Enhanced Extracellular Synthesis of Gold Nanoparticles by Soluble Extracts from Escherichia coli Transformed with Rhizobium tropici Phytochelatin Synthase Gene
Abstract
1. Introduction
2. Materials and Methods
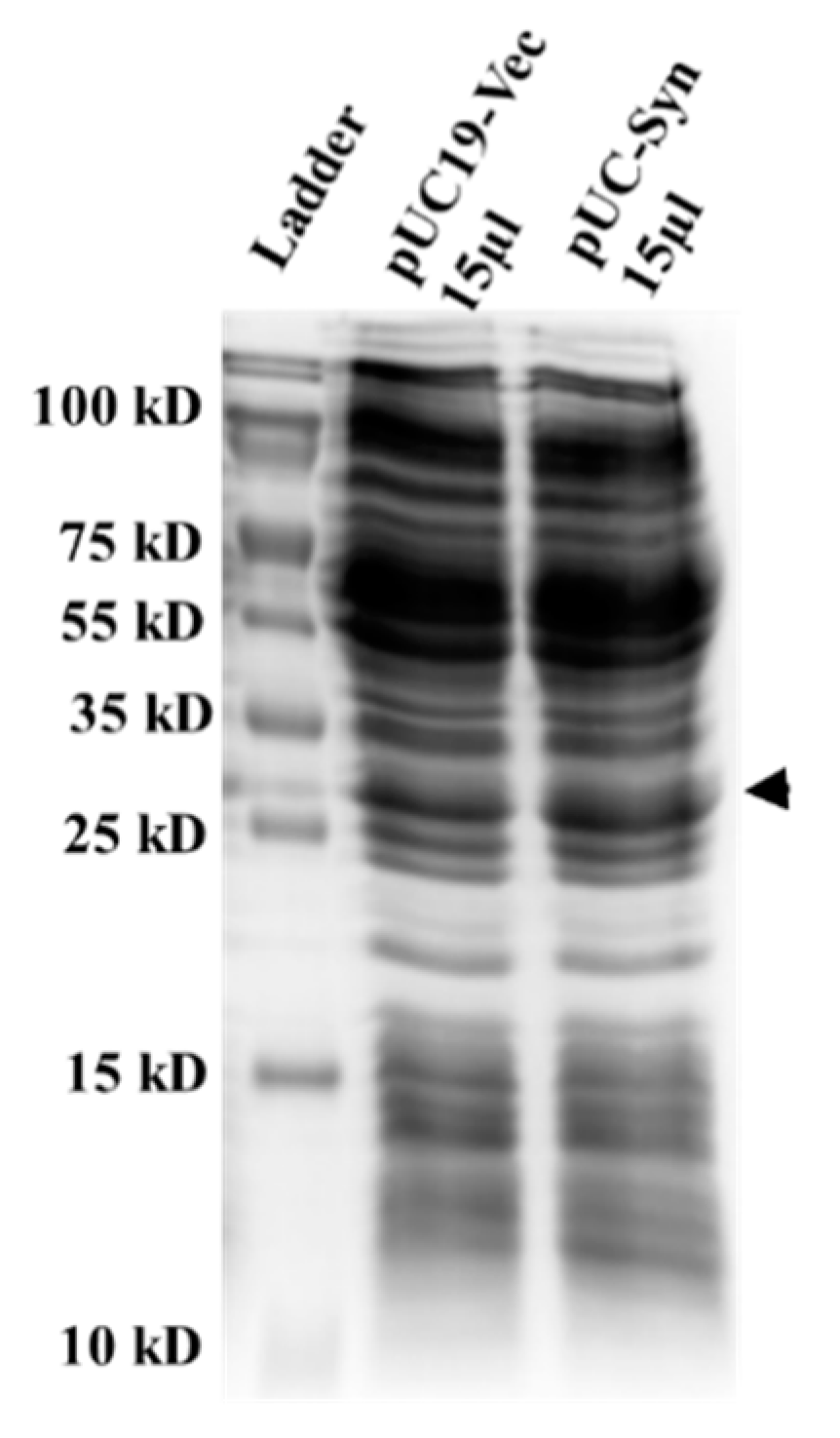
2.1. Determination of R. tropici Phytochelatin Synthase Protein Expression in Recombinant E
2.2. Synthesis of Gold Nanoparticles
2.3. UV–Vis Spectroscopy
2.4. Collection of Nanoparticles
2.5. Characterization of Gold Nanoparticles Using Energy-Dispersive X-ray Spectroscopy and Transmission Electron Microscopy
2.6. Zeta Potential Analysis of Gold Nanoparticles
2.7. Statistical Analysis
3. Results
3.1. Expression of R. tropici Phytochelatin Synthase in E. coli DH5α Cells
3.2. Synthesis and Yield of Nanoparticles by Soluble Intracellular Extracts
3.3. UV-Vis Spectral Characterization of Nanoparticles
3.4. Characterization of Gold Nanoparticles Using TEM
3.5. Compositional Analysis of Nanoparticles Using STEM and EDS
3.6. Measurement of Zeta Potential of Gold Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Lloyd, J.R. Microbial reduction of metals and radionuclides. FEMS Microbiol. Rev. 2003, 27, 411–425. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Grill, E.; Winnacker, E.-L.; Zenk, M.H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA 1987, 84, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Loffer, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific y-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Nishikori, S.; Iwabe, O.; Shiraki, K.; Miyasaka, H.; Takagi, M.; Hirata, K.; Miyamoto, K. Characterization of phytochelatin synthase-like protein encoded by alr0975 from a prokaryote, Nostoc sp. PCC 7120. Biochem. Biophys. Res. Commun. 2004, 315, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Varotto, C.; Borsò, M.; Rugnini, L.; Bruno, L.; Di Toppi, L.S. Eukaryotic and Prokaryotic Phytochelatin Synthases Differ Less in Functional Terms Than Previously Thought: A Comparative Analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407. Plants 2020, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and Metallothionins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead tolerance in plants: Strategies for phytoremediation. Environ. Sci. Pollut. Res. 2013, 20, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C. Phytochelatins and Their Roles in Heavy Metal Detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef]
