Selective Laser Melting of 316L Stainless Steel: Influence of Co-Cr-Mo-W Addition on Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Optimization of Process Parameters
3.2. Mechanical Properties and Phase Characteristic
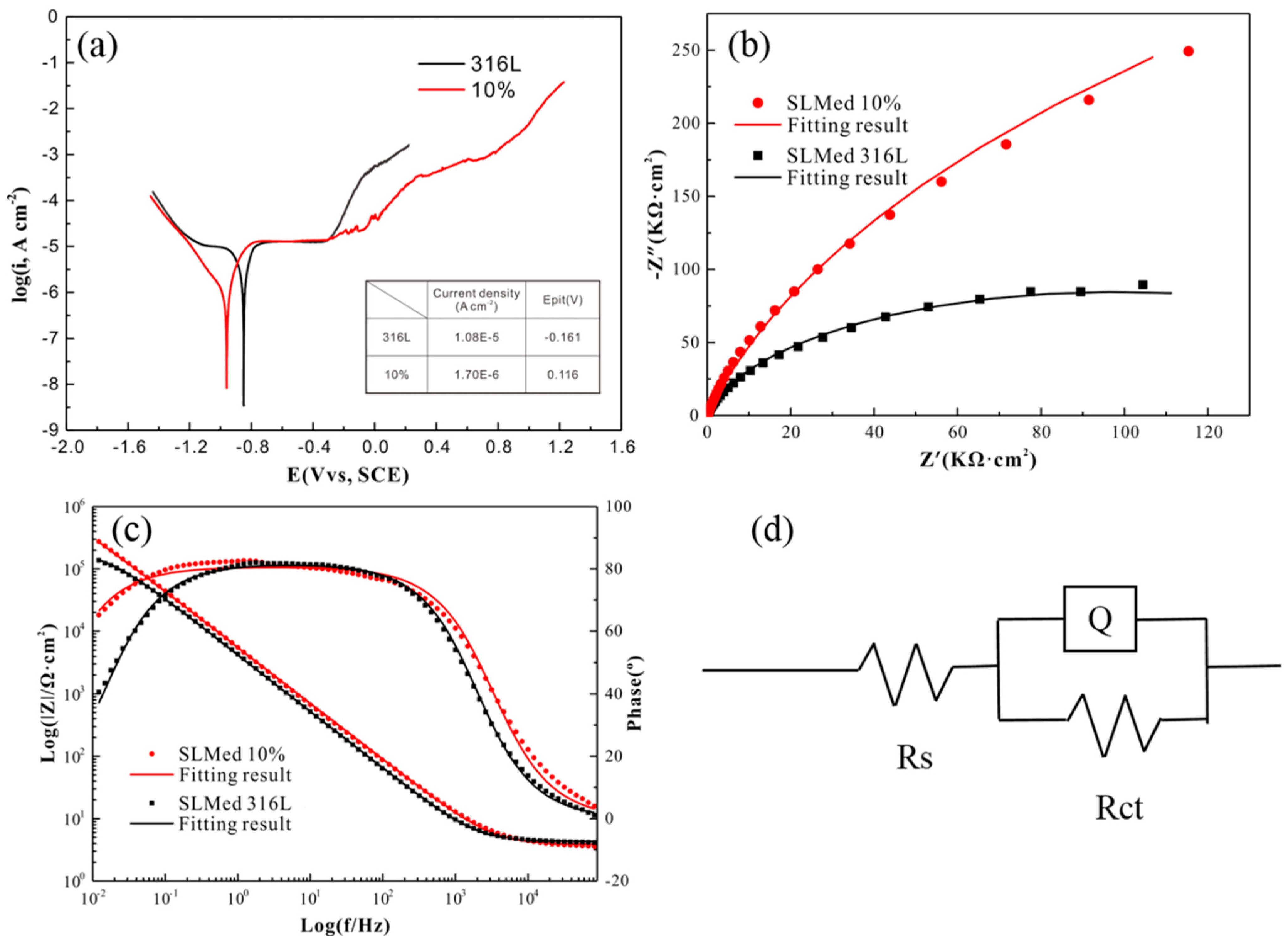
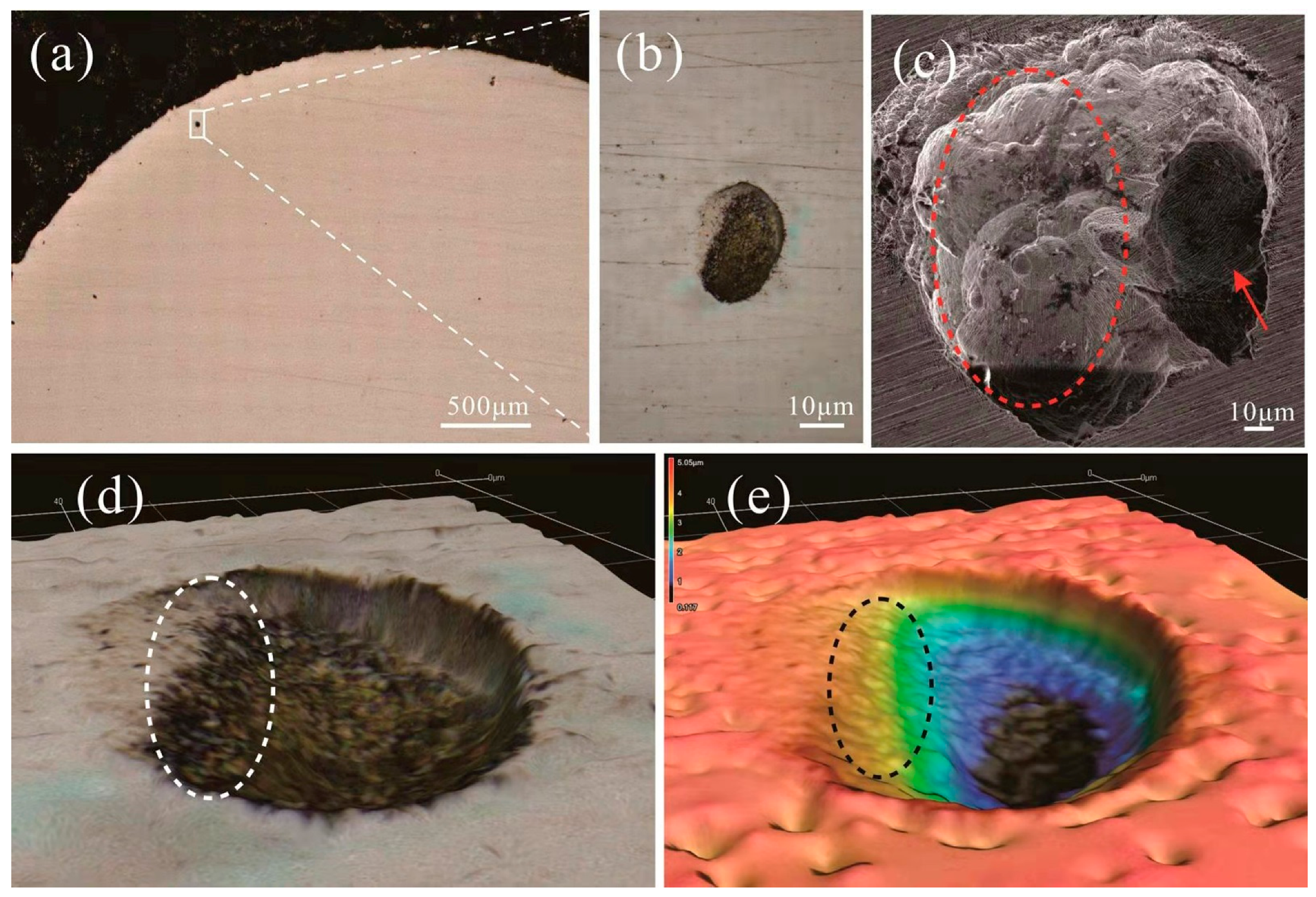
3.3. Corrosion Behavior of SLM-Processed Alloy
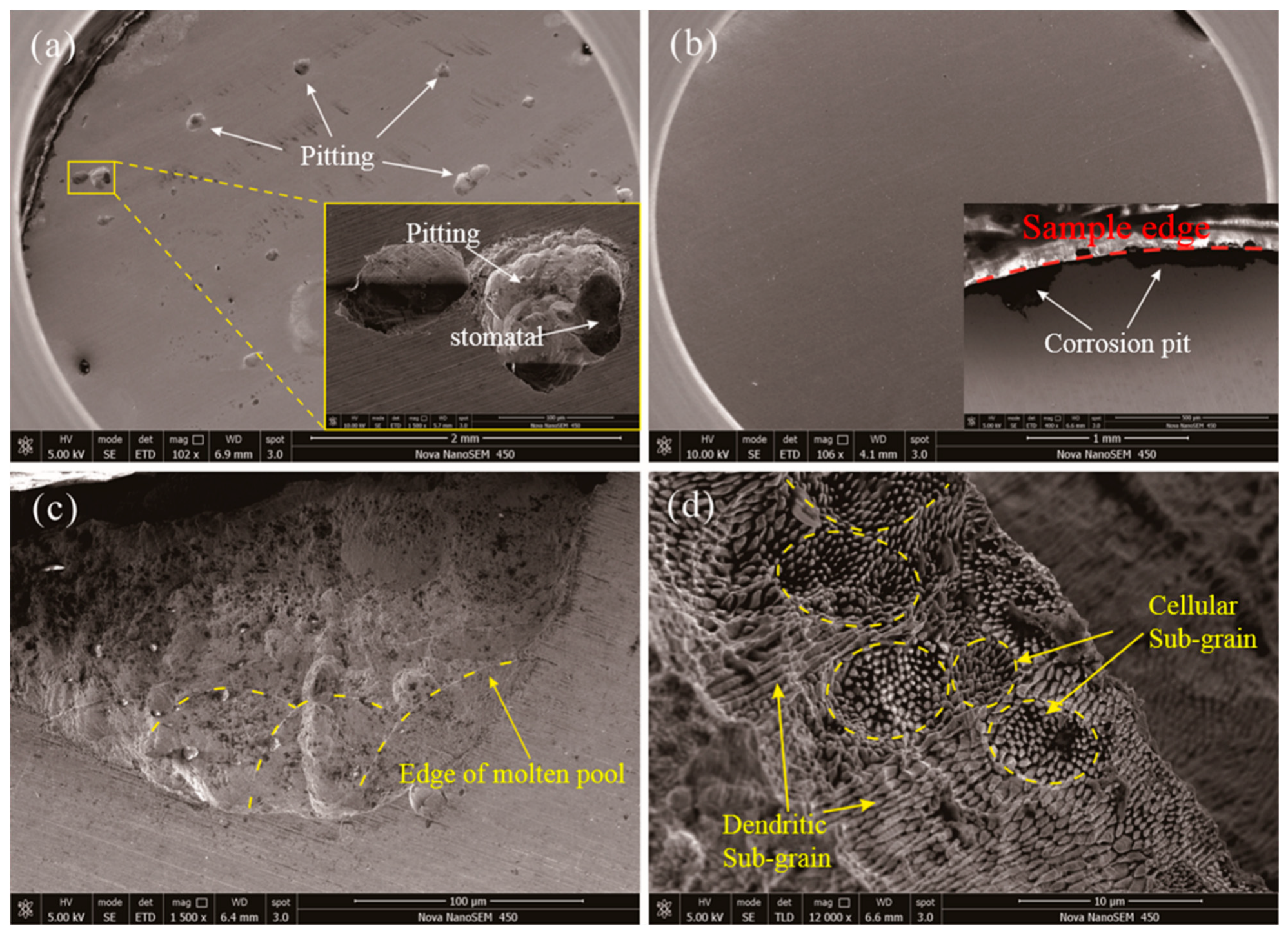
3.4. Microstructural Characterization of SLM-Processed Alloy
4. Conclusions
- (1)
- 316L SS and 316L + 10Co-Cr-Mo-W samples are fabricated using the SLM process. The optimization parameters of 316L SS and 316L + 10Co-Cr-Mo-W are 200 W, 170 W of laser power and 800 mm/s, 680 mm/s of scanning speed, respectively. Both relative densities reach to more than 99%.
- (2)
- Corrosion resistance of 316L is enhanced without loss of the strength. Although no new phase can be found in the matrix, the lattice distortion is caused by the dissolution of Co, Cr, Mo and W elements into the austenite grains. The pitting corrosion is related to the stability of the passive film formed on the bottom of the gas pores. The increasing corrosion resistance of 316L stainless steel is attributed to the addition of Cr and Mo element in the doped powder which contributes to the formation of the passivated film. The existence of Mo can increase the thickness of the passive film, as well as the content of Cr2O3 in the passive film. The higher density of dislocation in the 316L + 10Co-Cr-Mo-W should also be a reason that results in the formation of a thicker passive film on the 316L + 10Co-Cr-Mo-W.
