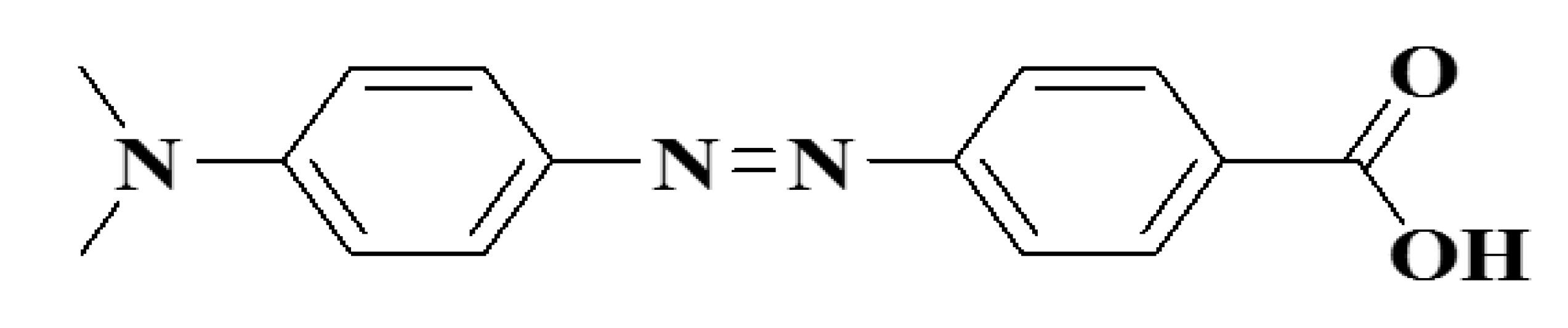Abstract
Noble metal NPs are highly attractive candidates because of their unique combination of physical, chemical, mechanical, and structural properties. A lot of developments in this area are still fascinating the materials research community, and are broadly categorized in various sectors such as chemical sensors, biosensors, Förster resonance energy transfer (FRET), and microelectronic applications. The related function and properties of the noble metals in these areas can be further tailored by tuning their chemical, optical, and electronic properties that are influenced by their size, shape, and distribution. The most widely used Au and Ag NPs in dispersed phase below 100 nm exhibit strong color change in the visible range which alters upon aggregation of the NPs. The chemical sensing of the analyte is influenced by these NPs aggregates. In this article, we have summarized the uniqueness of noble metal NPs, their synthesis methods, nucleation and growth process, and their important applications in chemical sensing, microelectronic packaging, and Förster resonance energy transfer.
1. Introduction
Nanomaterial is defined as a material in which the maximum value of one dimension can be 100 nm, which can be further defined as one billionth of meter or 10−9 m [1,2,3,4,5,6]. It is approximately 10 H or 5 Si atoms in a line. It is continuing to be the most rapidly growing R/D sector in last decades, which is evident from more than several billion dollars of annual investment in this particular field [7,8]. Due to its unique features, nanomaterials and NPs allow them to be used for a wide variety of applications in nanotechnology covering medical science, chemical, bio-network, applied physics, materials, microelectronic and metallurgy science, and engineering. There are lots of investments in the area of medical science, in particular, theragnostics, which refers to two kinds of word therapeutics and diagnostics [9]. It is an advanced technique in which cancer diagnosis and therapy is done simultaneously, for early detection and cure of the cancer [10,11]. To achieve this, some special metals in the periodic table include alkaline to alkaline metallics, rare metallics, and noble metallics used for theranostics application [12]. Compared to these metals, noble metals have exceptional resistance in corrosive environments (in acidic or basic medium) and they also resist to oxidation even at elevated temperature. Moreover, these metals, especially gold and silver at a nanometer scale, exhibit unique and tunable plasmonic properties that have fascinated the researcher greatly over the last decade and still receiving increased attention up to date. [13,14,15]. Their plasmonic features bring more advantages in the biomedical and biosensor fields [5]. The use of noble metals dates back to the ancient Roman and Greek era [5,16]. Precious metals such as gold and silver were used in fabrication for various purposes such as pots, liquids containers, and also in the manufacturing of coins. The silver colloids were extensively used in antimicrobial therapy until antibiotics were developed in the early 1940s [17]. In addition, in the ancient Chinese era, gold and its complexes were used for medicines. Moreover, Europeans, also used colloidal gold in medieval period particularly by chemist/alchemist in laboratories for the treatment of many infectious diseases and even recommended to provide long life [18]. The unique term nanotechnology was specified by Noble Laureate Dr. R. Feynman on his visionary lecture entitled “There is Plenty of Room at the Bottom” at the meeting of American Physical Society, at California Institute of Technology, USA in December 1959 [19].
Noble metals have always been dispersed in various supports or supporting materials for a range of catalytic and sensing applications [20,21]. However, particle growth at high temperatures is a serious issue faced by many researchers which causes a loss in sensitivity and catalytic activities [22]. Among methods of dispersion, Arnal et al. developed stabilization of Au/ZrO2 catalyst against sintering at high temperatures [23]. In very recent sudy, Yang et al. [24] studied the uniformly dispersed fluorescent Ag NPs in aqueous solution capped with poly-methacrylic acid for sensing the lead ions in water. Their study suggested the fluorescence intensity of Ag nanoclusters was increased in presence of Pb(II) ions in water upon excitation with 320 nm. The detection limit was estimated to be 60 nM. Additionally, hollow silica spheres encapsulated silver nanoparticles (Ag@SiO2) that can be useful for the sensing and drug delivery at the targeted site. A similar method has also been used to encapsulate Au NPs by hollow silica that can have a wider application, such as catalysis, detection, portable microelectronics, and antibacterial areas [23,24].
The future generation of metal NPs promises attractive chemical sensors that are expected to rely on various principles, such as plasmon sensors, nanobiosensors, coulometric and fluorometric principles. Therefore, in this review we have highlighted various aspects of Au NPs sensors for chemical sensing and flexible sensors for microelectronic packaging devices.
2. Surface Charge Determination on the NPS
The stability of the NPs in the colloidal solution is due the presence of surface charge on the nanoparticles surface. These charges are measured by a technique that is called zeta-potential analyzer that predict their stability [25,26]. If a measured zeta-potential of a surface modified nanoparticles showed a large positive or large negative, then the nanocolloids would have good physical stability due to electrostatic repulsion between the individual nanoparticles. The Zeta-potential value of nanoparticles +30 mV or −30 mV suggests good nanocolloids stability. Zeta-potential observed in the all colloidal system that has solid-liquid and liquid-liquid dispersion [27].
It is basically an electrical potential developed at the interfacial double layer which is dispersed nanoparticles and the continuous phase that could be the dispersion medium. It is determined through velocity measurement of the charged nanoparticles moving toward the electrode across the sample solution in the presence of an external electric field [25,26,27]. Rasmussen et al. determined the surface charge on various nanoparticles using salt gradient which caused a charge reversal of NPs in opposite direction. The increase in the NPs concentration and their spatial distribution provides both size (69–73 nm) and charge (−30 to −48 mV) on the surface of NPs [28]. Various surfactants are available in literature to provide anionic, ionic, or non-ionic ones to control the surface charge on the surface of NPs in the field of colloids, nanofluids, and nanocoatings [29,30,31].
3. Estimation of Surface to Volume Ratio
The reduction in particle range (nm) progressively scales the surface area of atoms and the surface to volume ratio of nano range particles. Consequently, particle size and surface atoms are two essential factors in nanomaterial research. For spherical shape particles, the particle radius (r) varies inversely with the surface to volume ratio (S/V), i.e.,
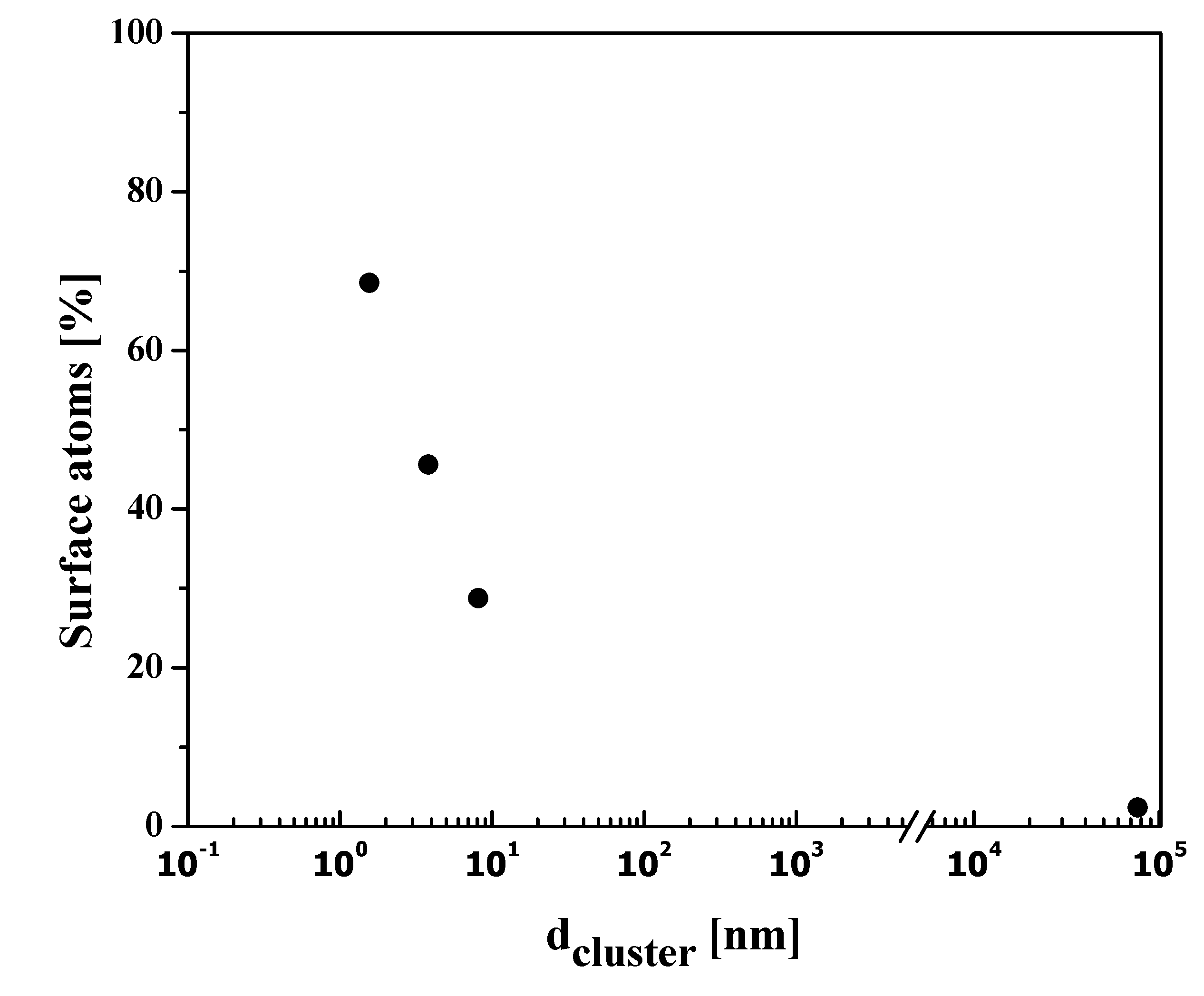
where r = particle radius. The surface to volume ratio is consequently interrelated, increasing with a decrease in the size of NPs. In an investigation, Nutzenadel et al. [32] reported the variation in the surface atoms of Pd-metal cluster (clusters are aggregates of atoms/molecules) over the range of 1 to 4 nm. They suggested that, with a diameter of approximately 4 nm, 30% of atoms are on the surface, and after decreasing the particle size close to 1 nm, the surface becomes 75% as depicted in Figure 1.
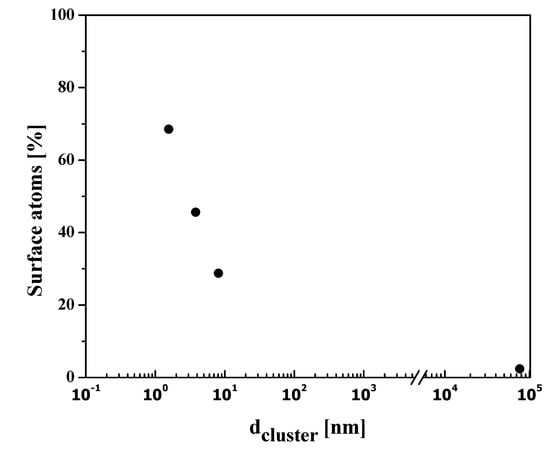
Figure 1.
Variation of the surface atoms in Pd nanoparticle clusters.
