Anisotropies in Elasticity, Sound Velocity, and Minimum Thermal Conductivity of Low Borides VxBy Compounds
Abstract
:1. Introduction
2. Method and Computation Details
3. Results and Discussion
3.1. Equilibrium Structure and Stability
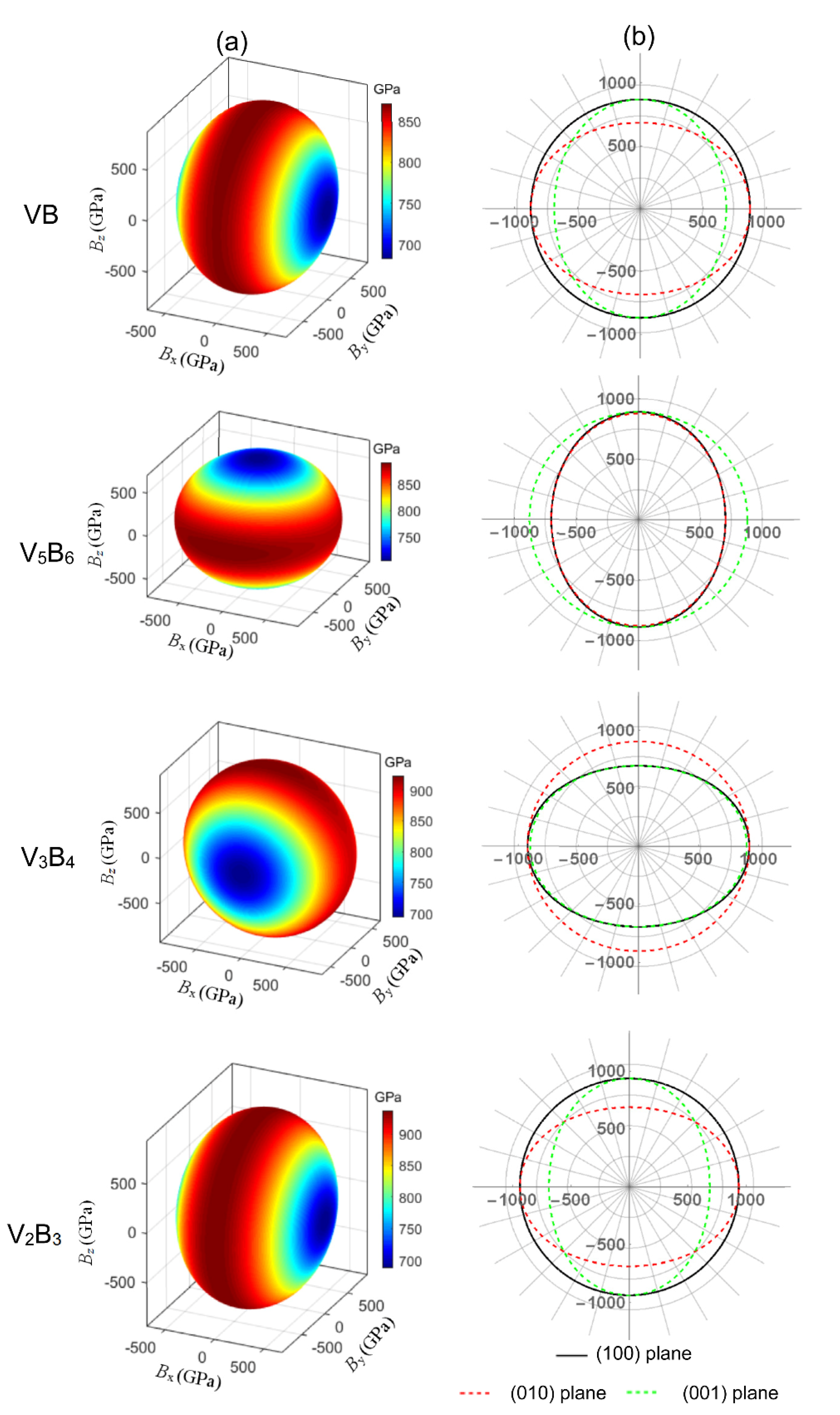
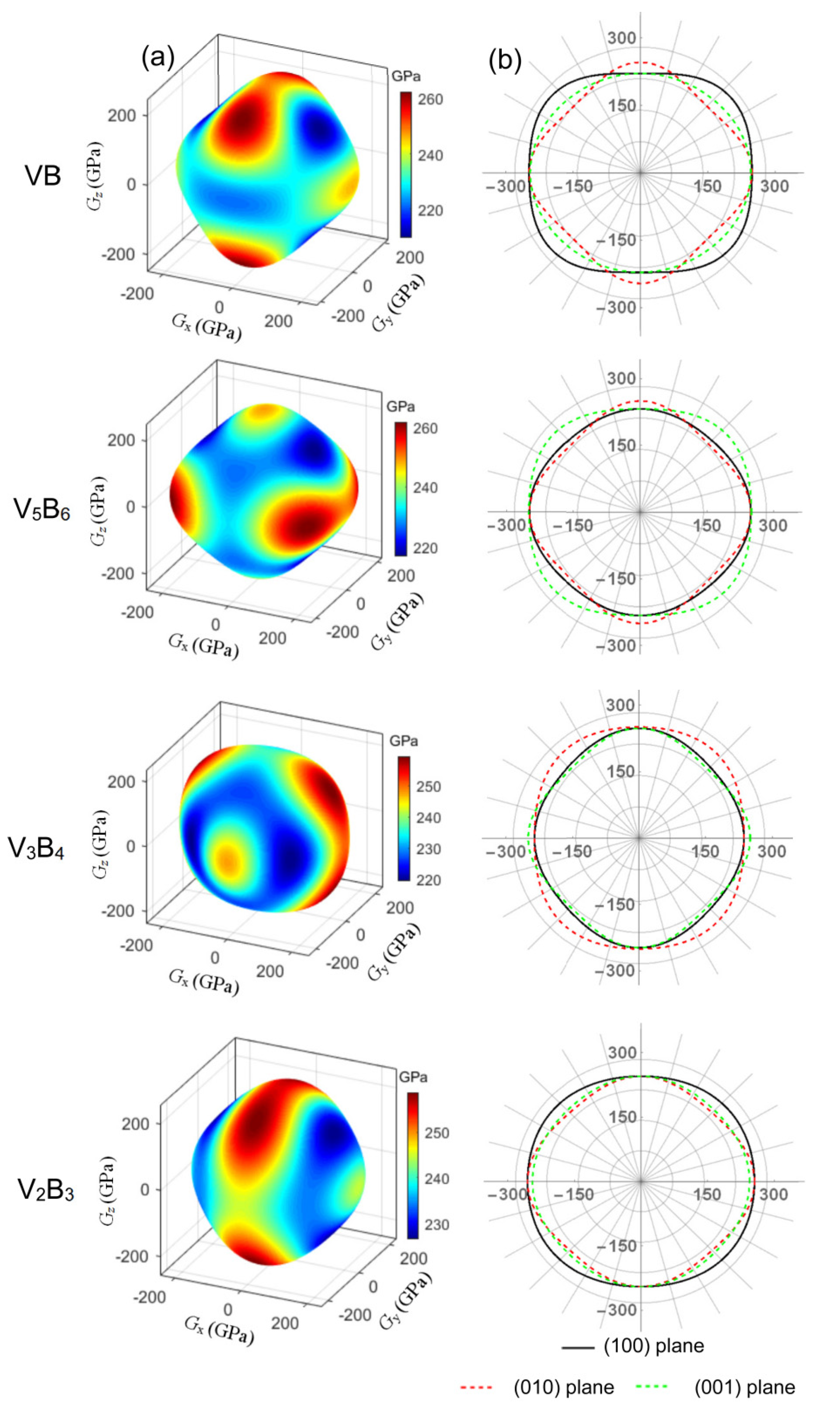
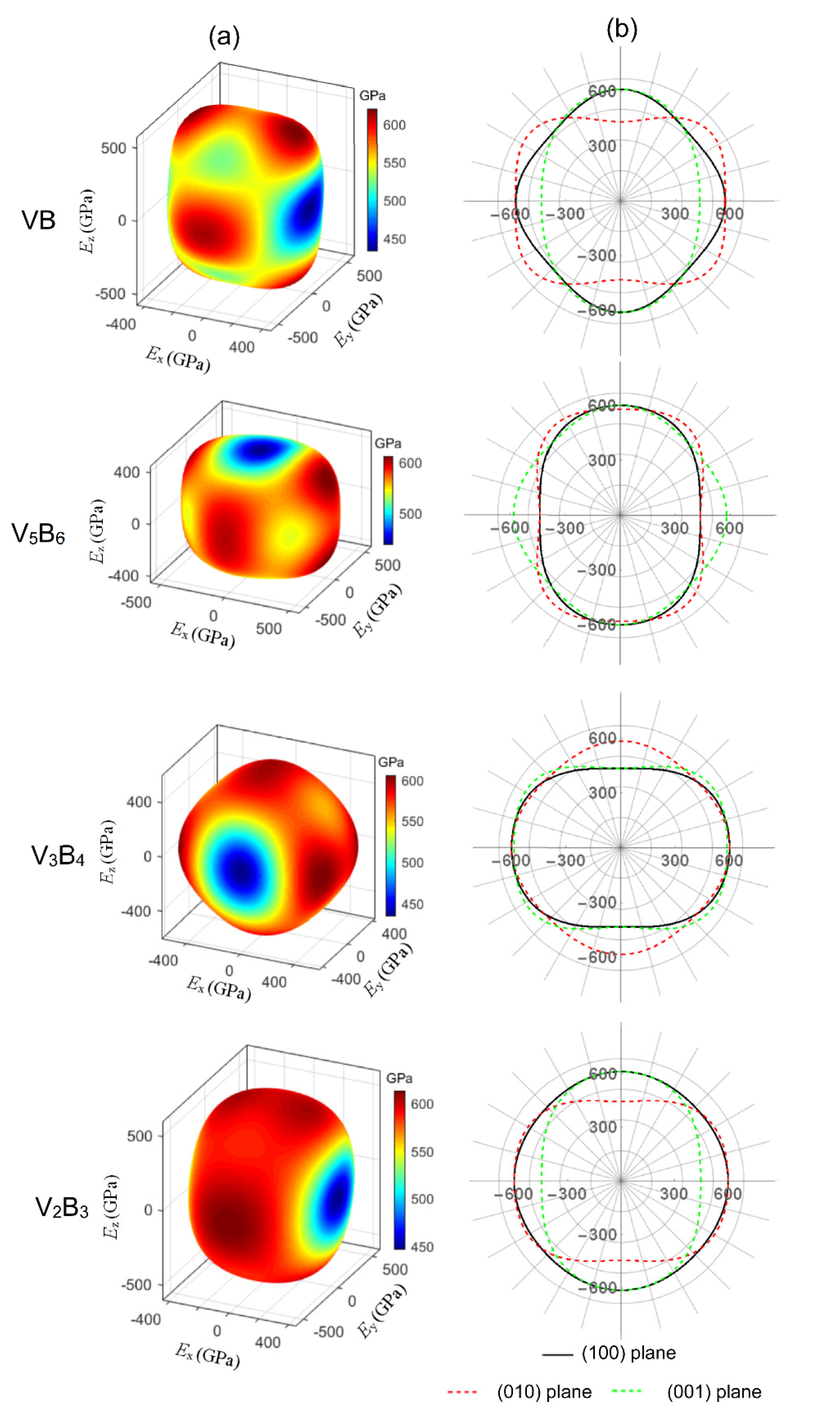
3.2. Elastic Properties
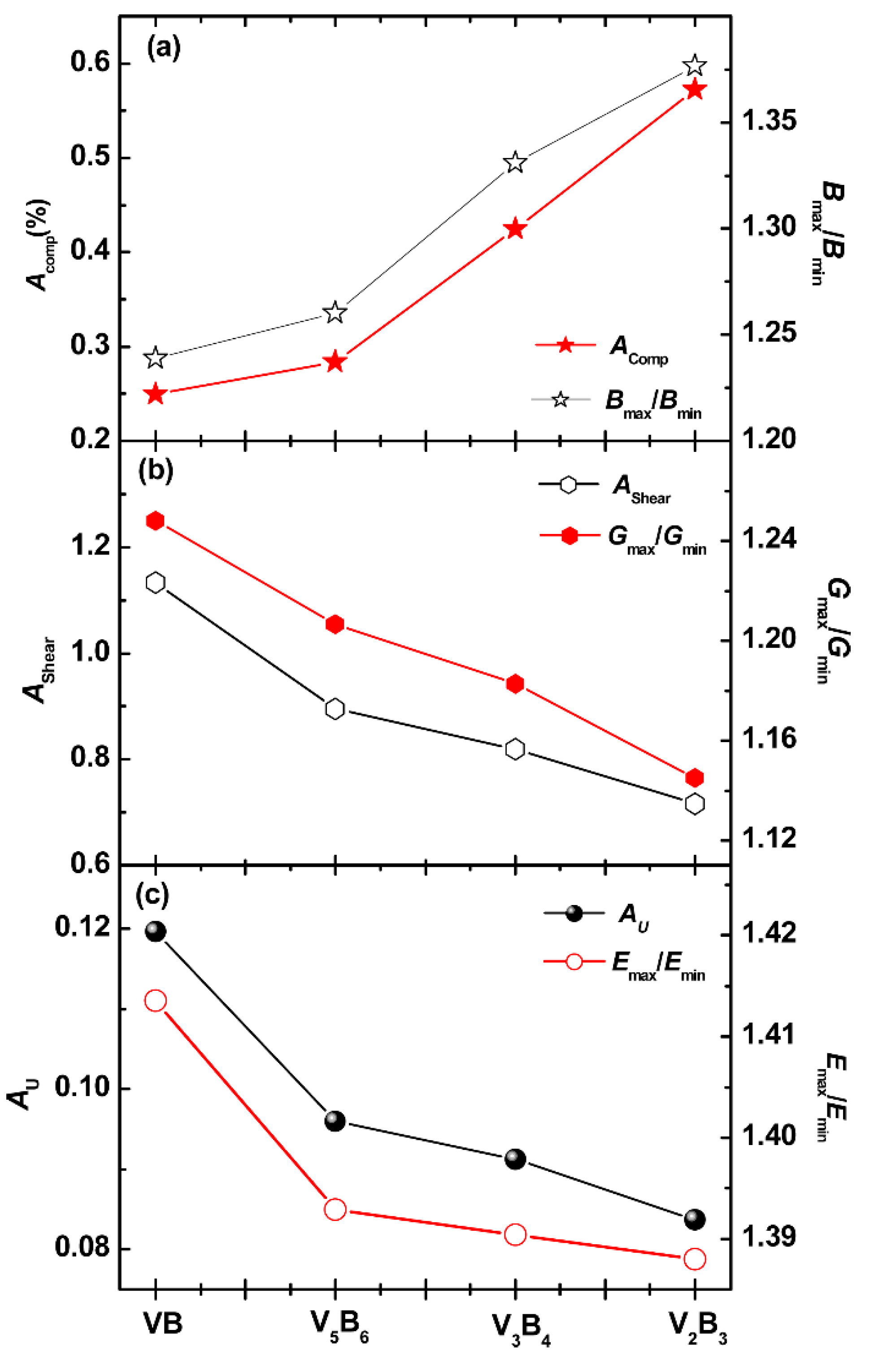
3.3. Elastic Anisotropy
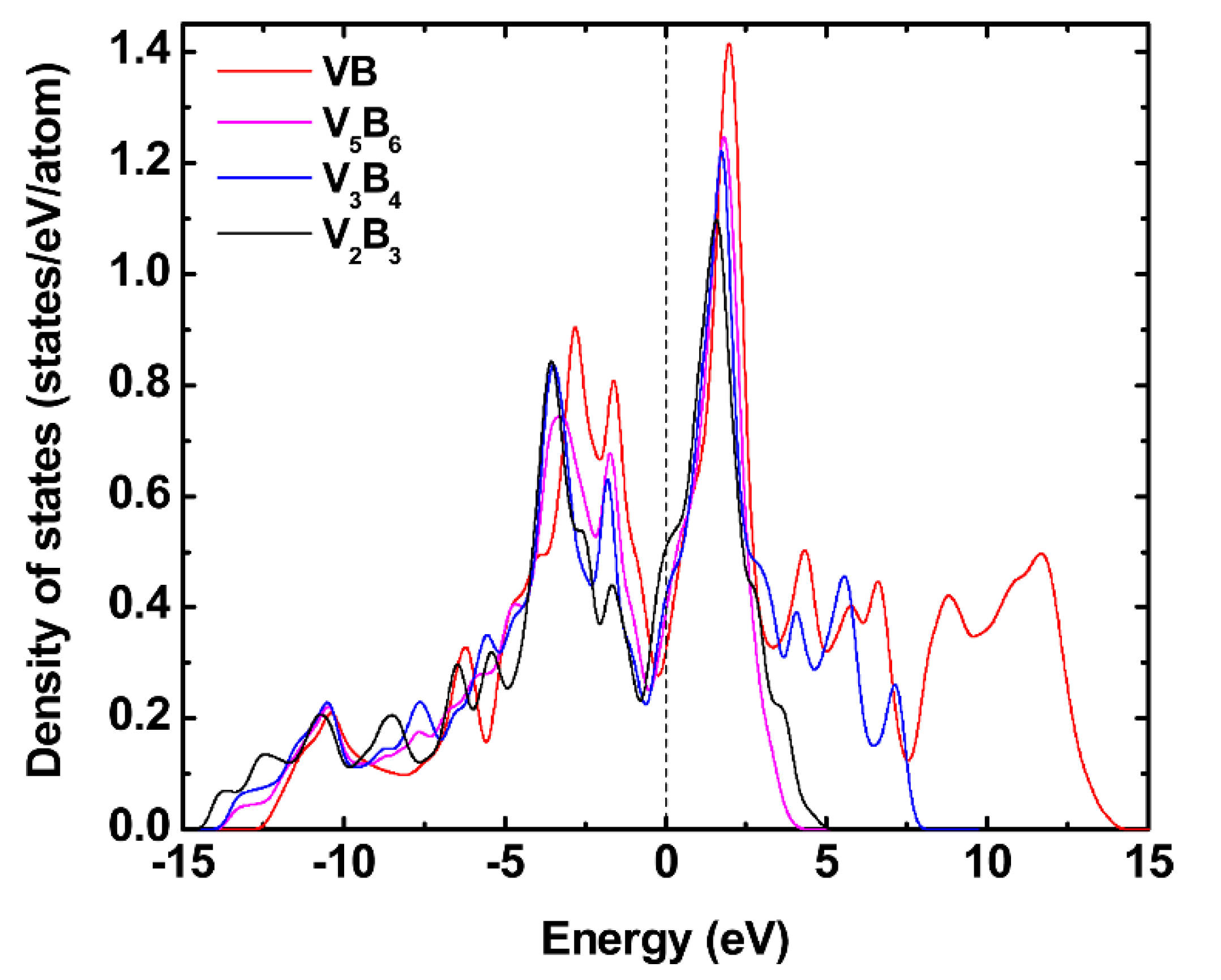
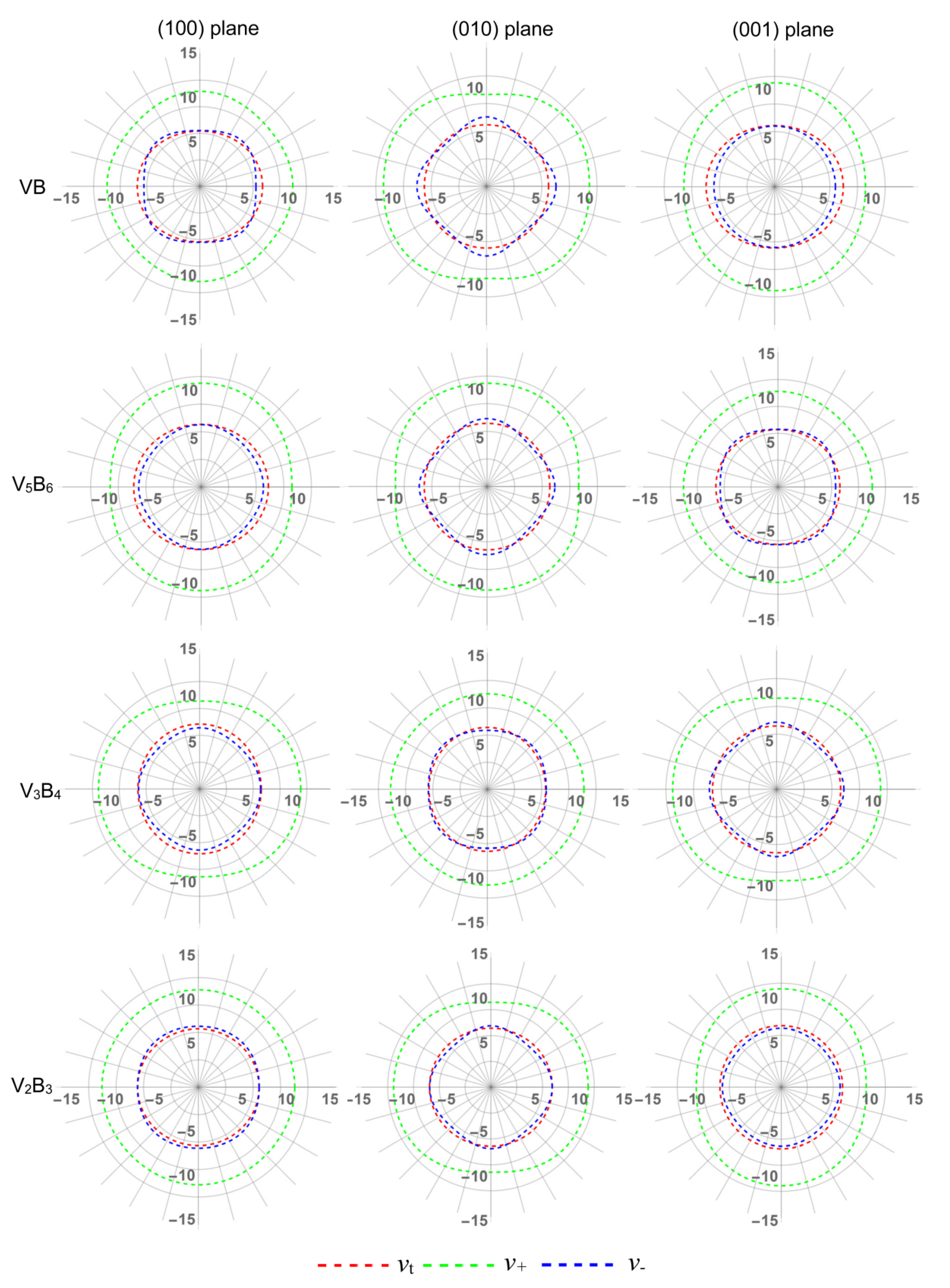
3.4. Anisotropy in Sound Velocity
3.5. Anisotropy in Minimum Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaner, R.B.; Gilma, J.J.; Tolbert, S.H. Designing superhard materials. Science 2005, 308, 1268–1269. [Google Scholar] [CrossRef] [Green Version]
- Burrage, K.C.; Lin, C.M.; Chen, W.C.; Chen, C.C.; Vohra, Y.K. Experimental and computational studies on superhard material rhenium diboride under ultrahigh pressures. Materials 2020, 13, 1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joha, P.; Polwart, N.; Troupe, C.E.; Wilson, J.I.B. The oxidation of (100) textured diamond, Diamond. Relat. Mater. 2002, 11, 861. [Google Scholar]
