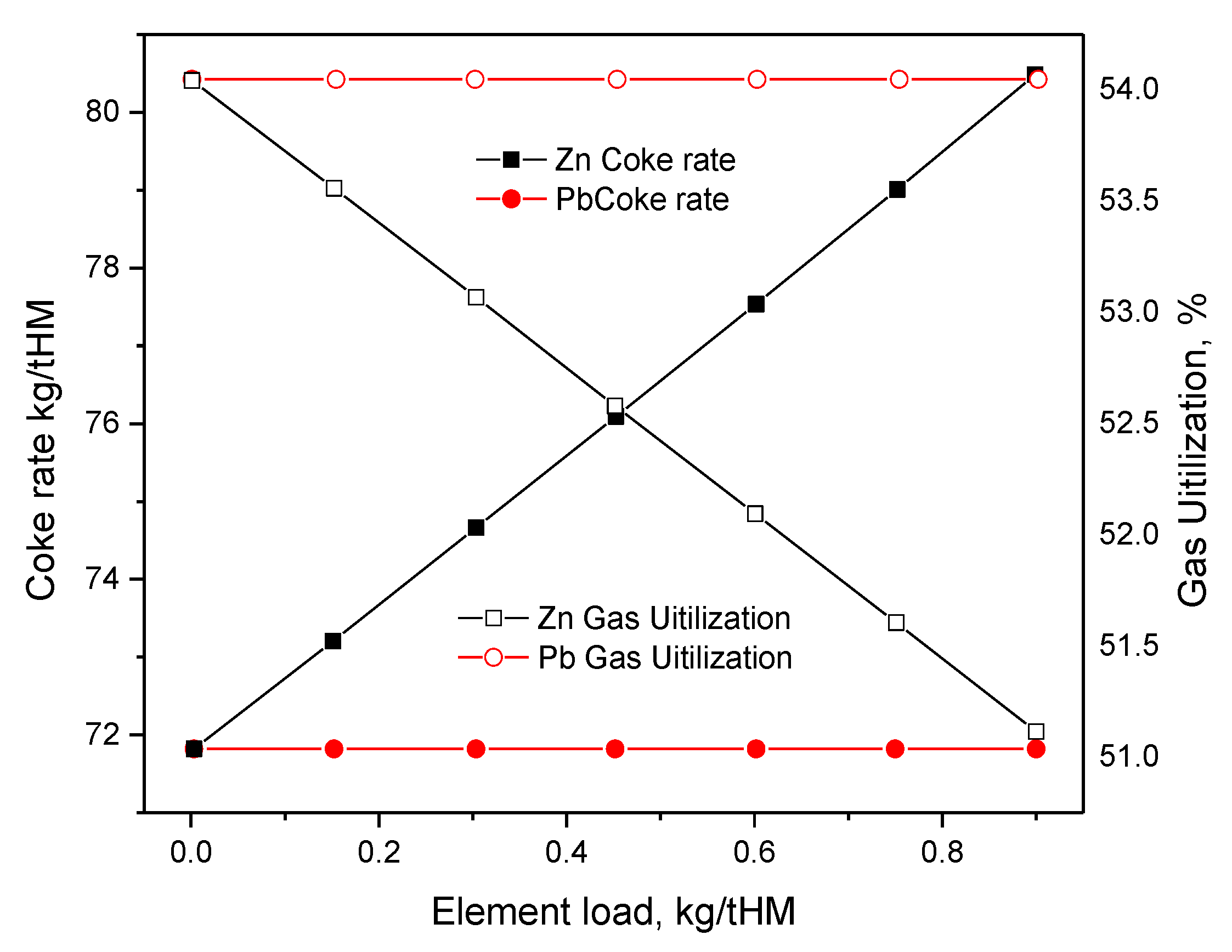
2.2. Effect of Zn on Ironmaking
2.3. Effect of Alkaline Elements on the Pb and Zn Present during the Ironmaking Process
2.4. Environmental Effect of Pb and Zn Impurities
3. Phase Changes and Characteristics in Metallurgical Reduction
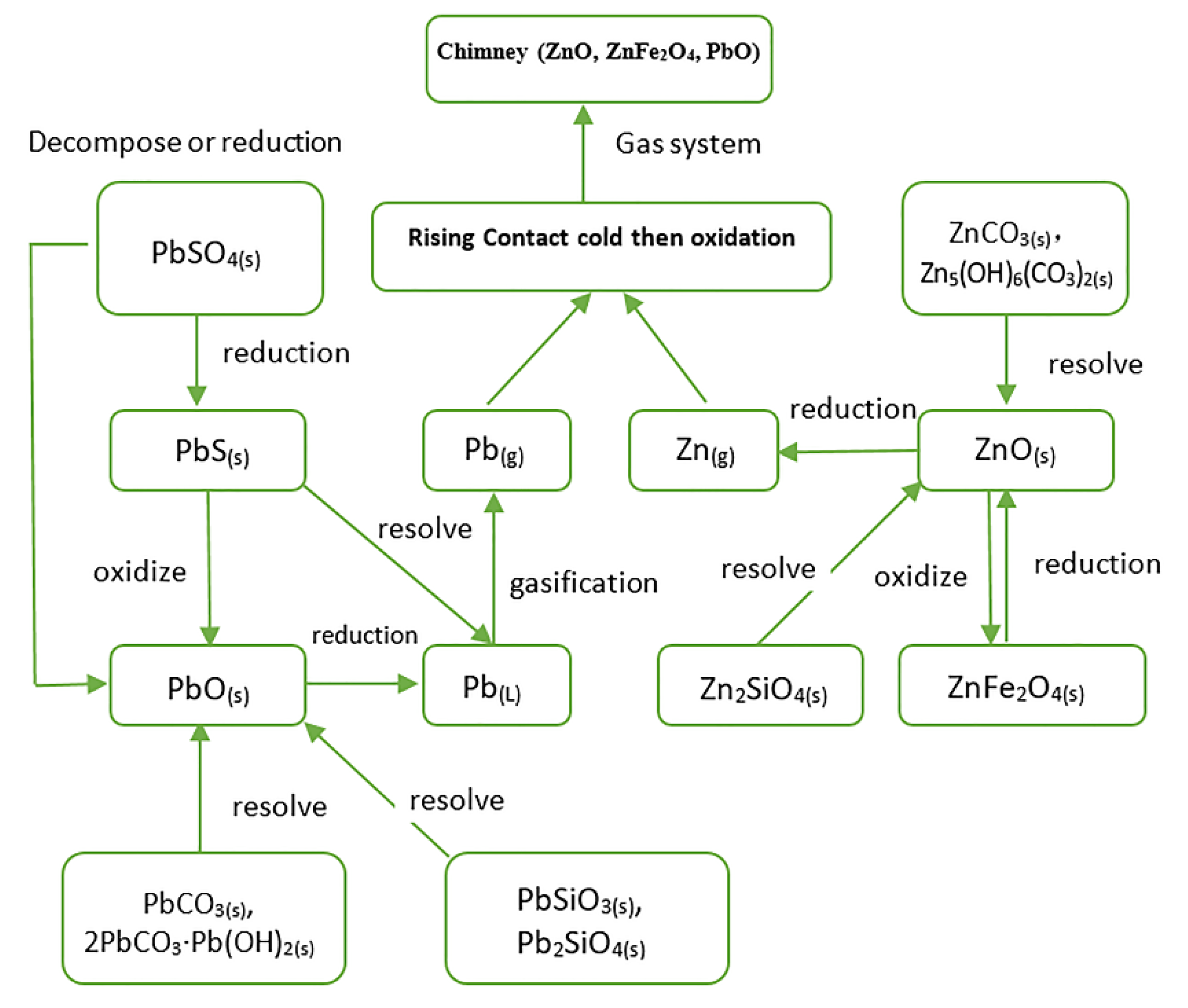
3.1. Characteristics of Pb and Zn Minerals during Ironmaking
3.2. Production and Occurrence State of Pb and Zn
3.3. Present Situation of the Pb–Zn Metallurgical Reduction Process