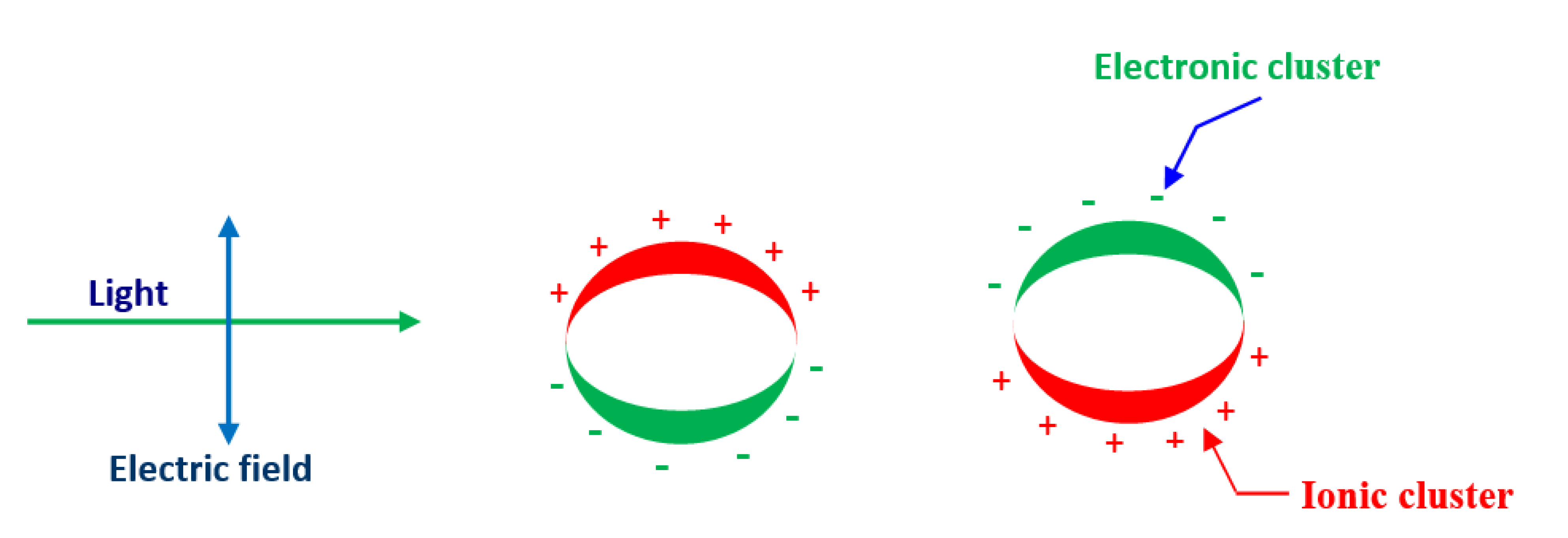
The substantial quantity of atoms in these materials lies on the surface boundary and therefore, there is improved contact and bonding at the nanoscale. For example, noble metal NPs have novel optical properties when exposed to electromagnetic radiations known as surface plasmon resonance (SPR). The SPR occurs because the conduction electron oscillates continuously close to the metallic surface. If the size of NPs is usually less than the wavelength of the incident light, they interact and obey Rayleigh’s theory rather than the Mie theory. In this state, SPR is subjected to electromagnet radiations primarily by dipolar mode as clearly shown in Figure 2.
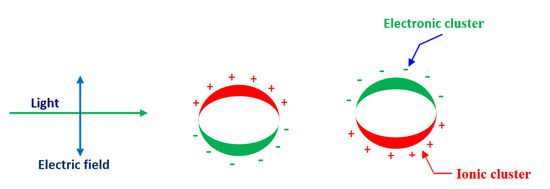
Figure 2.
Schematic diagram of surface plasmon resonance (SPR) incoherent interaction of the electrons in conduction band of metal NPs with an applied electromagnetic field.
All the electrons and nuclei oscillate in the same phase at the same frequency in the applied electric field [33,34]. Furthermore, as long as all NPs are uniform and smaller than the wavelength of the light, SPR does not reveal any noticeable changes due to the similar cross-section of the NPs.
4. Nucleation and Growth Process
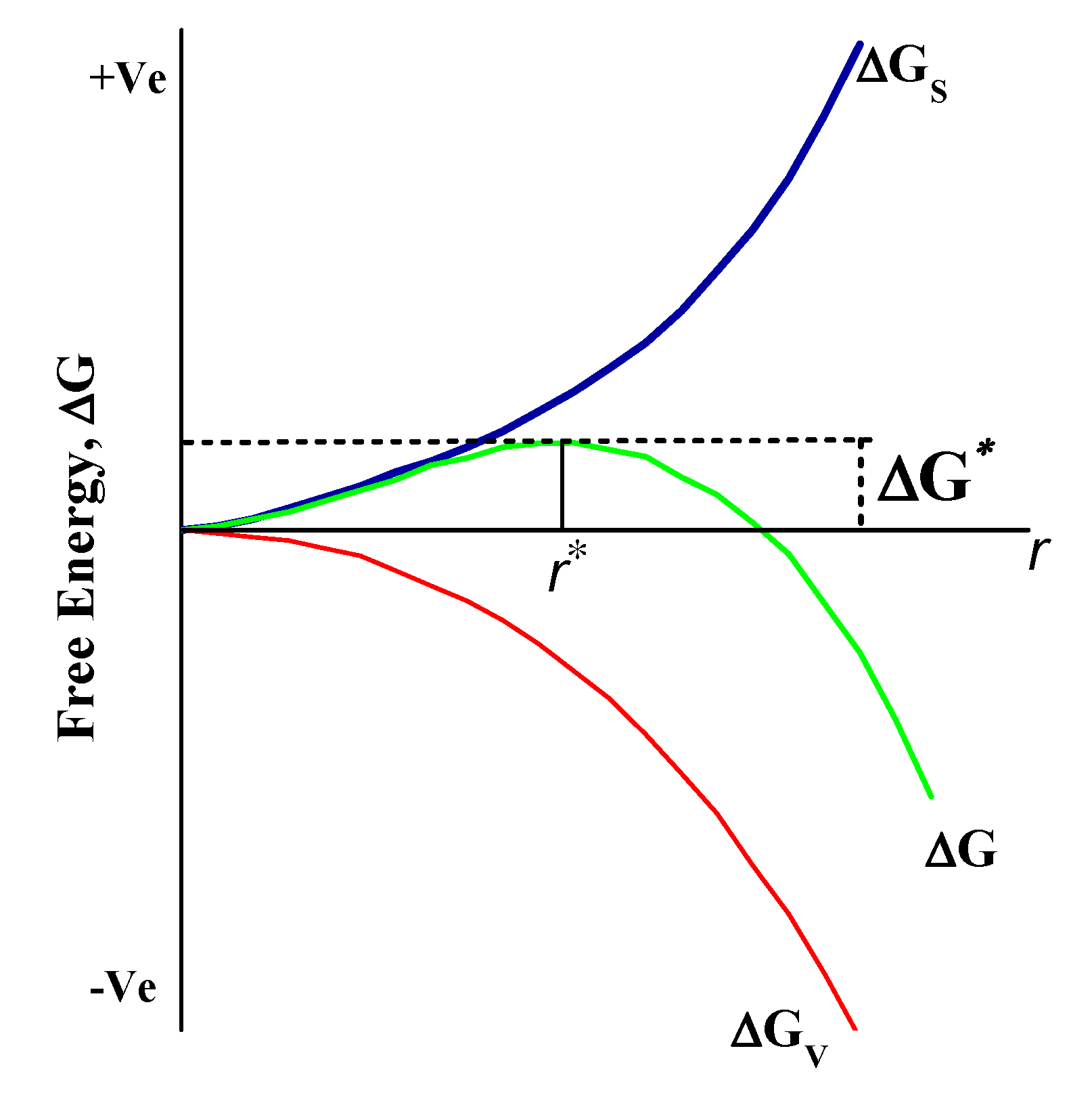
Nucleation is a framework that begins at the atomic scale where atoms serve as seeds in the liquid phase. These seeds such as atoms are arranged in particular patterns that create the final crystal structure of the specific compound. These seeds or nuclei are very prone to the external particles or impurity of the system, so they are intentionally applied in many industrial-scale processes to enhance the nucleation rate. The rate of nucleation can be further divided into two categories: homogeneous and heterogeneous. There is a greater chance of reaction in solution phase synthesis to proceed with nucleation through heterogeneous since structural inhomogeneities such as the surface of the vessel, stirrer rod, and impurities may act as a stabilizer surface for nucleating. Homogeneous nucleation, however, is more likely to begin somewhere in the bulk solution from the walls of the vessel. The process of homogeneous nucleation can be viewed, according to thermodynamics, as the change in the total energy of the particle, which is a sum of the surface and bulk volumetric energy as shown in Figure 3. Furthermore, for spherical particles with radius r and surface energy/area, the free energy/volume of crystals is Gv. The total Gibbs energy change is given by Equation (2):
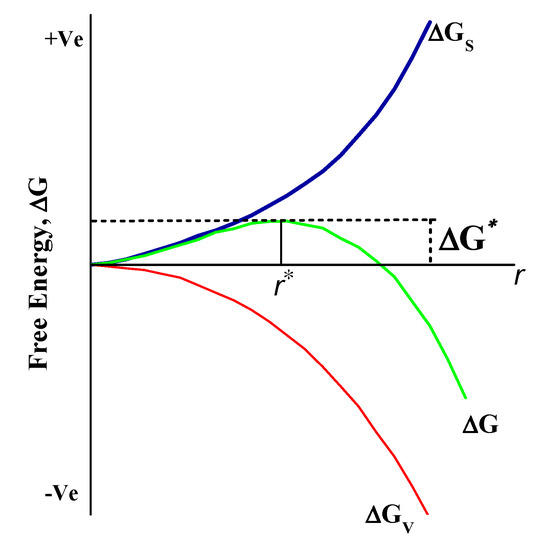
Figure 3.
Schematic representation of Gibbs free energy change in nucleation and growth process. ΔG represents total Gibb’s energy, and ΔG* are free energy and critical free energy (ΔG*) of the crystal.
Usually, the free energy of the crystal (ΔGv) can be written in correlation with temperature T, Boltzmann constant (kB), and supersaturating solution (S) as below Equation (3):
Further, the entire energy change of the crystal can be given as below
Furthermore, in order to obtain an equilibrium condition, we can obtain the total free energy barrier by the differentiation of Equation (4) (preserving d(ΔG)/dr = 0). In other words, the critical radius (r*) of a nanoparticle can be computed from Equation (5) [35].
Now from (5)
and
Substitution of Equation (7) in (4) provides the value of total Gibbs free energy change, for example,
The critical threshold of free energy (ΔG*) required for NPs to grow in the liquid phase is given by Equation (8).
5. Synthetic Methods of Noble Metal NPs
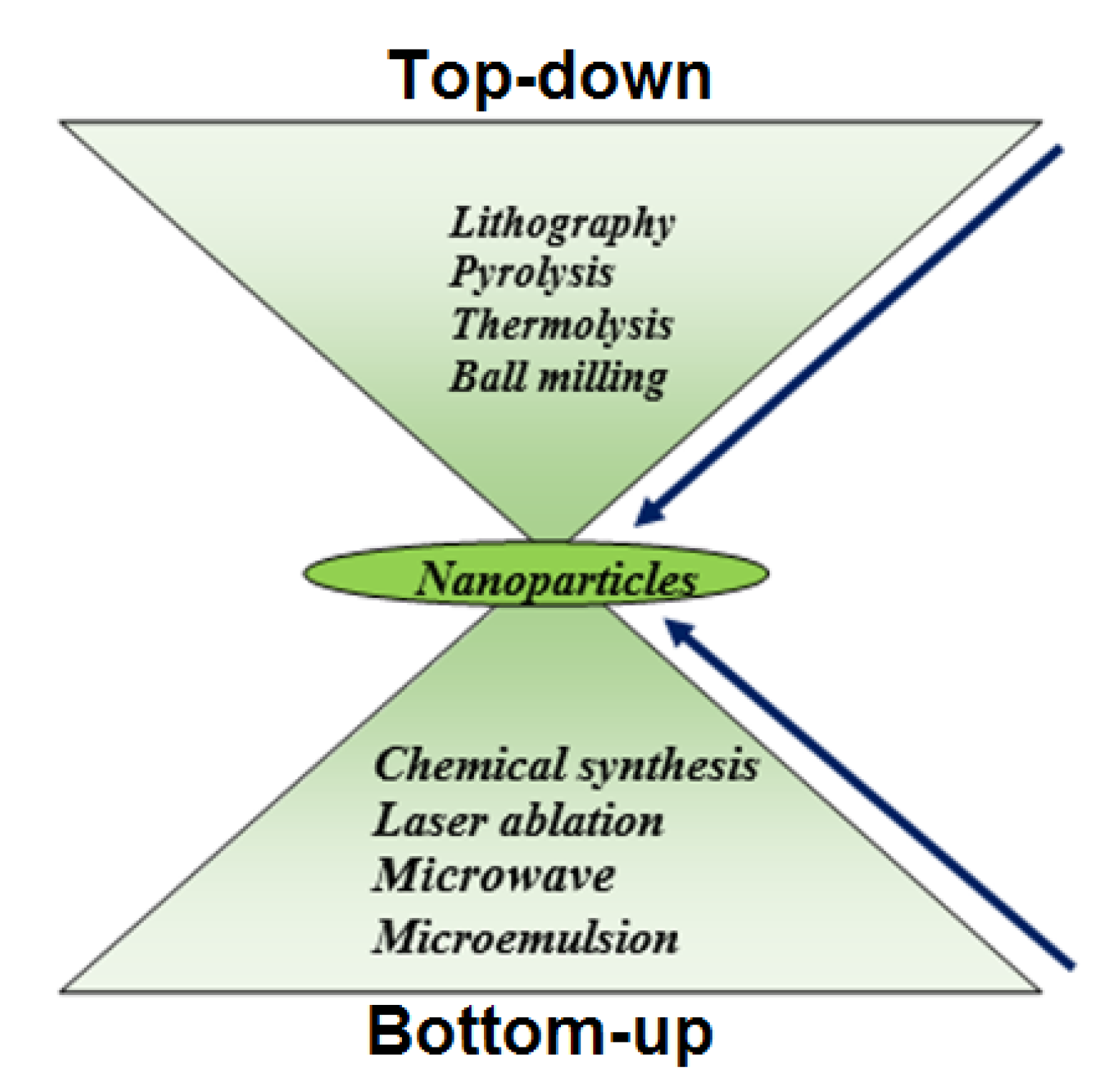
The most important groundbreaking fields of nanoscience are a vast variety of nanomaterials. The synthesis of NPs can essentially be accomplished via two major approaches: (1) Top-down and (2) Bottom-up technique, as clearly shown in Figure 4. The dimension of the bulk crystal reduces down to the range of nanometers in the top-down phase by applying different chemical and physical approaches. External variables, e.g., friction and pressure, constrain the particles in a desired shape and sizes to reduce in dimension [36]. For example, many approaches have been used in the processing of nanostructured materials in recent years, such as the ball milling process [37], the lithography process [38], the pyrolysis process [39], and the thermolysis process [40]. However, the top-down techniques have few drawbacks, e.g., large size variation, residual stresses, and therefore more surface defects. In addition, this process is less cost-effective because of the high-pressure environment, warm temperatures, and extremely advanced nanostructure material processing equipment.