- Dub, S.; Lytvyn, P.; Strelchuk, V.; Nikolenko, A.; Stubrov, Y.; Petrusha, I.; Taniguchi, T.; Ivakhnenko, S. Vickers hardness of diamond and CBN single crystals: AFM approach. Crystals 2017, 7, 369. [Google Scholar] [CrossRef] [Green Version]
- Lonsdale, K. Further comments on attempts by H. Moissan, J.B. Hannay and Sir Charles Parsons to make diamonds in the laboratory. Nature 1962, 196, 104–106. [Google Scholar] [CrossRef]
- Mohammadi, R.; Xie, M.; Lech, A.T.; Turner, C.L.; Kavner, A.; Tolbert, S.H.; Kaner, R.B. Toward inexpensive superhard materials, Tungsten tetraboride-based solid solutions. J. Am. Chem. Soc. 2012, 134, 20660–20668. [Google Scholar] [CrossRef]
- Chung, H.Y.; Weinberger, M.B.; Levine, J.B.; Kavner, A.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 2007, 316, 436–439. [Google Scholar] [CrossRef]
- Akopov, G.; Pangilinan, L.E.; Mohammadi, R.; Kaner, R.B. Perspective: Superhard metal borides: A look forward. APL Mater. 2018, 6, 070901. [Google Scholar] [CrossRef]