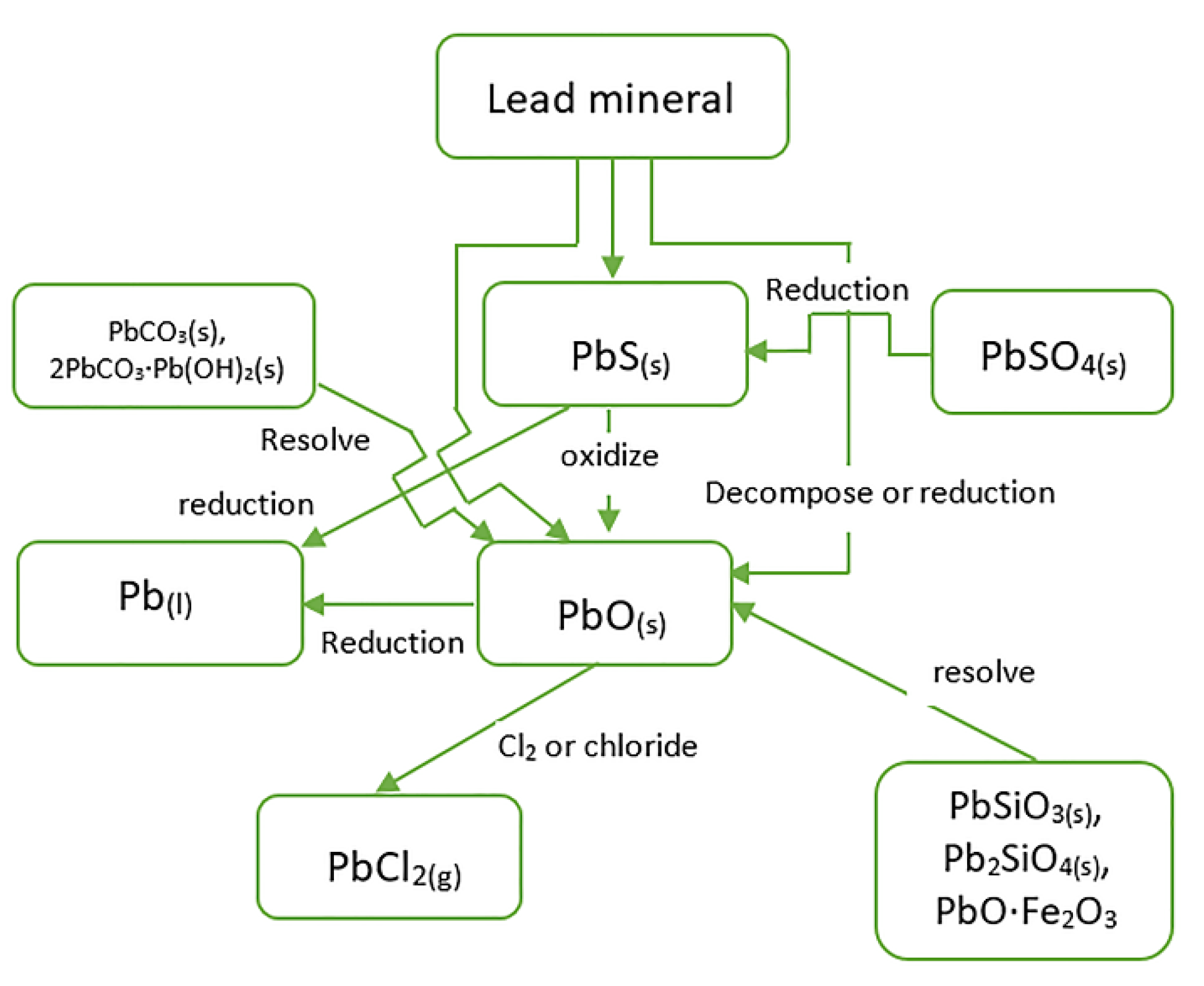
3.4. Reduction of Pb-Containing Minerals
3.5. Reduction of Zn-Bearing Minerals
4. Removing Lead and Zn Impurities from the Ironmaking Process
4.1. Removing Pb and Zn from Fe Dust
4.2. Selective Reduction of ZnFe2O4
4.3. Removing Pb and Zn from Sludge
4.4. Factors That Affect the Roasting Process
5. Conclusions
- (1)
- The distribution of the Pb–Zn–Fe in Fe ore is complicated, the particles are fine, and the removal of mineral phase at high temperature is difficult. Therefore, the production and occurrence of Pb and Zn impurities during the ironmaking process were analyzed.
- (2)
- The effect of Zn and Pb impurities in the iron industry, as well as the effect on the smelting output, is prominent on the melting furnace.
- (3)
- Analyzing phase shifts is important to separate Pb and Zn impurities in the iron industry properly. Therefore, phase transformations were examined during the smelting process.