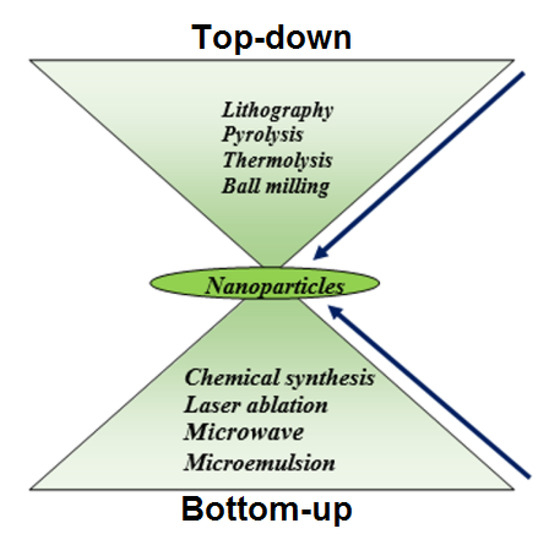
Figure 4.
Two major approach for synthesis of NPs.
Powder metallurgy is a method consisting of milling of microparticles down to nano level and subsequent densification to achieve bulk product. The balls are configured to rotate at some rev/min (RPM) in this process, the rpm offers the balls with energy that is eventually used to breakdown the particles from the micron size to the nano-sized particles. In addition, specific parameters of milling such as atmosphere, agent media, speed, ball to weight ratio, grinding time, and temperature must be further tuned to obtain optimum size of NPs.
Lithography is also another top-down process by which it is possible to design complex two-dimensional and three-dimensional nano-scale patterns with better control of the structure. By introducing photolithography, soft-lithography, imprint lithography, and scanning probe-lithography (SPL) to a substrate surface, the desired pattern can be developed in various ways. In the field of electronic circuit synthesis and processing of electronics components [41,42,43,44,45], this approach can be used.
Further, another top-down method used for the processing at an industrial scale is pyrolysis. In this process, the precursor content is injected at high pressure through a hole, followed by high-temperature annealing provided by the NPs. Since the higher pressure and temperature were involved in the synthesis method, the NPs obtained are often aggregated, so they have a large distribution of particle size. This method may not be very expensive since it takes more pressure and temperatures, but it is also used for processing on a commercial scale [46].
The creation of the blocks of atoms begins from the bottom in the bottom-up process and it goes atom by atom, creating the complete structure. These atoms, however, originate from their respective ions and salts by reduction with appropriate chemical reagents [47]. Bottom-up nanostructures have lower surface defects and good atomic packaging to minimize the total free energy of Gibb and provide greater reliability compared to the top-down method. This improvement in reliability will further expand the applications of NPs.
In previous times that it was used widely, for instance, the chemical reduction process is divided into green synthesis and the polyol synthesis methods [46,48]. There are a variety of familiar approaches from bottom to top. In the chemical reduction process, an effective reduction agent is typically used for removing metal ions from their respective salts. The polar or non-polar and universal solvents may be liquid such as water. The popular reducing agents, such as hydrazine (N2H4), alcohol (C2H5OH), lithium aluminum hydride (LiAlH4), and sodium borohydride (NaBH4) were reported earlier [48,49,50,51]. However, chemical reagents used as a reductant in the green synthesis method are moderate in nature as well as the most important sources are the reducing agents from green plants [52].
In the polyol process, polyalcohols such as polyethylene glycol and polyvinyl alcohols reduce metal ions into metallic NPs [53]. The polymer may also serve as a capping layer of metallic NPs that provides them longer longevity in a specific application. It is also an expense mechanism and can easily be customized to the demands. However, the NPs can be regulated by changing certain parameters including photovoltaic current, voltage, and temperature. Furthermore, Schulman et al. suggested a microemulsion process where at least one polar and non-polar phase is mixed with a stable surfactant [54]. The micro-emulsion is therefore characterized as a consistent, symmetric, and steady dispersion of two or more components. The surfactant forms the interfacial film that separates these two layers between the polar and the non-polar layer, producing micro-emulsions at this interfacial region. Micro-emulsion will usually occur in continuous water or a continuous oil process as a consequence of dispersing the oil drops [55,56,57,58].
Laser ablation is also a bottom-up method where we produce a coating by heating, vaporizing, or sublimating it using laser source energy used for the ablation. Consequently, the substance is fully transformed into the plasma phase. This technique has been beneficial over others because it does not need to evaporate the excess solvent that can be used for aqueous and non-aqueous synthesis purposes. In comparison to other approaches, this technique also provides some additional advantages, including a short period of time, industrial production, and easily controlled sizes and shapes.
Microwave processing is also a method of irradiation where the sample is irradiated with a microwave source of energy [59]. When irradiated in the presence of a surfactant, the metallic salts would decompose and lead to metal NPs.
6. Applications of Noble Metal NPs
Sensors are generally described by functional molecules or systems for detecting or sensing a given analyte molecule in the surrounding environment. The output signal is provided by a change in the analyte species concentration in the sample [60,61,62,63,64]. Therefore, NPs’ sensing application depends on three factors (i) analyte, (ii) a binding sensitivity detection part of an analyte, and (iii) transducers. It is possible to characterize the transducer as a system capable of converting binding events into comparable voltage or current. Therefore, the three aspects listed above are important for any sensor to be effective to produce a small error with higher signal-to-noise (S/N) ratio and a minimum analyte concentration. However, noble metal NPs are less stable in open environments and can have difficult surface chemicals that restrict their applications in the field of bio-sensing.
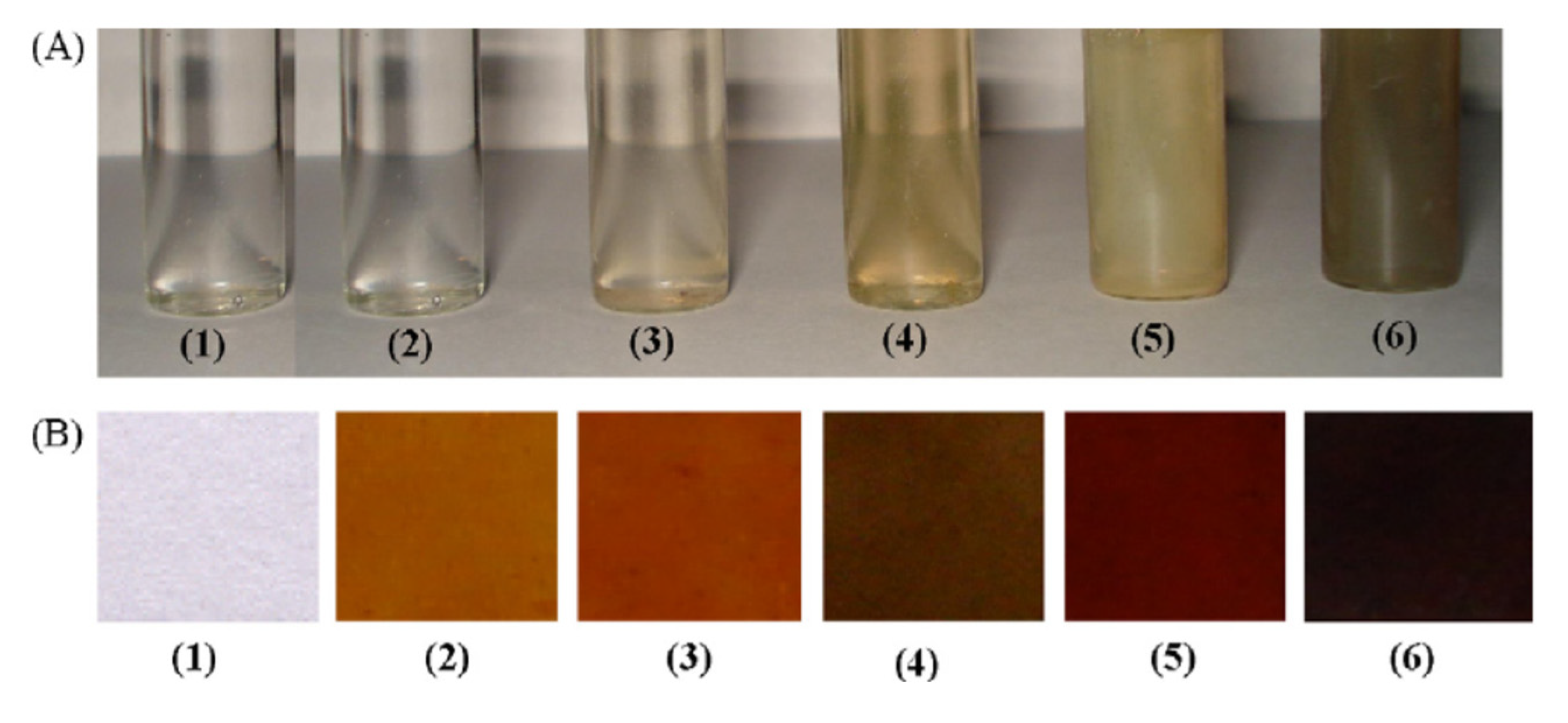
The functional NPs of the noble metal may therefore largely possess excellent photophysical characteristics such as high fluorescence and visible region absorption, conductivity, and redox properties to completely fulfill sensing requirements. The Ag NPs, in different sizes, reveal different colors, as shown in Figure 5. The Ag NPs are also very stable in colloidal suspension and in the form of nanocomposite films. Figure 5A,B displays the Ag colloidal NPs showing various colors due to particle size differences.
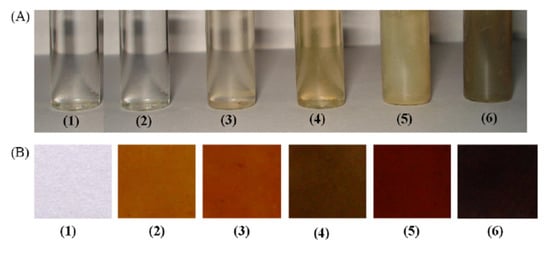
Figure 5.
Color variation in (A) Ag nanocolloids dispersed in water (B) nanocomposite films casted onto silicate glass. Samples 1–6 contain Ag in 0.0, 0.2, 0.5, 1.0, 2.0, and 5.0 wt%, respectively. [with permission reference [13]].
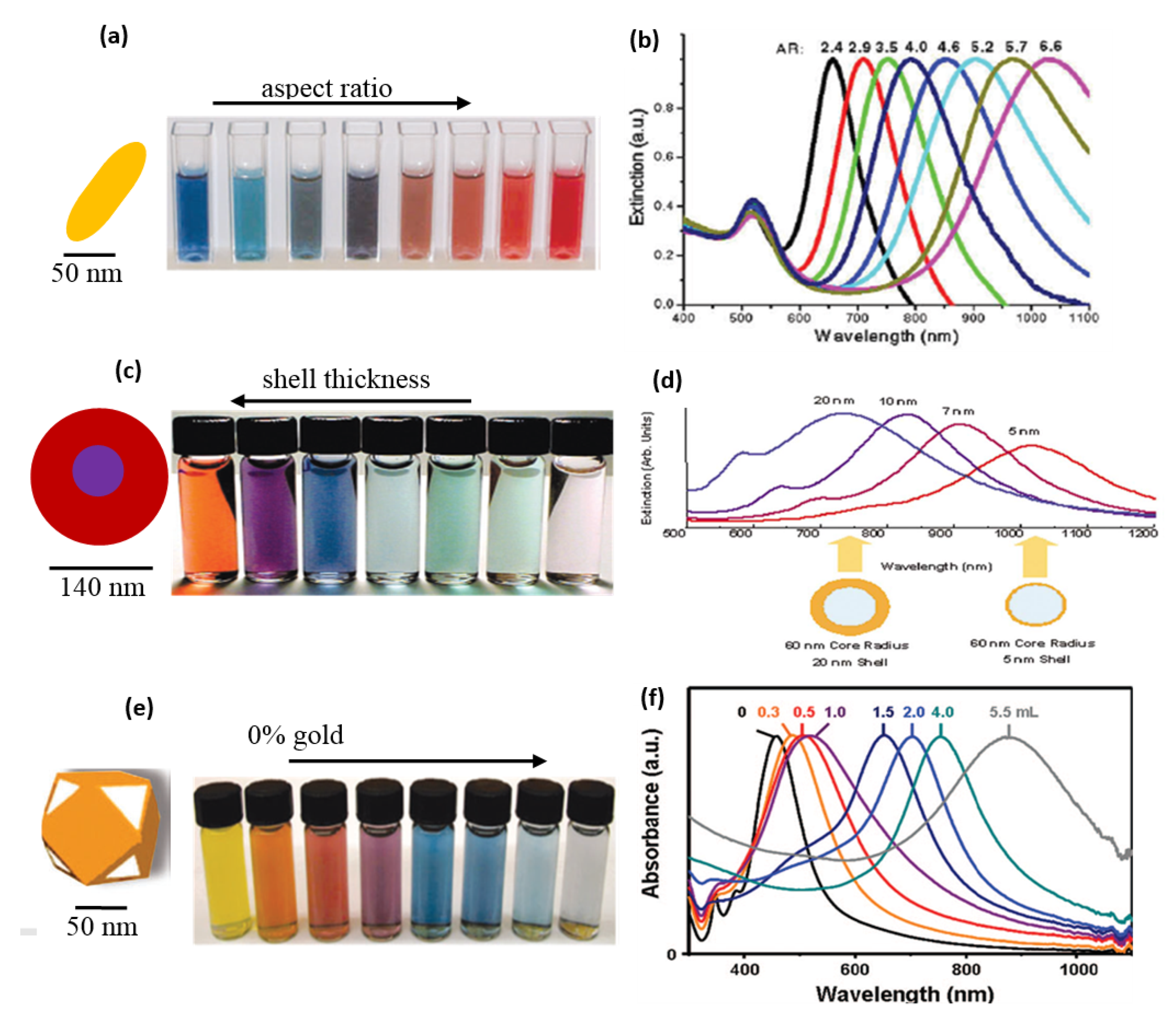
With the increasing Ag content, the various sizes of NPs were prepared using the polyol method. After that, a viscous solution was cast into glass petri dish in the form of film, the excessive water solvent was evaporated. The color shift in the film with the silver content increasing is seen in Figure 5b. The gold particles also displayed exceptional color change based on size and the wavelength of the absorption was tunable in the infrared zone (Figure 6). The change of colors in Au NPs was seen by El-Sayed and his coworkers [5,62] as their aspect ratio was different (as shown Figure 6a). In addition, the gold shell SPR on silica NPs was shown to be core and the silica NPs were tuned to around 600–11,000 nm (Figure 6b).