- (3)
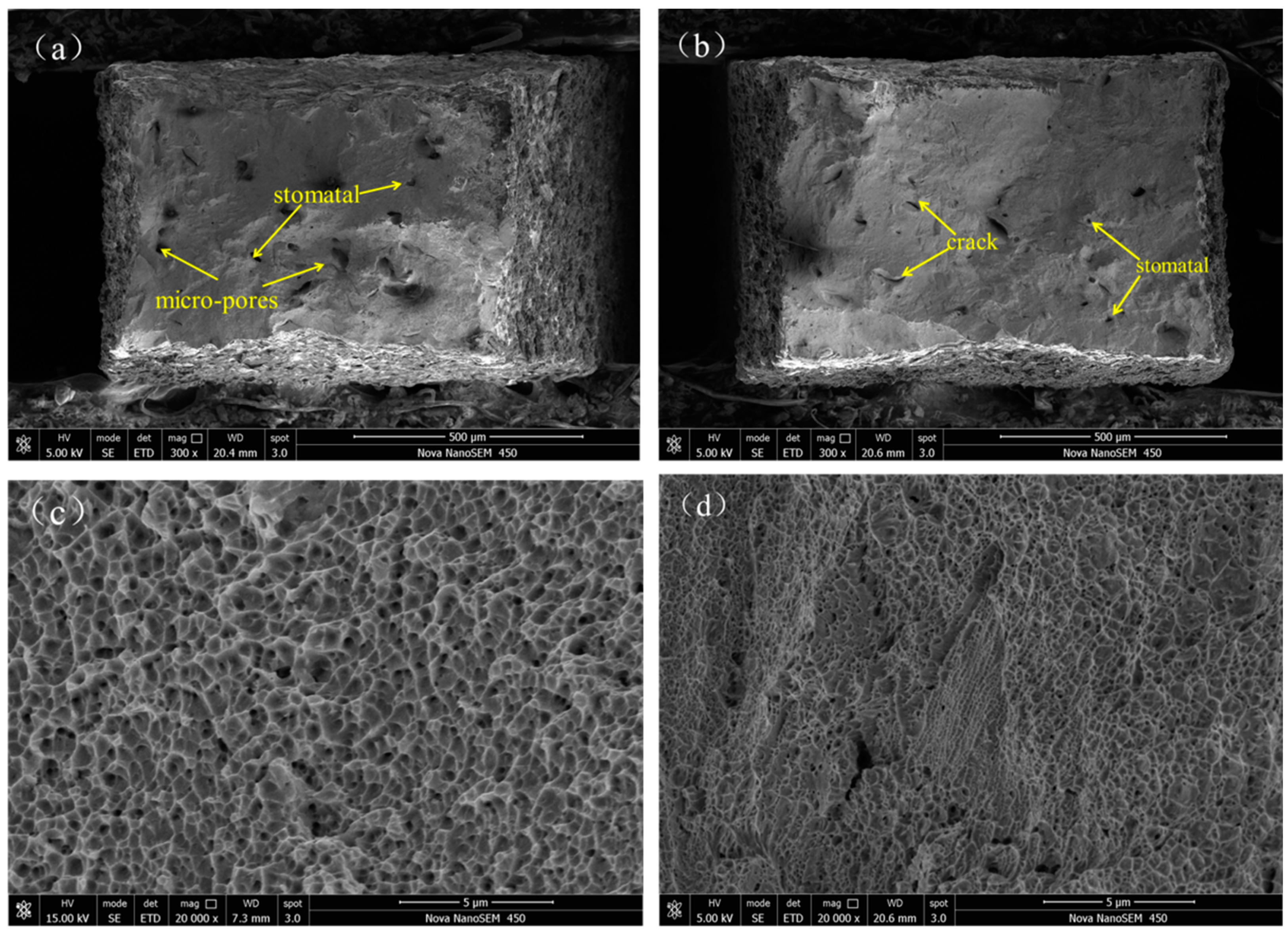
- The SEM analysis of corrosion surface of 316L and 316L + 10Co-Cr-Mo-W demonstrate that most of the pitting occurs preferring at the sub-grain boundary. More grain boundaries of cellular sub-grains are exposed in the solution than that of dendritic sub-grains, a larger amount of corrosion takes place and thus the cellular sub-grains become sparse and dark-coloured.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Qin, W.; Peng, P.; Chen, M.; Mao, Q.; Yue, W.; Kang, J.; Meng, D.; She, D.; Zhu, X.; et al. Effects of geometric dimension and grain size on impact properties of 316L stainless steel. Mater. Lett. 2021, 284, 128908. [Google Scholar] [CrossRef]
- Huang, S.H.; Liu, P.; Mokasdar, A.; Hou, L. Additive manufacturing and its societal impact: A literature review. Int. J. Adv. Manuf. Technol. 2013, 67, 1191–1203. [Google Scholar] [CrossRef]
- Calleja, A.; Tabernero, I.; Ealo, J.A.; Campa, F.J.; Lamikiz, A.; De Lacalle, L.N.L. Feed rate calculation algorithm for the homogeneous material deposition of blisk blades by 5-axis laser cladding. Int. J. Adv. Manuf. Technol. 2014, 74, 1219–1228. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Q.; Guo, K.; Wang, X.; Liu, J.; Sun, J. Effect of scanning strategies on the microstructure and mechanical behavior of 316L stainless steel fabricated by selective laser melting. Mater. Sci. Eng. A 2020, 793, 139879. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Lei, X.; Zhang, L.; Man, C.; Yao, J.; Cheng, X.; Li, X. Bio-functional and anti-corrosive 3D printing 316L stainless steel fabricated by selective laser melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Bains, P.S.; Sidhu, S.S.; Bahraminasab, M.; Prakash, C. Biomaterials in Orthopaedics and Bone Regeneration: Design and Synthesis; Springer: Singapore, 2019. [Google Scholar]
- Silva, M.; Felismina, R.; Mateus, A.; Parreira, P.; Malça, C. Application of a hybrid additive manufacturing methodology to produce a metal/polymer customized dental implant. Procedia Manuf. 2017, 12, 150–155. [Google Scholar] [CrossRef]