- (4)
- Most of the removal treatments eliminated the Pb and Zn impurities on dust or sludge, but those in the furnaces remained.
- (5)
- After this study, the removal of Pb and Zn impurities before the ironmaking process is suggested. Therefore, this implication can serve as the basis for further research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| BOF | Basic oxygen furnace |
| EAF | Electric arc furnaces |
| BF | Blast furnace |
| BFS | Blast furnace sludge |
| HM | Hectometers |
| EAFD | Electric arc furnaces dust |
| DES | Dust in a eutectic solvent |
| QSL | Queneau–Schuhmann–Lurgi |
| ISA | Isasmelt process |
| SKS | A method used in smelting |
| SL/RN | Stelco–Lurgi/Republic–National process |
| ZLR | Zinc leaching residues |
References
- Haldar, S.K. Chapter 13—Mineral Processing. In Mineral Exploration, 2nd ed.; Haldar, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 259–290. [Google Scholar]
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Dai, B.; Wang, W.; Schenk, J.; Xue, Z. Effect of iron ore type on the thermal behaviour and kinetics of coal-iron ore briquettes during coking. Fuel Process. Technol. 2018, 173, 11–20. [Google Scholar] [CrossRef]
- Van Herck, P.; Carlo, V.; Rudy, V.; Ronald, M. Zinc and lead removal from blast furnace sludge with a hydrometallurgical process. Environ. Sci. Technol. 2000, 34, 3802–3808. [Google Scholar] [CrossRef]
- Yang, Y.; Raipala, K.; Holappa, L. Chapter 1.1—Ironmaking. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 2–88. [Google Scholar]
- Ma, N. Recycling of basic oxygen furnace steelmaking dust by in-process separation of zinc from the dust. J. Clean. Prod. 2016, 112, 4497–4504. [Google Scholar] [CrossRef]
- Bailong, L. Recovery of gold and iron from the cyanide tailings by magnetic roasting. Rare Met. Mater. Eng. 2013, 42, 1805–1809. [Google Scholar] [CrossRef]
- Forster, J.; Pickles, C.A.; Elliott, R. Microwave carbothermic reduction roasting of a low grade nickeliferous silicate laterite ore. Miner. Eng. 2016, 88, 18–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Yu, X. Recovery of iron from cyanide tailings with reduction roasting–water leaching followed by magnetic separation. J. Hazard. Mater. 2012, 213, 167–174. [Google Scholar] [CrossRef]
- Peng, N.; Peng, B.; Li, C.; Li, M.; Wang, J.; Yan, H.; Yuan, Y. Recovery of iron from zinc calcines by reduction roasting and magnetic separation. Miner. Eng. 2012, 35, 57–60. [Google Scholar] [CrossRef]
- Han, J.; Liu, W.; Qin, W.; Peng, B.; Yang, K.; Zheng, Y. Recovery of zinc and iron from high iron-bearing zinc calcine by selective reduction roasting. J. Ind. Eng. Chem. 2015, 22, 272–279. [Google Scholar] [CrossRef]
- Gupta, S.; French, D.; Sakurovs, R.; Grigore, M.; Sun, H.; Cham, T.; Hilding, T.; Hallin, M.; Lindblom, B.; Sahajwalla, V. Minerals and iron-making reactions in blast furnaces. Prog. Energy Combust. Sci. 2008, 34, 155–197. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Liu, H.; Peng, B.; Liu, Z. Iron extraction from lead slag by bath smelting. Trans. Nonferrous Met. Soc. China 2017, 27, 1862–1869. [Google Scholar] [CrossRef]
- Bhateria, R.; Singh, R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J. Water Process Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Yang, T.; Rao, S.; Hu, W.; Liu, W.; Chen, L. Selective leaching of zinc from blast furnace dust with mono-ligand and mixed-ligand complex leaching systems. Hydrometallurgy 2017, 169, 219–228. [Google Scholar] [CrossRef]
- Min, X.; Xie, X.; Chai, L.; Liang, Y.; Li, M.; Ke, Y. Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue. Trans. Nonferrous Met. Soc. China 2013, 23, 208–218. [Google Scholar] [CrossRef]