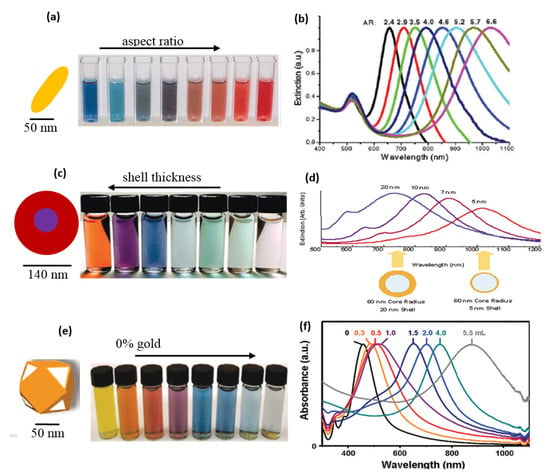
Figure 6.
Optical properties of Au (a) color change in Au nanorods with different aspect ratios of gold nanoparticles, (b) tunable SPR of gold nanoparticles with different aspect ratio of gold nanoparticles, (c) color change noted in Au nanoshells with different shell-thickness (d), and tunability of their wavelength at different shell thickness, (e) physical appearance of Au nanocages with different HAuCl4 content in their synthesis, and (f) tunability of their wavelength (with permission from [62]).
In other reports, Xia et al. [64], produced Ag nanocages via the Polyol method. The subsequent transformation of Ag into Au nanocages was achieved by adding auric chloride solution. The nanocages of gold were created by the silver metal galvanizing as given by:
3Ag (s) + HAuCl4 → Au (s) + 3AgCl (s) + HCl (aq)
The SPR could be tuned between about 470 nm and 1000 nm (Figure 6e). The change of color of gold particles found in the PVA solution was also seen by our group [15]. The PVA was a mild capping agent that stabilized the NPs [53]. These NPs were later reinforced it into PVA to produced Au-PVA composite films by evaporating water surplus at 70 °C.
6.1. Colorimetric Sensing
Colorimetric sensing involves color shift detection of the target solution which is caused by the NPs being aggregated. The aggregation of Ag or Au NPs is mainly correlated with the affinity to bind the targeted analytes of the surface ligands, so aggregation occurs primarily when the analyst binds the ligands on the gold NPs [13,65,66,67,68]. In general, however, the golden nanometers are red, their color after aggregation shifts blue, and their SPRs turn to red. Au NPs in the size of ~13 nm, for example, had maximum absorption at 523 nm when the analyte component is attached. In general, nanoparticle aggregation factors are affected by the changes in solution ion power, (ii) functioning of NPs that have DNA or carbohydrate, enzymes, or other threatening agents such as uranium compounds [69,70]. Furthermore, Au NPs were comfortably detected as harmful heavy metal ions (HMI) such as (Pb2+, Cd2+, Hg2+) [71]. Due to HMI chelation, the solution color has been switched from red to blue. On the region of MUA, the carboxylate group was HMI ionic receptors. The detection limit observed was above 400 μM for the entire HMI. The HMI was observed for the calorimetric sensing of aqueous Hg2+ ions, the Mirkin Group functionalized Gold NPs with DNA [72]. The sensing mechanism was related to the coordination compounds of thymine-Hg2+-thymine (T-T). The sensitivity was measured up to 100 nM in the designed sample. Furthermore, in colorimetric sensors for detecting Hg2+ ions, Liu group [73] used a very similar T-T mismatch method. The detection limit was 3 μM. Xie et al. [74] also published an ultra-sensitive Hg2+ detection sample based on fluorescent NPs from the gold. The quenchings are caused by interactions between Hg2+-Au+ and the surface of Au NPs with the small amount (~17%) of Au+ ions modified the surface of the Au metal NPs.
Recently the Hg2+ ions, produced by the reaction of tetrachlorinated uric acid in the presence of golden nanoclusters acting as a catalyst, were colorimetrically detected in another study by Zhou et al. The detection limit of Hg2+ was 10 nM, far lower than the allowed level in USA [75]. Silver NPs are also frequently used as optical sensor materials [76,77,78]. For instance, for Farhadi et al. [76], the newly synthesized silver nanostructure with plant extract was used for detecting Hg2+ ions. It has a scattered, yellowish-brown color that becomes color-free with the Hg2+ sample solution. They also researched the susceptibility of silver metal NPs for alkaline metallic ions, metal ions, and earth metallic alkaline. In comparison with other metals with a detection limit of 2.20 × 10−6 M, however, the findings are optimum for Hg2+ Ions. Sebastian et al. [77] studied the sensing property of stabilized Ag NPs of Agaricus bisporus in a further study These noble metal NPs were also used to detect possible hazards in addition to detecting drinking water toxic ions [78]. For example, uranium (UO22+), the calorimetric sensors that have excellent radioactive uranium selectivity, can be detected with gold NPs. The gold NPs detection limit for UO22+ was much lower than the infection level according to Environmental Protection Agency (EPA-USA) criterion for UO22+ in consumable water. To experiment, Liu et al. [79] identified the uranyl specific sensor DNAzyme with a detection limit of 45 pm with high selectivity. Similarly, Lee et al. [69] used both a labeled and label-free method for Uranil sensing and the findings were remarkable. The labeled method showed a higher detection limit of 50 nM and larger response time compared to the label-free method. The various developments in the chemical colorimetric sensing are given in Table 1.

Table 1.
Colorimetric sensing characteristics of the probes.
6.2. Fluorimetric Sensing
Fluorimetric detection is a fluorescence phenomenon regardless of the power losses typically in the solution between the excitation and the emission spectrum. Fluorescence is superior to absorption spectroscopy since the absorbent process is very fast (approximately 10–15 s), so that molecular motion does not occur during the absorbent process, as per the Frank-Condon theory. However, compared to the absorption process, fluorescence takes place over a longer time (environ 10 ns) imply that the fluorophore molecule stays in the exit for a longer period, thus, interacts with its neighboring molecule [61]. This relationship changes; the analyte may increase or decrease fluorescence intensity depending on the fluorophore alignment with the quencher, while fluorophore is linked to the analyte [70]. Furthermore, as stated in the literature in a few cases, static and dynamic quenching can take place together [70]. The major groups of the molecules are hydroxyl, amine, and halogens typically serve as quenchers [61].
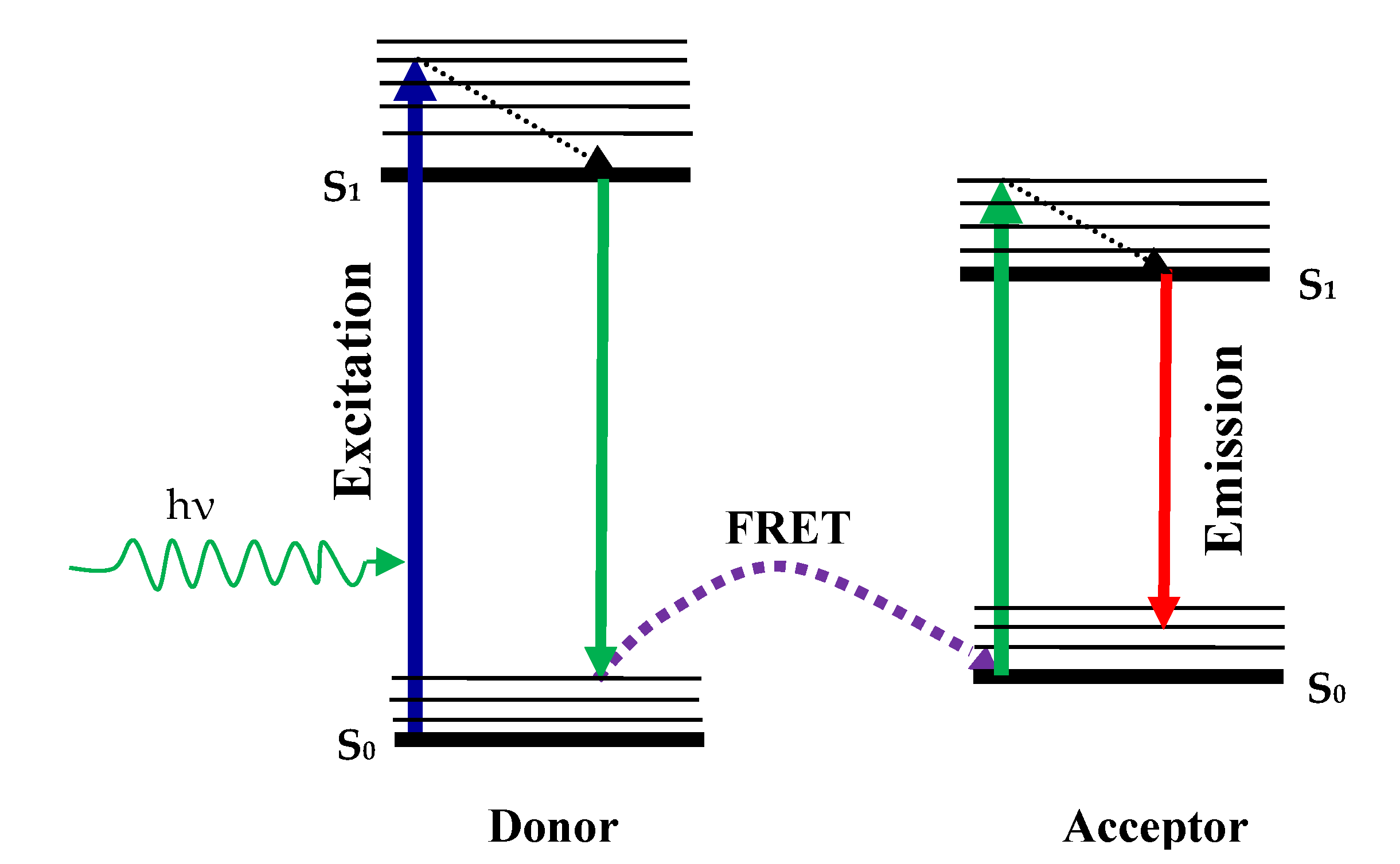
The difference in fluorophore intensity occurs due to the resonance energy transfer Förster (FRET). Theoretical calculations by Theodor Förster were based on the induced dipole-dipole interactions between donor molecules and the receiver molecule in 1949. In this phase, fluorophore (donor) initially goes to the excited state through absorption of a photon, when the FRET Mechanism model is followed by the radiation-free energy transfer that transfers energy to another molecule which is a recipient. Then, the acceptor becomes aroused and the acceptor molecule returns to the ground through photon emission, as shown in Figure 7. In this method, which is possible by the FRET method, the fluorescence intensity of the acceptor molecule will change. The interaction is studied at molecular levels and the distance from each other on a molecule or like the ligand-guided ion channel can be quantified [78].
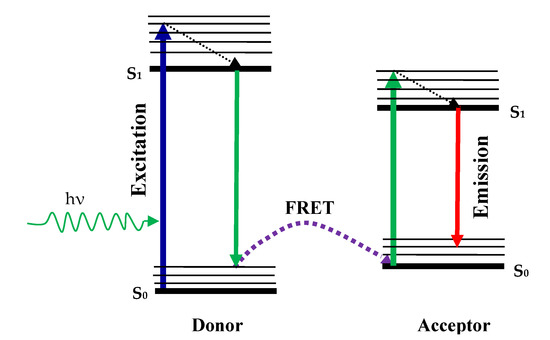
Figure 7.
The schematic of resonance energy transfer Förster (FRET) process.
Beyond the range of Förster distance, energy transfer takes place between the donor-acceptor molecules. The Förster gap usually lies between 10 Å and 60 Å and many proteins are comparable in size [61,83]. Another term RET is a special state of FRET when a donor’s emission spectrum overlaps with the absorption spectrum of the recipient. FRET is also called RET in this condition. The energy transfer efficiency can be calculated by the Förster Equation (10).
The Förster range, R0, is at that distance where the donor to acceptor distance is such that the energy transfer efficiency is 50%. The transfer of energies between the donor and the accepter depends, for example, on the medium refractive index for energy transmission, donor and acceptor spectrum form, and shape. Noble metal NPs such as gold are closely connected to biomolecules such as protein and nucleic acids and hence are used in fluorescent sensing. The Au NPs’ association with the biomolecule however depends on its composition and structure. For example, the spiral form single-stranded (ss)-DNA and double-stranded (ds-DNA) show different absorption patterns on the noble metal surface. There was a different scale. The ss-DNA was absorbed irreversibly and rigid ds-DNA (dorsale phosphate) was reflected back due to repulsion between the negative AuNPs stabilized. Au metal NPs can be used as an acceptor in the FRET phase because of its exceptionally high extinction coefficient of ~108 M−1 cm−1 for 15 nm Au NPs, whereas it is ~105 M−1cm−1 for traditional sensors. Furthermore, gold NPs also have additional characteristics such as high conductivity, simple surface alteration, high fluorescence abnormality, and a tunable absorption spectrum varying with their size and shape. For example, Au nanorods with different aspect ratios may be tuned to a visible infrared absorption spectrum (Figure 6), thus the spectrum of Au NPs can alter donor emissions across the range [60,62,84].