- Akopov, G.; Yeung, M.T.; Kaner, R.B. Rediscovering the Crystal Chemistry of Borides. J. Adv. Mater. 2017, 29, 1604506. [Google Scholar] [CrossRef]
- Yeung, M.T.; Mohammadi, R.; Kaner, R.B. Ultraincompressible, superhard materials. Annu. Rev. Mater. Res. 2016, 46, 465–485. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, D.; Zheng, W.; Ma, Y.; Chen, C. Anomalous Stress Response of Ultrahard WBn Compounds. Phys. Rev. Lett. 2015, 115, 185502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, M.T.; Lei, J.; Mohammadi, R.; Turner, C.L.; Wang, Y.; Tolbert, S.H.; Kaner, R.B. Superhard Monoborides: Hardness Enhancement through Alloying in W1−xTaxB. Adv. Mater. 2016, 28, 6993–6998. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Gao, Z.; Qin, P.; Gao, L.; Tang, C. The mechanism of anomalous hardening in transition-metal monoborides. Nanoscale 2017, 9, 9112–9118. [Google Scholar] [CrossRef]
- Liang, Y.; Qin, P.; Jiang, H.; Zhang, L.; Zhang, J.; Tang, C. Designing superhard metals: The case of low borides. AIP Adv. 2018, 8, 045305. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.L.; Li, D.; Lv, Y.Z.; Liu, Z.; Tian, F.B.; Duan, D.F.; Liu, B.B.; Cui, T. Strong covalent boron bonding induced extreme hardness of VB3. J. Alloys Compd. 2016, 688, 1101–1107. [Google Scholar] [CrossRef]