- Tennstedt, P.; Peisker, D.; Böttcher, C.; Trampczynska, A.; Clemens, S. Phytochelatin Synthesis Is Essential for the Detoxification of Excess Zinc and Contributes Significantly to the Accumulation of Zinc. Plant Physiol. 2009, 149, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Choia, Y.; Park, T.J.; Lee, D.C.; Lee, S.Y. Recombinant Escherichia coli as a biofactory for various single- and multi-element nanomaterials. Proc. Natl. Acad. Sci. USA 2018, 115, 5944–5949. [Google Scholar] [CrossRef]
- Zhang, D.; Yamamoto, T.; Tang, D.; Kato, Y.; Horiuchi, S.; Ogawa, S.; Yoshimura, E.; Suzuki, M. Enhanced Biosynthesis of CdS Nanoparticles Through Arabidopsis Thaliana Phytochelatin Synthase-Modified Escherichia Coli with Fluorescence Effect in Detection of Pyrogallol and Gallic Acid. Talanta 2019, 195, 447–455. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, D.; Yamamoto, T.; Kato, Y.; Horiuchi, S.; Ogawa, S.; Yoshimura, E.; Suzuki, M. Improving Biosynthesis of AuPd Core-Shell Nanoparticles Through Escherichia Coli with the Assistance of Phytochelatin for Catalytic Enhanced Chemiluminescence and Benzyl Alcohol Oxidation. J. Inorg. Biochem. 2019, 199, 110795. [Google Scholar] [CrossRef]
- Yuan, Q.; Bomma, M.; Hill, H.; Xiao, Z. Expression of Rhizobium tropici phytochelatin synthase in Escherichia coli resulted in increased bacterial selenium nanoparticle synthesis. J. Nanoparticle Res. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1.4 and their antimicrobial application. J. Pharm. Anal. 2018, 8, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green synthesis of nanoparticles with extracellular and intracellular extracts of basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R.; et al. Green synthesis of gold nanoparticles by thermophilic flamentous fungi. Sci. Rep. 2018, 8, 3943. [Google Scholar] [CrossRef]
- Camas, M.; Camas, A.S.; Kyeremeh, K. Extracellular Synthesis and Characterization of Gold Nanoparticles Using Mycobacterium sp. BRS2A-AR2 Isolated from the Aerial Roots of the Ghanaian Mangrove Plant, Rhizophora racemose. Indian J. Microbiol. 2018, 58, 214–221. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Vaidyanathan, R.; Gopalram, S.; Kalishwaralal, K.; Deepak, V.; Pandian, S.R.; Gurunathan, S. Enhanced silver nanoparticle synthesis by optimization of nitrate reductase activity. Colloids Surf. B Biointerfaces 2010, 75, 335–341. [Google Scholar] [CrossRef]
- Leng, Y.; Fu, L.; Ye, L.; Li, B.; Xu, X.; Xing, X.; He, J.; Song, Y.; Leng, C.; Guo, Y.; et al. Protein-directed synthesis of highly monodispersed, spherical gold nanoparticles and their applications in multidimensional sensing. Sci. Rep. 2016, 6, 28900. [Google Scholar] [CrossRef]