- Yang, X.; Chu, M.; Shen, F.; Zhang, Z. Mechanism of zinc damaging to blast furnace tuyere refractory. Acta Metall. Sin. (Engl. Lett.) 2009, 22, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Trinkel, V.; Aschenbrenner, P.; Thaler, C.; Rechberger, H.; Mallow, O.; Fellner, J. Distribution of Zn, Pb, K, and Cl in blast furnace lining. Steel Res. Int. 2017, 88, 7. [Google Scholar] [CrossRef]
- Zuo, H.B.; Wang, C.; Zhang, J.L.; Zhao, Y.G.; Jiao, K.X. Application of carbon composite bricks for blast furnace hearth. In 6th International Symposium on High-Temperature Metallurgical Processing; Jiang, T., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 595–602. [Google Scholar]
- Mikhailov, I.; Komarov, S.; Levina, V.; Gusev, A.; Issi, J.; Kuznetsov, D. Nanosized zero-valent iron as Fenton-like reagent for ultrasonic-assisted leaching of zinc from blast furnace sludge. J. Hazard. Mater. 2017, 321, 557–565. [Google Scholar] [CrossRef]
- Esezobor, D.E.; Balogun, S.A. Zinc accumulation during recycling of iron oxide wastes in the blast furnace. Ironmak. Steelmak. 2006, 33, 419–425. [Google Scholar] [CrossRef]
- Moradi, S.; Monhemius, A.J. Mixed sulphide–oxide lead and zinc ores: Problems and solutions. Miner. Eng. 2011, 24, 1062–1076. [Google Scholar] [CrossRef]
- Jiao, K.X.; Zhang, J.; Liu, Z.; Chen, C.; Liu, F. Circulation and accumulation of harmful elements in blast furnace and their impact on the fuel consumption. Ironmak. Steelmak. 2017, 44, 344–350. [Google Scholar] [CrossRef]
- Asadi Zeydabadi, B.; Mowla, D.; Shariat, M.; Kalajahi, J. Zinc recovery from blast furnace flue dust. Hydrometallurgy 1997, 47, 113–125. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, J. Study on the law of zinc absorption and change of metallurgical property of sinter and pellet. Iron Steel 2010, 45, 15–18. [Google Scholar]
- Kelebek, S.; Yörük, S.; Davis, B. Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Miner. Eng. 2004, 17, 285–291. [Google Scholar] [CrossRef]
- Li, Q.; Rao, X.; Xu, B.; Yang, Y.; Liu, T.; Jiang, T.; Hu, L. Extraction of manganese and zinc from their compound ore by reductive acid leaching. Trans. Nonferrous Met. Soc. China 2017, 27, 1172–1179. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Xu, R.; Yu, Y.; Jin, Y.; Xue, Z. Influence mechanism of zinc on the solution loss reaction of coke used in blast furnace. Fuel Process. Technol. 2017, 159, 118–127. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. Recycling of cupola furnace dust: Extraction and electrodeposition of zinc in deep eutectic solvents. J. Alloy. Compd. 2019, 771, 424–432. [Google Scholar] [CrossRef]
- Li, K.; Khanna, R.; Zhang, J.; Liu, Z.; Sahajwalla, V.; Yang, T.; Kong, D. The evolution of structural order, microstructure and mineral matter of metallurgical coke in a blast furnace: A review. Fuel 2014, 133, 194–215. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhang, Z.; Zhang, G. Removal of zinc from basic oxygen steelmaking filter cake by selective leaching with butyric acid. J. Clean. Prod. 2019, 209, 1–9. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Hursthouse, A.; Cuthbert, S. The potential of sequential extraction in the characterisation and management of wastes from steel processing: A prospective review. Int. J. Environ. Res. Public Health 2015, 12, 11724–11755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.A.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A review on lead slag generation, characteristics, and utilization. Resour. Conserv. Recycl. 2019, 146, 140–155. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Liu, H.; Peng, B. Clean strengthening reduction of lead and zinc from smelting waste slag by iron oxide. J. Clean. Prod. 2017, 143, 311–318. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Zhu, X.; Peng, J.; Li, S.; Ma, A.; Li, H.; Zhu, F. Role of manganese dioxide in the recovery of oxide–sulphide zinc ore. J. Hazard. Mater. 2018, 343, 315–323. [Google Scholar] [CrossRef]