Dubertret et al. [33] prepared the first study on gold-based FRET, which was useful for chemical fluorophores. The FRET was due to the interaction between the dipole and the dipole and the right orientation of the gold NPs. However, for gold spherical NPs with no dipole moment the energy may be transferred into gold NPs in either direction as opposed to dyes. This can gain from modern spherical-form metal NPs compared with the other types of NPs. Organic molecules that make the receiver excellent. Benzoic acid (DABCYL) is, for example, considered to be the most capable acceptor of organic quencher 4-((4’-(dimethylamino)phenyl) azobenzoic acid.
The fluorescence intensity of a given color is quenched to 99.0 percent. The efficiency for quenching is not standardized on the whole absorption spectrum, however, and the dyes’ efficiency decreases with long wavelengths. The DABCYL structure is given in Figure 8. The extended length is estimated to be about 1.2 nm, which is compatible with ~1.4 nm gold NPs in size. However, the gold NPs are more than 100-fold more effective than the DABCYL. Furthermore, as opposed to coloring, in the infrared zone the performance of gold NPs does not decrease [33,85].
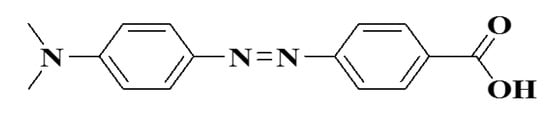
Figure 8.
4-((4’-(dimethylamino) phenyl) azobenzoic acid.
Although the gold NPs are successful quenchers, few reports also indicate a fluorescence enhancement of gold NPs for solid substrates at the required distances from the fluorophore metal. However, the increased fluorescence intensity is expected to be due to the far-reaching reflection of fluorophore radiation that has recurred to fluorophore [86]. Recently, Schietinger et al. published in another paper on the plasmon intensities of upgrade luminescence of 30 nm in diameter UCNPs attached to 30–60 nm gold NPs [87]. In recent years several reports on gold NPs for various fluorometric, quantum dots, and UCNP are summarized in Table 2 [88,89,90,91,92,93].

Table 2.
Au NPs as FRET acceptors.
Further, FRET performance improvers for the interaction with fluorophore silver NPs were reported as FRET in recent times [94,95,96]. The studied growth factor-BB identification of the human platelet using the enhanced silver nanoparticular FRET sensor of BHQ-2 quencher strands. In this study, fluorophore aptamer’s emission spectrum has been enhanced near silver NPs. These sensors were more sensitive than bare FRET and gold NPs dependent FRET with regard to sensitivity and selectivity. Their findings showed that silver NPs are used for improved FRET sensor selectivity and sensitivity of 0.8 ng/mL. Another research planned and developed improved FRET imagery by Zhao et al. [96].
6.3. Metal NPs in Microelectronic Packaging
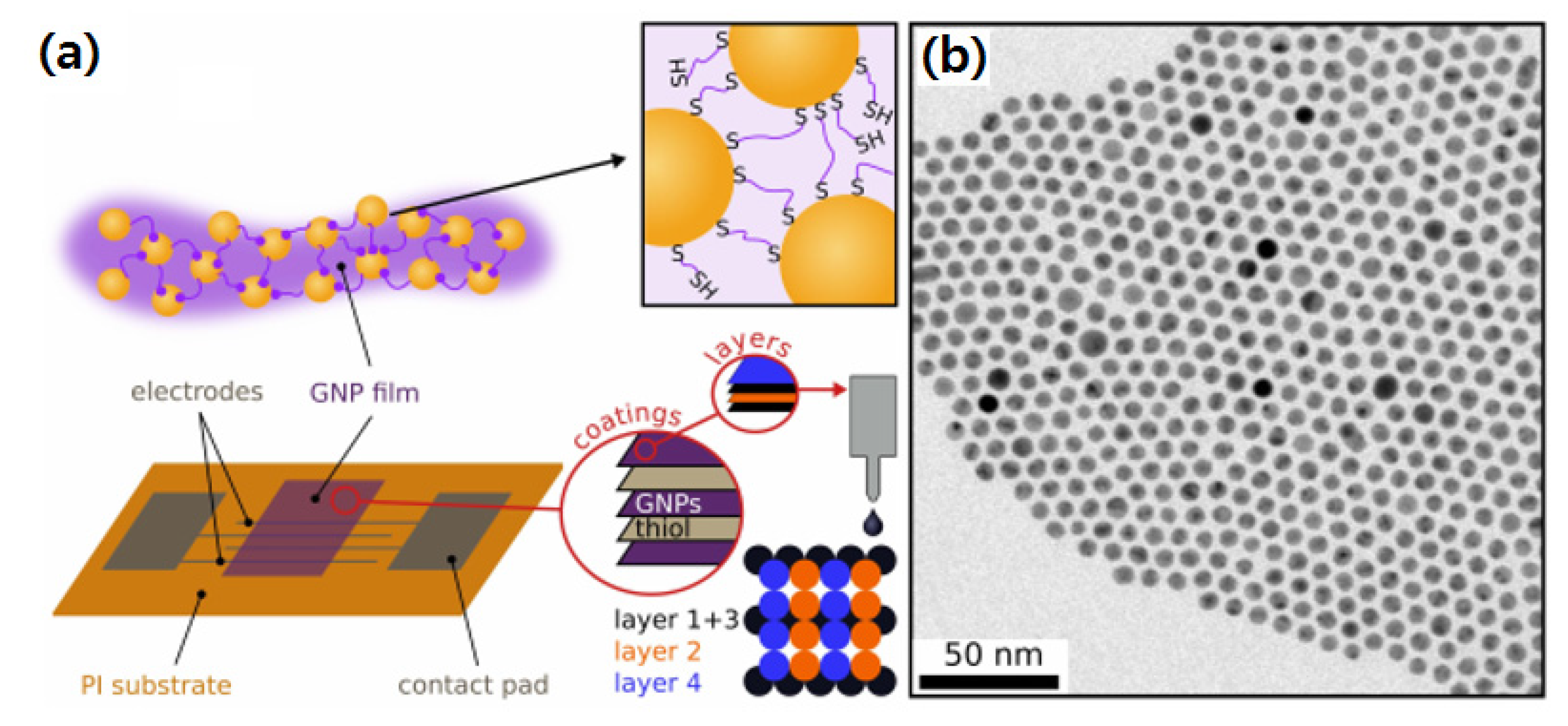
Metal NPs have been consistently used in microjoining of several electronic components such as electronic circuit boards, microfluidic devices, and nanodielectrics. Besides, enormous research efforts have been devoted in this area to develop metal NP pastes containing several hundred nm sizes for advanced microfluidic and stretchable electronics in medical applications. Generally, the metal NPs are prepared by using capping agents to control their growth and particle size for a variety of applications [97,98]. Monodispersed Au NPs have been successfully printed for chemoreceptive sensor circuit assembly on polyimide substrate as shown in Figure 9 [99]. The Ag interdigitated electrodes were printed on polyimide by a robotic dispenser. The printed Au NPs were of less than tens of nanometers. When heat-treated at high temperatures, these metal NPs decompose leaving behind the organic capping layer around the metal core surface which further forms a conducting thin layer over the circuit components.
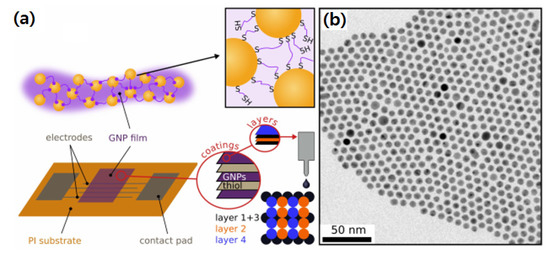
Figure 9.
(a) Schematic of chemoreceptive sensor circuit fabrication using Au NPs on polyimide, and (b) Au NPs size distribution [99].
However, compared to conventional microparticles decompose quickly at lower temperatures and form conducting layer at pretty low temperatures (300 °C) compared to the microparticles owing to quantum size effect. The thermal stability of Au NPs is also important for micropatterned sensors and flexible electronics.
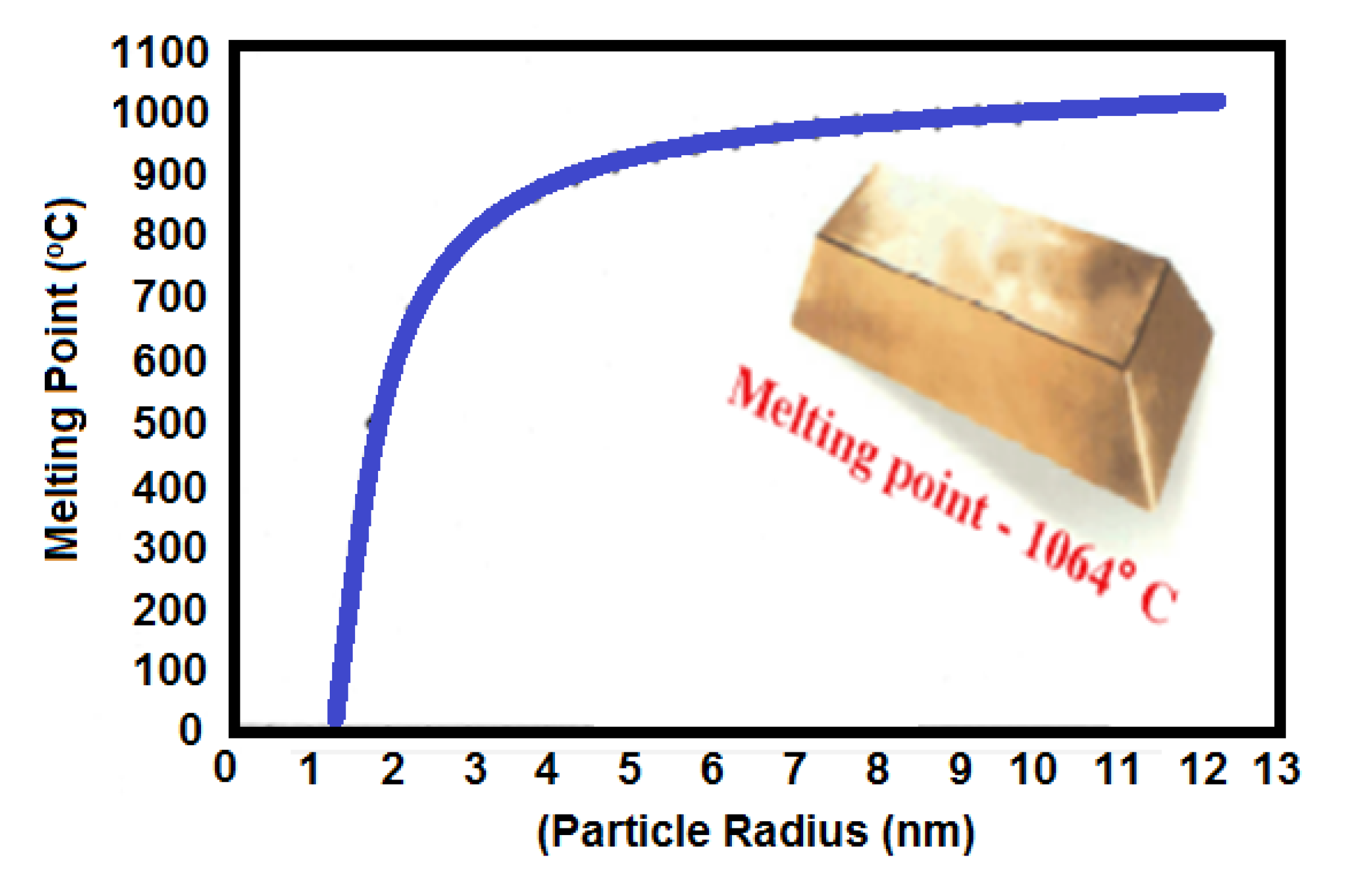
The most widely used Au NPs have excellent corrosion resistance and thermal and electrical conductivity, which enables them to bond with a fluxless process on plastic substrates. The melting points of Au NPs decrease compared to bulky Au (1064 °C) when the Au NPs size is below 10 nm as shown in Figure 10. Gibbs–Thomson equation shows the relationship of melting points of nanoparticle and bulk particle having a diameter of d as follows. This equation explains the depression in melting point of the nanostructured materials with particle size [100,101].
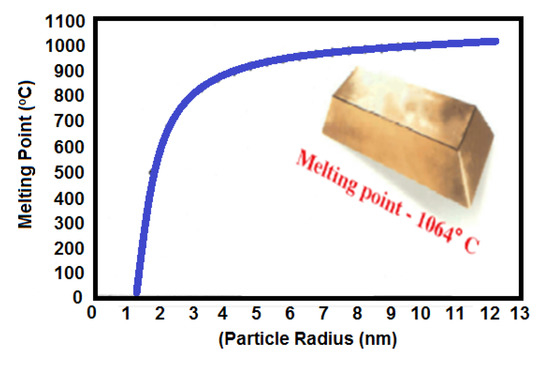
Figure 10.
Effect of particle radius on the melting point of Au NPs [100].