- Ravindra, P. Protein-mediated synthesis of gold nanoparticles. Mater. Sci. Eng. B 2009, 163, 93–98. [Google Scholar] [CrossRef]
- Engelbrekt, C.; Sørensen, K.H.; Zhang, J.; Welinder, A.C.; Jensen, P.S.; Ulstrup, J. Green synthesis of gold nanoparticles with starch–glucose and application in bioelectrochemistry. J. Mater. Chem. 2009, 19, 7839–7847. [Google Scholar] [CrossRef]
- Tajammul Hussain, S.; Iqbal, M.; Mazhar, M. Size control synthesis of starch capped-gold nanoparticles. J. Nanoparticle Res. 2009, 11, 1383–1391. [Google Scholar] [CrossRef]
- Xiao, Y.; Pavlov, V.; Levine, S.; Niazov, T.; Markovitch, G.; Willner, I. Catalytic growth of Au nanoparticles by NAD(P)H cofactors: Optical sensors for NAD(P)+-dependent biocatalyzed transformations. Angew. Chem. Int. Ed. 2004, 43, 4519–4522. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qin, G.; Raveendran, P.; Ikushima, Y. Facile “green” synthesis, characterization, and catalytic function of beta-d-glucose-stabilized Au nanocrystals. Chemistry 2006, 12, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of gold nanoparticles using various amino acids. J. Colloid Interface Sci. 2015, 447, 254–257. [Google Scholar] [CrossRef]
- Wangoo, N.; Bhasin, K.K.; Mehta, S.K.; Suri, C.R. Synthesis and capping of water-dispersed gold nanoparticles by an amino acid: Bioconjugation and binding studies. J. Colloid Interface Sci. 2008, 323, 247–254. [Google Scholar] [CrossRef]
- Bhattacharjee, R.R.; Das, A.K.; Haldar, D.; Si, S.; Banerjee, A.; Mandal, T.K. Peptide-assisted synthesis of gold nanoparticles and their self-assembly. J. Nanosci. Nanotechnol. 2005, 5, 1141–1147. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Marsic, D.; Hughes, R.C.; Byrne-Steele, M.L.; Ng, J.D. PCR-based gene synthesis to produce recombinant proteins for crystallization. BMC Biotechnol. 2008, 8, 44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, Q.; Bomma, M.; Xiao, Z. Enhanced Silver Nanoparticle Synthesis by Escherichia Coli Transformed with Candida Albicans Metallothionein Gene. Materials 2019, 12, 4180. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.C.; Chequer, N.A.; González, J.L.; Cordova, T. Alternative Methodology for Gold Nanoparticles Diameter Characterization Using PCA Technique and UV-VIS Spectrophotometry. Nanosci. Nanotechnol. 2012, 2, 184–189. [Google Scholar] [CrossRef]