- Wu, L.; Wan, B.; Zhao, Y.; Zhang, Y.; Liu, H.; Wang, Y.; Zhang, J.; Gou, H. Unraveling stable vanadium tetraboride and triboride by first-principles computations. J. Phys. Chem. C. 2015, 119, 21649–21657. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, Y.H.; Guo, J.M.; Wen, M. Correlation between hardness and bond orientation of vanadium borides. RSC Adv. 2014, 4, 47377. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerho, M.F. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Fan, C.Z.; Zeng, S.Y.; Li, L.X.; Zhan, Z.J.; Liu, R.P.; Wang, W.K.; Zhang, P.; Yao, Y.G. Potential superhard osmium dinitride with fluorite and pyrite structure: First-principles calculations. Phys. Rev. B 2006, 74, 125118. [Google Scholar] [CrossRef] [Green Version]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Chen, X.Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275. [Google Scholar] [CrossRef] [Green Version]
- Du, J.L.; Wen, B.; Melnik, R.; Kawazoe, Y. First-principles studies on structural, mechanical, thermodynamic and electronic properties of NieZr intermetallic compounds. Intermetallics 2014, 54, 110–119. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Gregoryanz, E. Synthesis of superhard materials. Mater. Today 2005, 8, 44–51. [Google Scholar] [CrossRef]
- Kong, Z.; Peng, M.; Sun, Y.; Qu, D.; Bao, L. Theoretical predictions of elastic anisotropies and thermal properties of TMRe2 (TM = Sc, Y, Zr and Hf). Phys. B Condens. Matter 2019, 571, 222. [Google Scholar] [CrossRef]
- Qu, D.; Bao, L.; Kong, Z.; Duan, Y. Anisotropy of elastic and thermal properties of TMOs2(TM = Sc, Y, Ti, Zr and Hf) from first-principles explorations. Mater. Res. Express 2019, 6, 116569. [Google Scholar] [CrossRef]
- Zhang, M.G.; Yan, H.Y. Elastic anisotropy and thermodynamic properties of iron tetraboride under high pressure and high temperature. Solid State Commun. 2014, 187, 53–58. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal elastic anisotropy index. Phys. Rev. Lett. 2008, 101, 055504–055507. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.H.; Buessem, W.R. The elastic anisotropy of crystals. J. Appl. Phys. 1967, 38, 2010–2012. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Liu, D.; Shi, Y.D.; Sun, Z.Y.; Wu, L.; Gao, Y.Q. Elastic anisotropy and physical properties of semi-transition-metal borides: First- principles calculation. Appl. Phys. Express 2020, 13, 015501. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Zhou, R.; Pan, W. Anisotropy in elasticity and thermal conductivity of monazite-type REPO4 (RE = La, Ce, Nd, Sm, Eu and Gd) from first-principles calculations. Acta Mater. 2013, 61, 7364–7383. [Google Scholar] [CrossRef]
- Lau, K.; McCurdy, A.K. Elastic anisotropy factor fot orthorhomic, tetragonal, and hexagonal crystals. Phys. Rev. B 1998, 58, 8980. [Google Scholar] [CrossRef]
- Cahill, D.G.; Watson, S.K.; Pohl, R.O. Lower limit to the thermal conductivity of disordered crystals. Phys. Rev. B 1992, 46, 6131. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, J.; Li, J.; Hu, Z.; Wang, J. Theoretical study on crystal structures, elastic stiffness, and intrinsic thermal conductivities of β-, γ-, and δ-Y2Si2O7. J. Mater. Res. 2015, 30, 493. [Google Scholar] [CrossRef]

| - | VB | V5B6 | V3B4 | V2B3 | |
|---|---|---|---|---|---|
| a | present | 3.047 | 2.972 | 2.975 | 3.039 |
| Ref. [13] | 3.050 | 2.976 | 2.980 | 3.039 | |
| Exp. [21] | 3.100 | 3.058 | - | 3.060 | |
| b | present | 8.035 | 21.213 | 3.041 | 18.392 |
| Ref. [13] | 8.036 | 21.220 | 3.042 | 18.408 | |
| Exp. [22] | 8.170 | 21.250 | - | 18.429 | |
| c | present | 2.966 | 3.046 | 13.199 | 2.977 |
| Ref. [13] | 2.969 | 3.049 | 13.208 | 2.982 | |
| Exp. [23] | 2.980 | 2.974 | - | 2.984 | |
| Ecoh | present | −8.517 | −8.414 | −8.354 | −8.280 |
| Eeff | present | −0.851 | −0.833 | −0.822 | −0.801 |
| - | VB | V5B6 | V3B4 | V2B3 |
|---|---|---|---|---|
| B | 266.5 | 272.8 | 276 | 279.2 |
| G | 235.5 | 237.7 | 237.9 | 242.4 |
| E | 545.7 | 552.5 | 554.4 | 564 |
| HV | 39.3 | 38.8 | 38.3 | 39.1 |
| - | VB | V5B6 | V3B4 | V2B3 | |
|---|---|---|---|---|---|
| vt | (100) plane | 1.129 | 1.075 | 1.050 | 1.041 |
| (010) plane | 1.009 | 1.005 | 1.061 | 1.044 | |
| (001) plane | 1.119 | 1.081 | 1.011 | 1.003 | |
| v+ | (100) plane | 1.057 | 1.142 | 1.151 | 1.019 |
| (010) plane | 1.143 | 1.139 | 1.031 | 1.147 | |