MB = bulk melting temperature, Hf = heat of fusion, σsl = solid-liquid interfacial energy, and ρs = solid material density. Further, if the diameter reaches around 5 nm, then the Au NPs can melt around room temperature as shown in [102,103]. Thus, nanosized particles are frequently used for lower melting point bonding or electronics packaging. Wang et al. suggested melting point of Au NPs entrapped in double layered graphene sheets increased when the length of Au NPs was decreased. The melting of the Au NPs begins from the innermost layer [103]. The NPs mixed with smaller and larger sizes showed an increased filling ratio during sintering for electronics packaging. German et al. reported the filling ratio of sintered particles as following equation [104]:
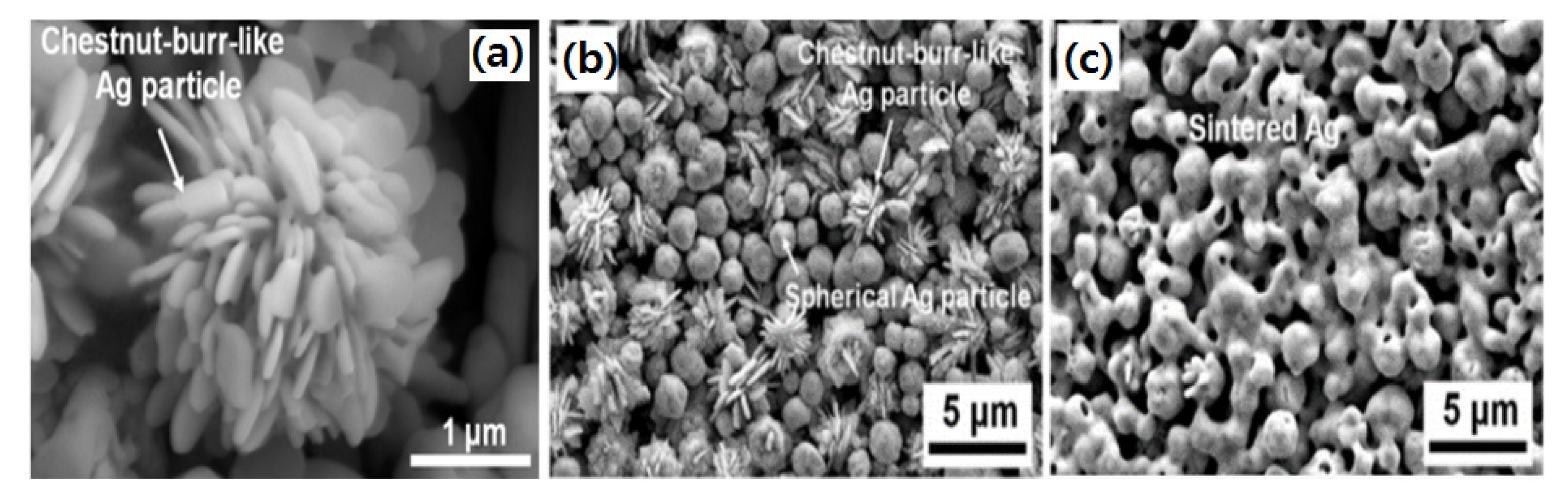
where fL and fS indicate the filling ratio of large and small particles, respectively. The shape of particles also affects the microjoining in noble metal NPs for die-attach materials. Figure 11a–c shows Ag micro powder having a submicron sized chestnut-burr-like shape. In this shape, the powder particles have a higher surface/volume ratio than the spherical powder, the bonding mechanism is almost similar to the Ag NPs during sintering [105,106].
fmax − fL + (1 − fL)fs
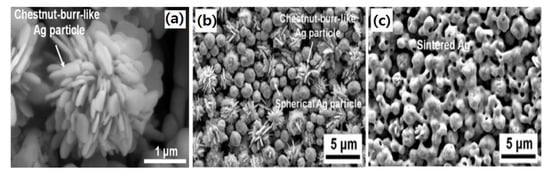
Figure 11.
(a) A single chestnut burr like Ag particle, (b) mixed chestnut burr and spherical particles, and (c) Ag particles after sintering [105].
If the pressure is applied during sintering, the bonding layer of powders can have a denser structure. The sintering driving force with applied pressure can be represented as the Equation (13).
where, γ = surface energy, Ω = molecular wt., K = curvature of voids, g = geometric constant, and Pa = applied pressure [107]. All these activities on Au and Ag NPs at low temperature joining materials suggest that the metal NPs can be successfully applied to flexible polymer substrates, glass and ceramics as well [97,108,109,110]. Fine electronic circuitries with line and space < 50 mm are usually formed by screen printing where traditional metal particles cannot be applied due to their bulk size. Further, the superior dispersion of metal NPs makes it possible to print fine traces in large scale integration devices in microjoining [97].
DE = γΩK +gPa
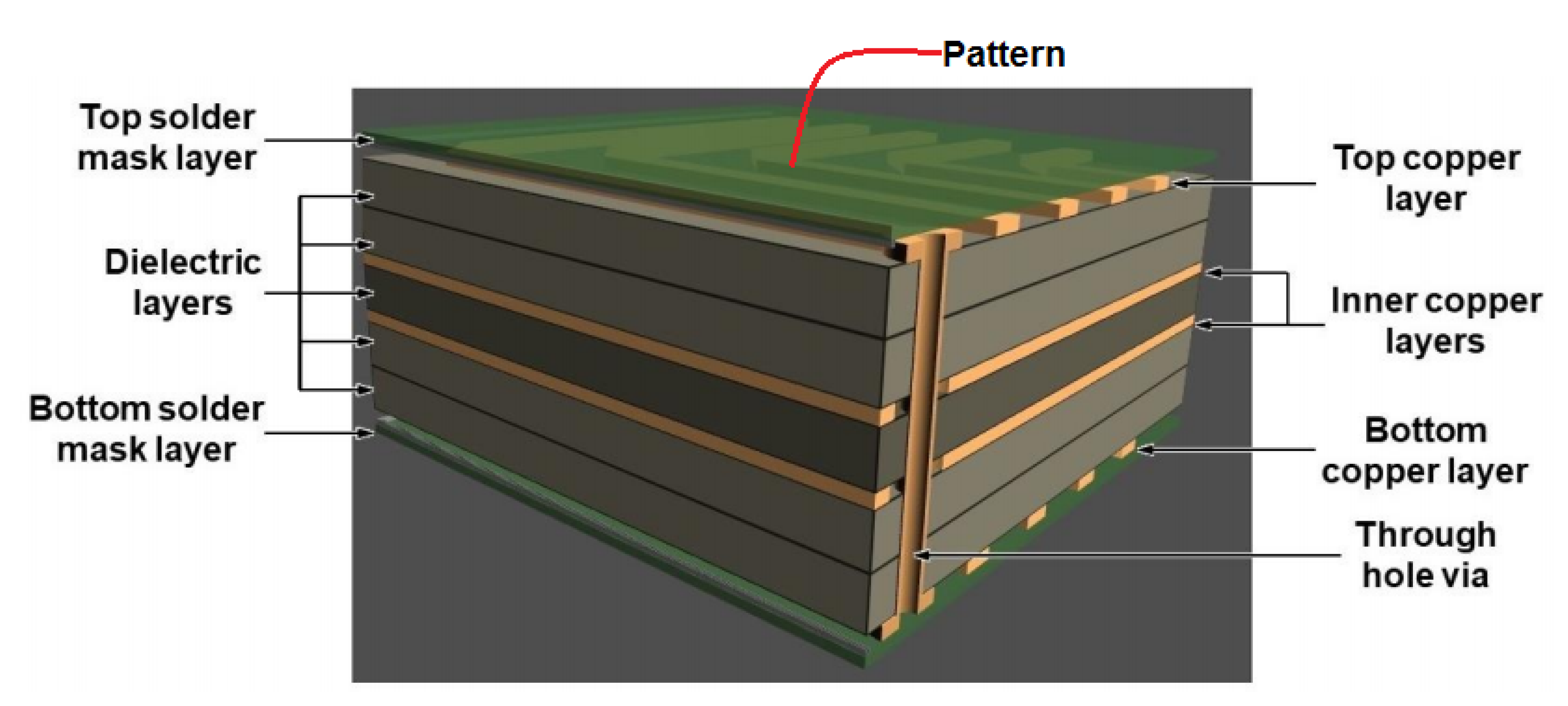
Various kinds of metal NPs have been used in screen printing of electronic circuit patterns such as Ag, Au, Pd, Ag-Pd, etc. [97,108,109,110]. Dispersed Au and Ag NPs with average size smaller than 10 nm [97] were widely prepared by evaporation routes. These metal NPs were dispersed in an organic solvent which is further activated by heating around 200 °C to leave behind a conductive film (0.1 to 10 µm thick) with a resistivity of 3 to 50 µΩcm [108,109]. Figure 12 shows a typical design of a multilayer circuit board consisting of copper layers, dielectric, and mask layers for generating patterns.
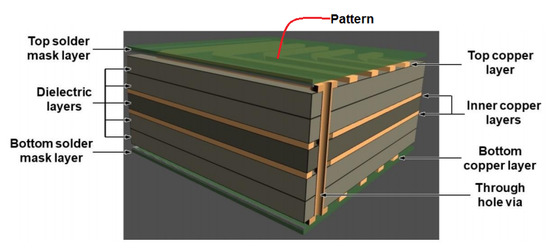
Figure 12.
Schematic diagram of a multilayered circuit board [111].
The copper layers are filled through the substrate for electrical continuity to the patterned electric circuit over the top surface [111]. In some reports, Ag-Pd NPs have been utilized for the printing of electronic devices [110]. Ag85-Pd15 nanoparticle paste affords the 4 µm thick films with resistivity of 12 µΩcm, exhibiting anti electromigration behavior. Some researchers have used hybrid Ag paste composed of micron and nanoparticle sized Ag prepared by an evaporation method (diameter 3–5 µm) [97,112]. Highly viscous Ag nanoparticle pastes are also used for printing up to 18 µm width by dispensing method. This dispensing method can also repair any disconnected part through heat treatment and laser irradiation [112].
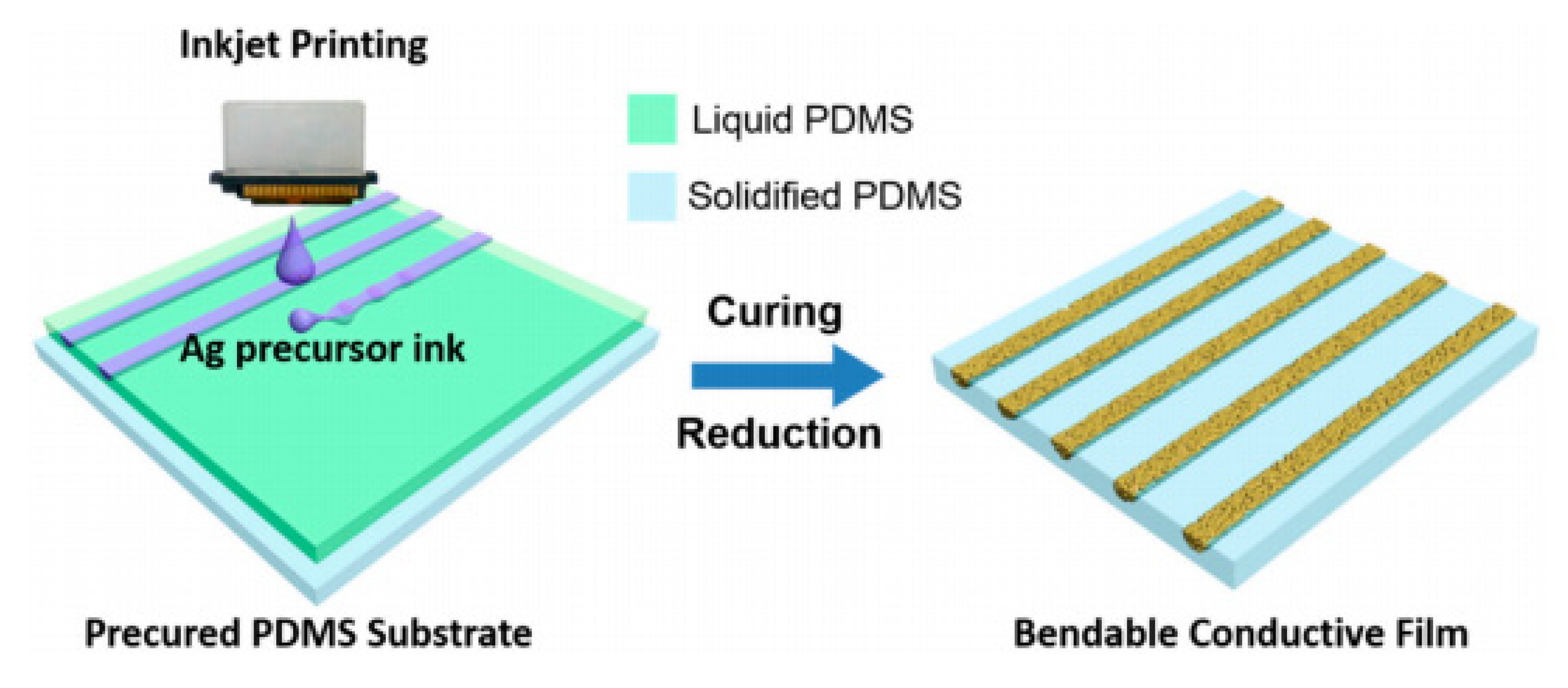
Inkjet printing and dispersed metal NPs have shown promise for superfine patterning with several microns line width which is hardly possible via screen printing. For inkjet printing, around 2 pL of liquid can produce a 15–16 µm size dot and form a line by connecting each dot by inkjet printing. As compared to paste and screen printing, metal NPs are less viscous and well dispersed in inkjet printing. The fine electronic pattern fabricated on procured flexible polydimethylsiloxane (PDMS) substrate with Ag nanoink is inkjet printing is shown in Figure 13.
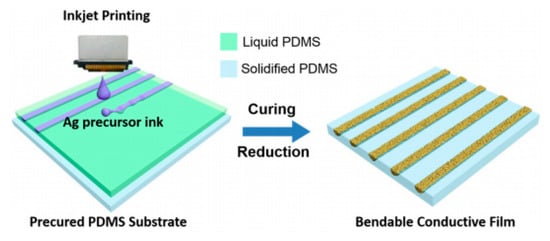
Figure 13.
Flexible circuit pattern fabricated using Ag inkjet printing [113].
The Ag precursor ink was printed on the PDMS substrate and cured. Further reduction of printed deposits with hydrazine was done to produce the semi-wrapped patterns directly. Recent inkjet printing uses femtoliter drops of dispersed Ag nano-ink to generate fine patterns enclosing fine lines in a given space which is a viable technique in developing flexible electronics [114,115]. In addition, multilayer inkjet printing is beneficial for embedded IC circuits for large scale integration for flexible electronics, medical and chemical sensing applications [116,117].