- Wang, H.G. A novel hydrothermal method for zinc extraction and separation from zinc ferrite and electric arc furnace dust. Int. J. Miner. Metall. Mater. 2016, 23, 146–155. [Google Scholar] [CrossRef]
- Kukurugya, F.; Rahfeld, A.; Möckel, R.; Nielsen, P.; Horckmans, L.; Spooren, J.; Broos, K. Recovery of iron and lead from a secondary lead smelter matte by magnetic separation. Miner. Eng. 2018, 122, 17–25. [Google Scholar] [CrossRef]
- Martins, F.M.; Neto, J.M.d.R.; Cunha, C.J.d. Mineral phases of weathered and recent electric arc furnace dust. J. Hazard. Mater. 2008, 154, 417–425. [Google Scholar] [CrossRef]
- Pickles, C.A. Thermodynamic analysis of the selective carbothermic reduction of electric arc furnace dust. J. Hazard. Mater. 2008, 150, 265–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Li, X. Zinc recovery from franklinite by sulphation roasting. Hydrometallurgy 2011, 109, 211–214. [Google Scholar] [CrossRef]
- Jankovic, B.; Stopić, S.; Güven, A.; Friedrich, B. Kinetic modeling of thermal decomposition of zinc ferrite from neutral leach residues based on stochastic geometric model. J. Magn. Magn. Mater. 2014, 358, 105–118. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Olivas-martinez, M. Chapter 2.3—lead and zinc production. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 671–700. [Google Scholar]
- Pickles, C.A. Thermodynamic analysis of the selective chlorination of electric arc furnace dust. J. Hazard. Mater. 2009, 166, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, Y.; Lu, M.; Su, Z.; Li, G.; Jiang, T. Extraction and separation of manganese and iron from ferruginous manganese ores: A review. Miner. Eng. 2019, 131, 286–303. [Google Scholar] [CrossRef]
- Boyanova, B.; Peltekov, A.; Petkova, V. Thermal behavior of zinc sulfide concentrates with different iron content at oxidative roasting. Thermochim. Acta 2014, 586, 9–16. [Google Scholar] [CrossRef]
- Leclerc, N.; Meux, E.; Lecuire, J.-M. Hydrometallurgical extraction of zinc from zinc ferrites. Hydrometallurgy 2003, 70, 175–183. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wei, C.; Liu, C.; Jiang, J.; Wang, K. Sulfidation roasting of low grade lead-zinc oxide ore with elemental sulfur. Miner. Eng. 2010, 23, 563–566. [Google Scholar] [CrossRef]
- Leclerc, N.; Meux, E.; Lecuire, J.-M. Hydrometallurgical recovery of zinc and lead from electric arc furnace dust using mononitrilotriacetate anion and hexahydrated ferric chloride. J. Hazard. Mater. 2002, 91, 257–270. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Liu, Z.; Li, Y.; Li, S.; Zhang, W.; Li, Q. Leaching of iron concentrate separated from kiln slag in zinc hydrometallurgy with hydrochloric acid and its mechanism. Trans. Nonferrous Met. Soc. China 2017, 27, 901–907. [Google Scholar] [CrossRef]
- Luo, L.; Nguyen, A.V. A review of principles and applications of magnetic flocculation to separate ultrafine magnetic particles. Sep. Purif. Technol. 2017, 172, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Peng, Z.; Yan, J.; Li, Z.; Yang, H.; Wang, J.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Clean. Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- Jaafar, I.; Griffiths, A.; Hopkins, A.; Steer, J.; Griffiths, M.; Sapsford, D. An evaluation of chlorination for the removal of zinc from steelmaking dusts. Miner. Eng. 2011, 24, 1028–1030. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Peng, B.; Min, X.; Hu, M.; Peng, N.; Yuang, Y.; Lei, J. Study on separating of zinc and iron from zinc leaching residues by roasting with ammonium sulphate. Hydrometallurgy 2015, 158, 42–48. [Google Scholar] [CrossRef]