| (001) plane | 1.146 | 1.041 | 1.144 | 1.158 | |
| v- | (100) plane | 1.110 | 1.043 | 1.058 | 1.032 |
| (010) plane | 1.179 | 1.145 | 1.063 | 1.098 | |
| (001) plane | 1.004 | 1.084 | 1.130 | 1.043 | |
| - | VB | V5B6 | V3B4 | V2B3 | |
|---|---|---|---|---|---|
| κmin | (100) plane | 1.027 | 1.037 | 1.048 | 1.007 |
| (010) plane | 1.053 | 1.054 | 1.014 | 1.073 | |
| (001) plane | 1.027 | 1.019 | 1.060 | 1.066 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhang, Y.; Zhao, Y.; Ma, Y. Anisotropies in Elasticity, Sound Velocity, and Minimum Thermal Conductivity of Low Borides VxBy Compounds. Metals 2021, 11, 577. https://doi.org/10.3390/met11040577
Yu J, Zhang Y, Zhao Y, Ma Y. Anisotropies in Elasticity, Sound Velocity, and Minimum Thermal Conductivity of Low Borides VxBy Compounds. Metals. 2021; 11(4):577. https://doi.org/10.3390/met11040577
Chicago/Turabian StyleYu, Jing, Yongmei Zhang, Yuhong Zhao, and Yue Ma. 2021. "Anisotropies in Elasticity, Sound Velocity, and Minimum Thermal Conductivity of Low Borides VxBy Compounds" Metals 11, no. 4: 577. https://doi.org/10.3390/met11040577