6.4. Impact of Nanoparticles of Human Health and Enviroments
The rapid progress has been taking place in the nanotechnology leading to concerns about the potential risk associated with the use and application of nanoparticles on human health and the environment. Obviously, the nanoparticles are more active with their bulk counterpart that may need some more careful investigation before it really becomes applied to the human. In this regard, nanoparticles have been investigated in different models such as cell model, mouse model and human model. The available data suggest that the over exposure can lead to accumulation in the organs. For instance, Mats Hulander et al. [118] reported that noble metal can be used as a biomaterial because these metals have a special antimicrobial and anti-allergic capability. The use of noble metals such as silver colloidal NPs often increases the risk of night vision, bow pain, and respiratory and neurological disorders significantly. Additionally, some skin allergies have also been noted with the use of colloidal noble metal nanoparticles [119]. In addition, Zhu et al. [120] have found that noble metals have been applied as a multifunctional cancer therapy because of their remarkable surface plasmon responses. Furthermore, a number of hazardous pollutants are also being generated as byproducts due to the rapid development of chemical industries. This is very difficult and huge challenge to decompose these NPs with traditional biological treatment. Such issues can be easily solved with the help of noble metal nanomaterials-based catalysts because they have outstanding absorption and dissociating ability [121]. In this regard, the noble metals not only contribute to human life but also minimize environment pollution.
7. Summary and Conclusions
We have overviewed the various routes utilized for the synthesis of metallic NPs, their nucleation and growth behavior. The properties of the bulk materials depend upon the surface atoms density that determines the chemical sensing and electronic behavior. Besides, we have summarized the various developments in this metal NPs technology related to Au and Ag NPs in various chemical analysis, biosensing, FRET, and microelectronic applications. Precious noble metal NPs have shown remarkable usage in sensing of water contamination at much lower detection limits as compared to traditional sensors. Au NPs have been shown to sense various radioactive uranyl compounds and saved the human life. We have also discussed the shape and size of NPs, such as how spherical NPs have more advantages over the other shapes of the NPs. Moreover, we discussed why the gold NPs have superiority in FRET process compare to the traditional FRET sensor such as DABCYL which also retain their efficiency in the infrared region. However, in contrast to Au, Ag NPs have been established as FRET efficiency enhancer when interacted with fluorophore. The technology of metal NPs is growing across microelectronic systems for screen printing and inkjet printing as a new way to fabricate fine pitch circuit and multilayer printing for systems in packaging all at once [3]. The future trends in chemical sensing are expected to depend on the development of SPR sensors, nanobiosensors, FRET, etc., for chemical, flexible portable diagnostic sensors in micro- and nanoelectronics. Nanobiosensors have yet to be fulfill their potential in chemical sensing with a high sensitivity and selectivity, as they still need comprehensive developments in future. The future generation of nanobiosensors based on AuNPs will definitely revolutionize the field of chemical sensing and monitoring at reduced costs and widespread usage of medicines and therapies at ease. Therefore, the employment of metal NPs technology has further advanced the field of electronics, medical, chemical, and bio-sensing to design smart engineering devices and applications.
Author Contributions
Conceptualization and methodology, A.G. and J.P.J.; formal analysis and validation, P.K. and P.G.; resources and supervision, J.P.J.; writing—original draft preparation, A.G. and J.P.J.; writing—review and editing, A.S. and N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 2020 Research Fund of the University of Seoul for Jae Pil Jung.
Institutional Review Board Statement
Not applicable.
Acknowledgments
This work was supported by the 2020 Research Fund of the University of Seoul for Jae Pil Jung.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-Dimensional Metal Nanostructures: From Colloidal Syntheses to Applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef]
- West, J.L.; Halas, N.J. Engineered Nanomaterials for Biophotonics Applications: Improving Sensing, Imaging, and Therapeutics. Annu. Rev. Biomed. Eng. 2003, 5, 285–292. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Y.; Liu, H.; Wu, X.; Li, Y.; Miao, L.; Shen, Z.; Wu, A. High-Performance Colorimetric Detection of Hg2+ Based on Triangular Silver Nanoprisms. ACS Sens. 2016, 5, 521–527. [Google Scholar] [CrossRef]
- Gautam, A.; Komal, P. Probable ideal size of Ln3+-based upconversion NPs for single and multimodal imaging. Coord. Chem. Rev. 2018, 376, 393–404. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold NPs for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold NPs in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Paull, R.; Wolfe, J.; Hébert, P.; Sinkula, M. Investing in nanotechnology. Nat. Biotechnol. 2003, 21, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Jia, L. Global Governmental Investment in Nanotechnologies. Curr. Nanosci. 2005, 1, 263–266. [Google Scholar] [CrossRef]
- Gharatape, A.; Salehi, R. Recent Progress in Theranostic Applications of Hybrid Gold NPs. Eur. J. Med. Chem. 2017, 138, 221–233. [Google Scholar] [CrossRef]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold Nanocages: From Synthesis to Theranostic Applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, J.; Chen, Q.; Liu, Z. Nanoscale metal-organic frameworks and coordination polymers as theranostic platforms for cancer treatment. Coord. Chem. Rev. 2019, 398, 113009–113023. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Shen, Y. Hydrothermal Synthesis of Gold Nanoplates and their Structure-Dependent LSPR Properties. J. Mater. Res. 2018, 33, 2671–2679. [Google Scholar] [CrossRef]
- Gautam, A.; Ram, S. Preparation and thermomechanical properties of Ag-PVA nanocomposite films. Mater. Chem. Phys. 2010, 119, 266–271. [Google Scholar] [CrossRef]
- Ram, S.; Tripathy, P.; Fecht, H.J. Gold NPs reinforced poly (vinyl alcohol) of self-standing optical films. J. Nanosci. Nanotechnol. 2007, 7, 3200–3206. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Gautam, A.; Fecht, H.J.; Cai, J.; Bansmann, J.; Behm, R.J. A new allotropic structure of silver nanocrystals nucleated and grown over planar polymer molecules. Philos. Mag. Lett. 2007, 87, 361–372. [Google Scholar] [CrossRef]
- Cametti, M.; Džolić, Z. New frontiers in hybrid materials: Noble metal NPs Supramolecular gel systems. Chem. Commun. 2014, 50, 8273–8286. [Google Scholar] [CrossRef]
- Alexander, J. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Gold NPs in Biology and Medicine: Recent Advances and Prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef]
- “Plenty of Room” Revisited. Nat. Nanotechnol. 2009, 4, 781. [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalyst 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Arnal, P.M.; Comotti, M.; Schüth, F. High-Temperature-Stable Catalysts by Hollow Sphere Encapsulation. Angew. Chem. Int. Ed. 2006, 45, 8224–8227. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Zhao, F.; Wang, Y.; Ye, Z.; Hua, C.; Liu, Z.; Bohinc, K.; Guo, X. Spherical Polyelectrolyte Brushes as Templates to Prepare Hollow Silica Spheres Encapsulating Metal Nanoparticles. Nanomaterials 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Bohinc, K.; Bossa, G.V.; May, S. Incorporation of ion and solvent structure into mean-field modeling of the electric double layer. Adv. Colloid Interface Sci. 2017, 249, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Mojarad, N.; Krishnan, M. Measuring the size and charge of single nanoscale objects in solution using an electrostatic fluidic trap. Nat. Nanotechnol. 2012, 7, 448. [Google Scholar] [CrossRef]
- Skaug, M.J.; Schwemmer, C.; Fringes, S.; Rawlings, C.D.; Knoll, A.W. Nanofluidic rocking brownian motors. Science 2018, 359, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Smith, G.N.; Grillo, I.; Rogers, S.E.; Eastoe, J. Surfactants with colloids: Adsorption or absorption? J. Colloid Interface Sci. 2015, 449, 205–214. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Chaichan, M.t.; Kazem, H.A.; Sopian, K. Evaluation and analysis of nanofluid and surfactant impact onphotovoltaic-thermal systems. Case Stud. Therm. Eng. 2019, 13, 100392. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Pulse Electroplating of Ultrafine Grained Tin Coating. In Electroplating of Nanostructures; IntechOpen: Rijeka, Croatia, 2015; pp. 105–129. [Google Scholar]
- Nützenadel, C.; Züttel, A.; Chartouni, D.; Schmid, G.; Schlapbach, L. Critical Size and Surface Effect of the Hydrogen Interaction of Palladium Clusters. Eur. Phys. J. D 2000, 8, 245–250. [Google Scholar] [CrossRef]
- Dubertret, B.; Calame, M.; Libchaber, A.J. Single-Mismatch Detection Using Gold-Quenched Fluorescent Oligonucleotides. Nat. Biotechnol. 2001, 19, 365–370. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Shape and Size Dependence of Radiative, Non-radiative and Photothermal Properties of Gold Nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of NPs in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-Dimensional Metal Nanomaterials: Synthesis, Properties, and Applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, X.; Gao, B.; Zou, W.; Dong, L. Facile Ball-Milling Synthesis of CuO/Biochar Nanocomposites for Efficient Removal of Reactive Red 120. ACS Omega 2020, 5, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Korczagin, I.; Golze, S.; Hempenius, M.A.; Vancso, G.J. Surface Micropatterning and Lithography with Poly(Ferrocenylmethylphenylsilane). Chem. Mater. 2003, 15, 3663–3668. [Google Scholar] [CrossRef]
- Kim, S.H.; Liu, B.Y.H.; Zachariah, M.R. Synthesis of Nanoporous Metal Oxide Particles by a New Inorganic Matrix Spray Pyrolysis Method. Chem. Mater. 2002, 14, 2889–2899. [Google Scholar] [CrossRef]
- Susha, A.S.; Ringler, M.; Ohlinger, A.; Paderi, M.; LiPira, N.; Carotenuto, G.; Rogach, A.L.; Feldmann, J. Strongly Luminescent Films Fabricated by Thermolysis of Gold−Thiolate Complexes in a Polymer Matrix. Chem. Mater. 2008, 20, 6169–6175. [Google Scholar] [CrossRef]
- Zhu, M.; Baffou, G.; Meyerbröker, N.; Polleux, J. Micropatterning Thermoplasmonic Gold Nanoarrays To Manipulate Cell Adhesion. ACS Nano. 2012, 6, 7227–7233. [Google Scholar] [CrossRef]
- Marques-Hueso, J.; Morton, J.A.S.; Wang, X.; Bertran-Serra, E.; Desmulliez, M.P.Y. Photolithographic Nanoseeding Method for Selective Synthesis of Metal-Catalysed Nanostructures. Nanotechnology 2019, 30, 15302–15309. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for Micro- and Nanoscale Patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- Sreenivasan, S.V. Nanoimprint Lithography Steppers for Volume Fabrication of Leading-Edge Semiconductor Integrated Circuits. Microsyst. Nanoeng. 2017, 3, 17075–17093. [Google Scholar] [CrossRef] [PubMed]
- Garno, J.C.; Yang, Y.; Amro, N.A.; Cruchon-Dupeyrat, S.; Chen, S.; Liu, G.-Y. Precise Positioning of NPs on Surfaces Using Scanning Probe Lithography. Nano Lett. 2003, 3, 389–395. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic NPs. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Gautam, A.; Mukherjee, S.; Ram, S. Controlled Novel Route to Synthesis and Characterization of Silver Nanorods. J. Nanosci. Nanotechnol. 2010, 10, 4329–4334. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Singh, G.P.; Ram, S. A Simple Polyol Synthesis of Silver Metal Nanopowder of Uniform Particles. Synth. Met. 2007, 157, 5–10. [Google Scholar] [CrossRef]
- Evanoff, D.D.; Chumanov, G. Size-Controlled Synthesis of NPs. 1. “Silver-Only” Aqueous Suspensions via Hydrogen Reduction. J. Phys. Chem. B 2004, 108, 13948–13956. [Google Scholar] [CrossRef]
- Tatarchuk, V.V.; Sergievskaya, A.P.; Korda, T.M.; Druzhinina, I.A.; Zaikovsky, V.I. Kinetic Factors in the Synthesis of Silver NPs by Reduction of Ag+ with Hydrazine in Reverse Micelles of Triton N-42. Chem. Mater. 2013, 25, 3570–3579. [Google Scholar] [CrossRef]
- Fountoulaki, S.; Daikopoulou, V.; Gkizis, P.L.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Mechanistic Studies of the Reduction of Nitroarenes by NaBH4 or Hydrosilanes Catalyzed by Supported Gold NPs. ACS Catal. 2014, 4, 3504–3511. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green Nanotechnology: A Review on Green Synthesis of Silver NPs An Ecofriendly Approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Tripathy, P.; Ram, S. Microstructure, topology and X-ray diffraction in Ag-Metal Reinforced Polymer of Polyvinyl Alcohol of Thin Laminates. J. Mater. Sci. 2006, 41, 3007–3016. [Google Scholar] [CrossRef]