- Oustadakis, P.; Tsakiridis, P.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD): Part I: Characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Herrero, D.; Arias, P.; Güemez, B.; Barrio, V.; Cambra, J.; Requies, J. Hydrometallurgical process development for the production of a zinc sulphate liquor suitable for electrowinning. Miner. Eng. 2010, 23, 511–517. [Google Scholar] [CrossRef]
- Han, J.; Liu, W.; Qin, W.; Yang, K.; Wang, D.; Luo, H. Innovative methodology for comprehensive utilization of high iron bearing zinc calcine. Sep. Purif. Technol. 2015, 154, 263–270. [Google Scholar] [CrossRef]
- Suetens, T.; Guo, M.; Acker, K.; Blanpain, B. Formation of the ZnFe2O4 phase in an electric arc furnace off-gas treatment system. J. Hazard. Mater. 2015, 287, 180–187. [Google Scholar] [CrossRef]
- Yu, G.; Peng, N.; Zhou, L.; Liang, Y.; Zhou, X.; Peng, B.; Chai, L.; Yang, Z. Selective reduction process of zinc ferrite and its application in treatment of zinc leaching residues. Trans. Nonferrous Met. Soc. China 2015, 25, 2744–2752. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Xiao, X. Recovery of metals from the roasted lead-zinc tailings by magnetizing roasting followed by magnetic separation. J. Clean. Prod. 2017, 158, 73–80. [Google Scholar] [CrossRef]
- Li, M.; Peng, B.; Chai, L.; Peng, N.; Yan, H.; Hou, D. Recovery of iron from zinc leaching residue by selective reduction roasting with carbon. J. Hazard. Mater. 2012, 237, 323–330. [Google Scholar] [CrossRef]
- Zhu, D.; Li, S.; Pan, J.; Yang, C.; Shi, B. Migration and distributions of zinc, lead and arsenic within sinter bed during updraft pre-reductive sintering of iron-bearing wastes. Powder Technol. 2019, 342, 864–872. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T. Improved removal of zinc from blast furnace sludge by particle size separation and microwave heating. Miner. Eng. 2018, 127, 265–276. [Google Scholar] [CrossRef]
- Jiang, G.-M.; Peng, B.; Lian, Y.; Chian, L.; Wang, Q.; Li, Q.; Hu, M. Recovery of valuable metals from zinc leaching residue by sulfate roasting and water leaching. Trans. Nonferrous Met. Soc. China 2017, 27, 1180–1187. [Google Scholar] [CrossRef]
- Cantarino, M.V.; de Carvalho Filho, C.; Borges Mansur, M. Selective removal of zinc from basic oxygen furnace sludges. Hydrometallurgy 2012, 111, 124–128. [Google Scholar] [CrossRef]
- Rath, S.S.; Rao, D.S.; Mishra, B.K. A novel approach for reduction roasting of iron ore slime using cow dung. Int. J. Miner. Process. 2016, 157, 216–226. [Google Scholar] [CrossRef]
- Luong, V.T.; Kang, D.; An, J.; Dao, D.; Kim, M.; Tran, T. Iron sulphate roasting for extraction of lithium from lepidolite. Hydrometallurgy 2014, 141, 8–16. [Google Scholar] [CrossRef]
- Peng, T.F.; Gao, X.; Li, Q.; Xu, L.; Luo, L.; Xu, L. Phase transformation during roasting process and magnetic beneficiation of oolitic-iron ores. Vacuum 2017, 146, 63–73. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, L.; Yang, C.; Ye, Q.; Chen, J.; Peng, J.; Srinivasakannan, C.; Li, W. Kinetics of microwave roasting of zinc slag oxidation dust with concentrated sulfuric acid and water leaching. Chem. Eng. Process. Process Intensif. 2015, 97, 75–83. [Google Scholar] [CrossRef]
- Faris, N.; Tardio, J.; Ram, R.; Bhargava, S.; Pownceby, M. Investigation into coal-based magnetizing roasting of an iron-rich rare earth ore and the associated mineralogical transformations. Miner. Eng. 2017, 114, 37–49. [Google Scholar] [CrossRef]
- Luo, L.Q.; Huang, H.; Yu, Y.F. Characterization and technology of fast reducing roasting for fine iron materials. J. Cent. South Univ. 2012, 19, 2272–2278. [Google Scholar] [CrossRef]





| Fe Concentrate | Sinter and Pellet | Ironmaking Furnace Dust | Furnace Tumor |
|---|---|---|---|
| PbS, PbO, PbSO4 | PbSiO3 | PbO | Middle part of the furnace’s body is CaSiO3 |
| ZnS, ZnCO3 | ZnS, ZnFe2O4, Zn2SiO4 | ZnO, ZnFe2O4, Zn2SiO4 | Upper part of the furnace’s body is ZnO |
| Source | Component | Specificity |
|---|---|---|