- Tang, J.; Gao, K.; Ou, Q.; Fu, X.; Man, S.Q.; Guo, J.; Liu, Y. Calculation extinction cross sections and molar attenuation coefficient of small gold nanoparticles and experimental observation of their UV–vis spectral properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 513–520. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Shedbalkar, U.; Richa, S.; Sweety, W.; Sharvari, G.; Chopade, B.A. Microbial synthesis of gold nanoparticles: Current status and future prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Homberger, M.; Simon, U. On the application potential of gold nanoparticles in nanoelectronics and biomedicine. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1405–1453. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Li, Z.Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Cho, H.-Y.; Choi, H.K.; Lee, J.-Y.; Choi, J.-W. Application of Gold Nanoparticle to Plasmonic Biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef] [PubMed]






| D1 (pUC19-Vec Cell Group After Spin at 3000 g) | D2 (pUC19-Vec Cell Group After Spin at 17,000 g) | S1 (pUC-Syn Recombinant Cell Group After Spin at 3000 g) | S2 (pUC-Syn Recombinant Cell Group After Spin at 17,000 g) | D1 + D2 | S1 + S2 | |
|---|---|---|---|---|---|---|
| Diameter (nm) (mean ± SD) | 26.49 ± 14.75 | 25.87 ± 8.95 | 28.95 ± 12.13 | 24.72 ± 12.30 | 26.06 ± 11.05 | 26.63 ± 12.40 |
| Number of Nanoparticles | 137 | 309 | 234 | 285 | 446 | 519 |
| p value | D1 vs. D2, p > 0.05 D1 vs. S1, p > 0.05 D1 vs. S2, p > 0.05 | D2 vs. D1, p > 0.05 D2 vs. S1, p < 0.001 D2 vs. S2, p > 0.05 | S1 vs. D1, p > 0.05 S1 vs. D2, p < 0.001 S1 vs. S2, p < 0.001 | S2 vs. D1, p > 0.05 S2 vs. D2, p > 0.05 S2 vs. S1, p < 0.001 | D1+D2 vs. S1+S2, p > 0.05 | D1+D2 vs. S1+S2, p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Q.; Bomma, M.; Xiao, Z. Enhanced Extracellular Synthesis of Gold Nanoparticles by Soluble Extracts from Escherichia coli Transformed with Rhizobium tropici Phytochelatin Synthase Gene. Metals 2021, 11, 472. https://doi.org/10.3390/met11030472
Yuan Q, Bomma M, Xiao Z. Enhanced Extracellular Synthesis of Gold Nanoparticles by Soluble Extracts from Escherichia coli Transformed with Rhizobium tropici Phytochelatin Synthase Gene. Metals. 2021; 11(3):472. https://doi.org/10.3390/met11030472
Chicago/Turabian StyleYuan, Qunying, Manjula Bomma, and Zhigang Xiao. 2021. "Enhanced Extracellular Synthesis of Gold Nanoparticles by Soluble Extracts from Escherichia coli Transformed with Rhizobium tropici Phytochelatin Synthase Gene" Metals 11, no. 3: 472. https://doi.org/10.3390/met11030472
APA StyleYuan, Q., Bomma, M., & Xiao, Z. (2021). Enhanced Extracellular Synthesis of Gold Nanoparticles by Soluble Extracts from Escherichia coli Transformed with Rhizobium tropici Phytochelatin Synthase Gene. Metals, 11(3), 472. https://doi.org/10.3390/met11030472