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of Formation and Structure of Micro Emulsions by Electron Microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Tissandier, C.; Diop, N.; Martini, M.; Roux, S.; Tillement, O.; Hamaide, T. One-Pot Synthesis of Hybrid Multifunctional Silica NPs with Tunable Coating by Click Chemistry in Reverse W/O Microemulsion. Langmuir 2012, 28, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; van Veggel, F.C.J.M. Synthesis of InN@SiO2 Nanostructures and Fabrication of Blue LED Devices. ACS Appl. Mater. Interfaces 2012, 4, 3902–3909. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Ganguly, A.; Vaidya, S. Microemulsion-Based Synthesis of Nanocrystalline Materials. Chem. Soc. Rev. 2010, 39, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Alejo, C.J.; D’Alfonso, C.; Pacioni, N.L.; González-Béjar, M.; Grenier, M.; Lanzalunga, O.; Alarcon, E.I.; Scaiano, J.C. Ultraclean Derivatized Monodisperse Gold NPs through Laser Drop Ablation Customization of Polymorph Gold Nanostructures. Langmuir 2012, 28, 8183–8189. [Google Scholar] [CrossRef]
- Dahal, N.; García, S.; Zhou, J.; Humphrey, S.M. Beneficial Effects of Microwave-Assisted Heating versus Conventional Heating in Noble Metal Nanoparticle Synthesis. ACS Nano. 2012, 6, 9433–9446. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold NPs in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold NPs: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.-H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-Enabled Photonics-Based Imaging and Therapy of Cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Au, L.; Li, X.; Xia, Y. Facile Synthesis of Ag Nanocubes and Au Nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef]
- Polavarapu, L.; Pérez-Juste, J.; Xu, Q.-H.; Liz-Marzán, L.M. Optical Sensing of Biological, Chemical and Ionic Species Through Aggregation of Plasmonic NPs. J. Mater. Chem. C 2014, 2, 7460–7476. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold NPs with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, X.; Kumar, A.; Zou, G.; Zhang, X.; Liang, X.-J. Long Genomic DNA Amplicons Adsorption onto Unmodified Gold NPs for Colorimetric Detection of Bacillus Anthracis. Chem. Commun. 2013, 49, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing Colorimetric Approaches Based on Gold and Silver NPs Aggregation: Chemical Creativity Behind the Assay. A Review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef]
- Lee, J.H.; Wang, Z.; Liu, J.; Lu, Y. Highly Sensitive and Selective Colorimetric Sensors for Uranyl (UO22+): Development and Comparison of Labeled and Label-Free DNAzyme-Gold Nanoparticle Systems. J. Am. Chem. Soc. 2008, 130, 14217–14226. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K. Functionalized Gold Nanoparticle Supported Sensory Mechanisms Applied in Detection of Chemical and Biological Threat agents: A review. Anal. Chim. Acta 2012, 715, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Johnson, R.C.; Hupp, J.T. Gold Nanoparticle-Based Sensing of “Spectroscopically Silent” Heavy Metal Ions. Nano Lett. 2001, 1, 165–167. [Google Scholar] [CrossRef]
- Lee, J.-S.; Han, M.S.; Mirkin, C.A. Colorimetric Detection of Mercuric ion (Hg2+) in Aqueous Media Using DNA-Functionalized Gold NPs. Angew. Chem. Int. Ed. Engl. 2007, 46, 4093–4096. [Google Scholar] [CrossRef]
- Xue, X.; Wang, F.; Liu, X. One-Step, Room Temperature, Colorimetric Detection of Mercury (Hg2+) Using DNA/Nanoparticle Conjugates. J. Am. Chem. Soc. 2008, 130, 3244–3245. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Highly Selective and Ultrasensitive Detection of Hg2+ Based on Fluorescence Quenching of Au Nanoclusters by Hg2+–Au+ Interactions. Chem. Commun. 2010, 46, 961–963. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z. Colorimetric Detection of Hg2+ by Au NPs Formed by H2O2 Reduction of HAuCl4 Using Au Nanoclusters as the Catalyst. Sens. Actuat. B Chem. 2017, 241, 1063–1068. [Google Scholar] [CrossRef]
- Farhadi, K.; Forough, M.; Molaei, R.; Hajizadeh, S.; Rafipour, A. Highly Selective Hg2+ Colorimetric Sensor Using Green Synthesized and Unmodified Silver NPs. Sens. Actuat. B Chem. 2012, 161, 880–885. [Google Scholar] [CrossRef]
- Sebastian, M.; Aravind, A.; Mathew, B. Green Silver-Nanoparticle-Based Dual Sensor for Toxic Hg(II) Ions. Nanotechnology 2018, 29, 355502. [Google Scholar] [CrossRef] [PubMed]
- Alagan, J.; Dhesingh, R.S. Functionalized Silver Nanoparticle Probe for Visual Colorimetric Sensing of Mercury. Mater. Res. Bull. 2016, 83, 48–55. [Google Scholar]
- Liu, J.; Brown, A.K.; Meng, X.; Cropek, D.M.; Istok, J.D.; Watson, D.B.; Lu, Y. A Catalytic Beacon Sensor for Uranium with Parts-Per-Trillion Sensitivity and Millionfold Selectivity. Proc. Natl. Acad. Sci. USA 2007, 104, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Wang, C.; Wang, T.; Li, L.; Su, Z. Colorimetric Detection of Pb2+ Using Glutathione Functionalized Gold NPs. ACS Appl. Mater. Interfaces 2010, 2, 1466–1470. [Google Scholar] [CrossRef]
- Hung, Y.-L.; Hsiung, T.-M.; Chen, Y.-Y.; Huang, Y.-F.; Huang, C.-C. Colorimetric Detection of Heavy Metal Ions Using Label-Free Gold NPs and Alkanethiols. J. Phys. Chem. C 2010, 114, 16329–16334. [Google Scholar] [CrossRef]
- Patel, K.; Bhamore, J.R.; Park, T.J.; Kailasa, S.K. Selective and Sensitive Colorimetric Recognition of Ba2+ Ion Using Guanine-Functionalized Silver NPs. Chem. Sel. 2018, 3, 10182–10187. [Google Scholar]
- Dau, A.; Komal, P.; Truong, M.; Morris, G.; Evans, G.; Nashmi, R. RIC-3 Differentially Modulates α4β2 and α7 Nicotinic Receptor Assembly, Expression, and Nicotine-Induced Receptor Upregulation. BMC Neurosci. 2013, 14, 14–47. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tian, F.; Lyu, J.; Yang, M. Nanoparticle Based Fluorescence Resonance Energy Transfer (FRET) for Biosensing Applications. J. Mater. Chem. B 2015, 3, 6989–7005. [Google Scholar] [CrossRef] [PubMed]
- Gersten, J.; Nitzan, A. Spectroscopic Properties of Molecules Interacting with Small Dielectric Particles. J. Chem. Phys. 1981, 75, 1139–1152. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-Enhanced Fluorescence and Plasmon Emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef]
- Mendez-Gonzalez, D.; Melle, S.; Calderón, O.G.; Laurenti, M.; Cabrera-Granado, E.; Egatz-Gómez, A.; López-Cabarcos, E.; Rubio-Retama, J.; Díaz, E. Control of Upconversion Luminescence by Gold Nanoparticle Size: From Quenching to Enhancement. Nanoscale 2019, 11, 13832–13844. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cao, L.; Xu, K.; Zhuo, L.; Ge, J.; Li, Q.; Yu, L. A New Nanobiosensor for Glucose with High Sensitivity and Selectivity in Serum Based on Fluorescence Resonance Energy Transfer (FRET) between CdTe Quantum Dots and Au NPs. Chem. A Eur. J. 2008, 14, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Hong, M.-Y.; Lee, D.; Nam, S.-H.; Yoon, H.C.; Kim, H.-S. Inhibition Assay of Biomolecules Based on Fluorescence Resonance Energy Transfer (FRET) Between Quantum Dots and Gold NPs. J. Am. Chem. Soc. 2005, 127, 3270–3271. [Google Scholar] [CrossRef] [PubMed]
- Angelatos, A.S.; Radt, B.; Caruso, F. Light-Responsive Polyelectrolyte/Gold Nanoparticle Microcapsules. J. Phys. Chem. B 2005, 109, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Zhao, S.; Lu, X.; Tian, J. Gold NPs-based fluorescence resonance energy transfer for competitive immunoassay of biomolecules. Analyst 2012, 137, 5885–5890. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.M.; Kim, G.B.; Kim, Y.-P. Gold Nanoparticle-Based Fluorescence Quenching Via Metal Coordination for Assaying Protease Activity. Gold Bull. 2012, 45, 213–219. [Google Scholar] [CrossRef][Green Version]
- Mayilo, S.; Kloster, M.A.; Wunderlich, M.; Lutich, A.; Klar, T.A.; Nichtl, A.; Kürzinger, K.; Stefani, F.D.; Feldmann, J. Long-Range Fluorescence Quenching by Gold NPs in a Sandwich Immunoassay for Cardiac Troponin T. Nano Lett. 2009, 9, 4558–4563. [Google Scholar] [CrossRef]
- Amjadi, M.; Abolghasemi-Fakhri, Z.; Hallaj, T. Carbon Dots-Silver NPs Fluorescence Resonance Energy Transfer System as a Novel Turn-on Fluorescent Probe for Selective Determination of Cysteine. J. Photochem. Photobiol. A Chem. 2015, 309, 8–14. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Wang, C.; Li, W.; Qiang, W.; Xu, D. Silver Nanoparticle-Enhanced Fluorescence Resonance Energy Transfer Sensor for Human Platelet-Derived Growth Factor-BB Detection. Anal. Chem. 2013, 85, 4492–4499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, T.; Liu, Y. Silver Nanoparticle Plasmonic Enhanced Förster Resonance Energy Transfer (FRET) Imaging of Protein-Specific Sialylation on the Cell Surface. Nanoscale 2017, 9, 9841–9847. [Google Scholar] [CrossRef]
- Oda, J.M. Metal Nano-Particles. Jpn. Inst. Electron. Packag. 2002, 5, 523–528. [Google Scholar] [CrossRef]
- Wu, B.-H.; Yang, H.-Y.; Huang, H.-Q.; Chen, G.X.; Zheng, N.-F. Solvent Effect on the Synthesis of Monodisperse Amine-Capped Au Nanoparticles. Chin. Chem. Lett. 2013, 24, 457–462. [Google Scholar] [CrossRef]
- Ketelsen, B.; Tjarks, P.P.; Schlicke, H.; Liao, Y.-C.; Vossmeyer, T. Fully Printed Flexible Chemiresistors with Tunable Selectivity Based on Gold NPs. Chemosensors 2020, 8, 116. [Google Scholar] [CrossRef]
- Schmid, G.; Corain, B. Nanoparticulated Gold: Syntheses, Structures, Electronics, and Reactivities. Eur. J. Inorg. Chem. 2003, 3081–3098. [Google Scholar] [CrossRef]
- Castro, T.; Reifenberger, R.; Choi, E.; Andres, R.P. Size-dependent melting temperature of individual nanometer-sized metallic clusters. Phys. Rev. B 1990, 42, 8548–8556. [Google Scholar] [CrossRef]
- Chin, H.; Cheong, K.; Ismail, A. A Review on Die Attach Materials for SiC-Based High-Temperature Power Device. Metall. Mater. Trans. B 2010, 41, 824. [Google Scholar] [CrossRef]
- Wang, G.; Wu, N.; Wang, J.; Shao, J.; Zhu, X.; Lu, X.; Guo, L. Abnormal change of melting points of gold NPs confined between two-layer graphene nanosheets. RSC Adv. 2016, 6, 108343–108346. [Google Scholar] [CrossRef]
- German, R.M. Prediction of Sintered Density for Bimodal Powder Mixtures. Metall. Trans. A 1992, 23, 1455–1465. [Google Scholar] [CrossRef]
- Roh, M.H.; Nishikawa, H.; Jung, J.P. A Review of Ag Paste Bonding for Automotive Power Device Packaging. J. Microelectron. Packag. Soc. 2015, 22, 15–23. [Google Scholar] [CrossRef]
- Jung, D.-H.; Roh, M.H.; Lee, J.H.; Kim, K.H.; Jung, J.P. Transient Liquid Phase (TLP) Bonding of Device for High Temperature Operation. J. Microelectron. Packag. Soc. 2017, 24, 17–25. [Google Scholar] [CrossRef]
- Skandan, G.; Hahn, H.; Kear, B.H.; Roddy, M.; Cannon, W.R. The Effect of Applied Stress on Densification of Nano-structured Zirconia during Sinter-forging. Mater. Lett. 1994, 20, 302–309. [Google Scholar] [CrossRef]
- Matsuba, Y. Direct Patterning Using Metal Nano-Particles. J. Jpn. Inst. Electron. Packag. 2003, 6, 130–135. [Google Scholar] [CrossRef]
- Bishop, P.T.; Ashfield, L.J.; Berzins, A.; Boardman, A.; Buche, V.; Cookson, J.; Gordon, R.J.; Salcianu, C.; Sutton, P.A. Printed Gold for Electronic Applications. Gold Bull. 2010, 43, 181–185. [Google Scholar] [CrossRef]
- Nakamoto, M.; Yamamoto, M.; Kashiwagi, Y.; Kakiuchi, H.; Tsujimoto, T.; Yoshida, Y. In Proceedings of Microelectronics Symposium, 2005; pp. 241–244.
- Shamkhalichenar, H.; Bueche, C.J.; Choi, J.-W. Printed Circuit Board (PCB) Technology for Electrochemical Sensors and Sensing Platforms. Biosensors 2020, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Ohsako, Y. In: Abstract of Chemical Engineering, 34th Meeting, K122, 2001.
- Sun, J.; Jiang, J.; Bao, B.; Wang, S.; He, M.; Zhang, X.; Song, Y. Fabrication of Bendable Circuits on a Polydimethylsiloxane (PDMS) Surface by Inkjet Printing Semi-Wrapped Structures. Materials 2016, 9, 253. [Google Scholar] [CrossRef]
- Yin, Z.P.; Huang, Y.A.; Bu, N.B.; Wang, X.M.; Xiong, Y.L. Inkjet Printing for Flexible Electronics: Materials, Processes and Equipments. Chin. Sci. Bull. 2010, 55, 3383–3407. [Google Scholar] [CrossRef]
- Jang, J. Displays Develop A New Flexibility. Mater. Today 2006, 9, 46–52. [Google Scholar] [CrossRef]
- Sharma, A.; Lim, D.U.; Jung, J.P. Microstructure and brazeability of SiC nanoparticles reinforced Al–9Si–20Cu produced by induction melting. Mater. Sci. Technol. 2016, 32, 773–779. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Pulse Electrodeposition of Lead-Free Tin-Based Composites for Microelectronic Packaging. In Electrodeposition of Composite Materials; InTech: Rijeka, Croatia, 2016; pp. 253–274. [Google Scholar]
- Hulander, M.; Hong, J.; Andersson, M.; Gervén, F.; Ohrlander, M.; Tengvall, P.; Elwing, H. Blood interactions with noble metals: Coagulation and immune complement activation. ACS Appl. Mater. Interfaces 2009, 1, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 2001806. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cao, Y.; Yao, Q.; Chai, O.J.H.; Xie, J. Engineering Noble Metal Nanomaterials for Pollutant Decomposition. Ind. Eng. Chem. Res. 2020, 59, 20561–20581. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).