| Natural Pb mineral | PbS, lead oxide ore, and bauxite (PbCO3) | Most of the Pb in nature exists in the form of PbS |
| Fe ore | PbS, PbO, PbSO4, and PbCO3 or basic lead carbonate and lead silicate | The Pb content in BF is minimal. The main Pb minerals are PbO and Pb |
| Pb-bearing sinter and pellet | The order of mineral the content of Pb is PbO > PbO·SiO2 and 2PbO·SiO2 > lead ferrate > PbSO4; metal Pb, and PbS. | Roasting causes desulfurization of sulfides to form oxides |
| Natural Zn mineral | Mainly sphalerite (ZnS), part red zinc ore ZnO, rhomsonite (ZnCO3), Zn2SiO4, and ZnFe2O4 | Zn in nature often exists in the forms of ZnS, but some exist as oxidized ores |
| Fe ore | Zn exists in the form of ZnCO3, ZnS, ZnFe2O4, and Zn2SiO4 | The content of Zn in BF is minimal, mainly in the form of ZnO and Zn |
| Zn-bearing sinter and pellets | ZnO, Zn2SiO4, ZnFe2O4, and a small amount of ZnS | The ZnSO4 and ZnS contents given good desulphurization by sintering is minimal |
| Zn dust | The order of the contents of Fe and Zn minerals is ZnFe2O4 > ZnO > Zn2SiO4 > ZnS and ZnSO4 | ZnO is easy to leach, but ZnFe2O4 is difficult to dissolve |
| Classification | Characteristics | Technology | Specificity | |
|---|---|---|---|---|
| Pyrogenic process of Pb smelting | Modern Pb productions are almost in the form of pyrometallurgical lead smelting, which can be divided into BF and direct Pb smelting | BF | Separation of the oxidation stage of the Pb concentrate from the reduction stage | |
| Smelting in suspension | Kivcet method | Oxygen-rich smelting | ||
| Pool melting | QSL method | Oxygen-rich bottom blowing furnace | ||
| ISA, Asmelt method | Oxygen-rich top blowing furnace | |||
| Vanukov method | Oxygen-enriched side blowing furnace | |||
| SKS method | Bottom-blowing oxidation BF reduction | |||
| Both | Kaldo method | Oxygen top blowing Pb smelting process | ||
| Pyrogenic process of Zn smelting | Accounts for 30% of the total Zn production in China. The vertical tank method accounts for 60%, the ISP method accounts for 31.6%, and the other methods cover a small percentage | Vertical tank method | The recovery rate of Zn is 95% and the utilization rate of sulfur is more than 94% | |
| ISP method | The recovery rate of Zn is 93.8%, Pb and Zn can be produced at the same time, and the processing cost is low | |||
| Electric furnace method | Zn products containing more than 99.99% Zn can be obtained | |||
| Other soil methods | Small-scale and requires low investment | |||
| Recovery of Zn-containing dust through fire method | Rotary kiln process, where the rotary bottom furnace process is the most representative | Rotary kiln | Comprehensive utilization of Fe and Zn from low Zn dust | |
| Rotary hearth furnace | High efficiency and low energy requirements, effective recovery of metals (e.g., Fe and Zn) | |||
| Circulating fluidized bed | Use of good gas dynamics conditions in fluidized bed to reduce energy consumption | |||
| Melt reduction | Zinc removal complete and the hot metal quality is high, but the object contains abundant Zn dust | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, S.; Luo, L.; Zheng, B.-T.; Wei, C.-X.; Christophe, N. Effect of Lead and Zinc Impurities in Ironmaking and the Corresponding Removal Methods: A Review. Metals 2021, 11, 407. https://doi.org/10.3390/met11030407
Mustafa S, Luo L, Zheng B-T, Wei C-X, Christophe N. Effect of Lead and Zinc Impurities in Ironmaking and the Corresponding Removal Methods: A Review. Metals. 2021; 11(3):407. https://doi.org/10.3390/met11030407
Chicago/Turabian StyleMustafa, Sayaf, Liqun Luo, Bo-Tao Zheng, Chen-Xi Wei, and Niyonzima Christophe. 2021. "Effect of Lead and Zinc Impurities in Ironmaking and the Corresponding Removal Methods: A Review" Metals 11, no. 3: 407. https://doi.org/10.3390/met11030407