- Koutsoukis, T.; Zinelis, S.; Eliades, G.; Al-Wazzan, K.; Al Rifaiy, M.; Al Jabbari, Y.S. Selective laser melting technique of co-Cr dental alloys: A review of structure and properties and comparative analysis with other available techniques. J. Prosthodont. 2015, 24, 303–312. [Google Scholar] [CrossRef]
- Shih, C.-C.; Su, Y.-Y.; Su, L.H.J.; Chang, M.-S.; Lin, S.-J. Effect of surface oxide properties on corrosion resistance of 316L stainless steel for biomedical applications. Corros. Sci. 2004, 46, 427–441. [Google Scholar] [CrossRef]
- Gnanavel, S.; Ponnusamy, S.; Loganathan, M.; Muthamizhchelvan, C. In vitro corrosion behaviour of Ti–6Al–4V and 316L stainless steel alloys for biomedical implant applications. J. Bio-Tribo-Corros. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chater, R.J.; Hutton, B.M.; McPhail, D.S. Why stainless steel corrodes. Nat. Cell Biol. 2002, 415, 770–774. [Google Scholar] [CrossRef]
- Sailer, I.; Philipp, A.; Zembic, A.; Pjetursson, B.E.; Hämmerle, C.H.F.; Zwahlen, M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin. Oral Implant. Res. 2009, 20, 4–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laleh, M.; Hughes, A.E.; Xu, W.; Gibson, I.; Tan, M.Y. Unexpected erosion-corrosion behaviour of 316L stainless steel produced by selective laser melting. Corros. Sci. 2019, 155, 67–74. [Google Scholar] [CrossRef]
- Trelewicz, J.R.; Halada, G.P.; Donaldson, O.K.; Manogharan, G. Microstructure and corrosion resistance of laser additively manufactured 316L stainless steel. JOM 2016, 68, 850–859. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Y.; Jing, H.; Lin, D.; Zhao, L.; Xu, L.; Xin, P. Selective laser melting of low-content graphene nanoplatelets reinforced 316L austenitic stainless steel matrix: Strength enhancement without affecting ductility. Addit. Manuf. 2020, 34, 101381. [Google Scholar] [CrossRef]
- Xiang, N.; Xin, X.-Z.; Chen, J.; Wei, B. Metal–ceramic bond strength of Co–Cr alloy fabricated by selective laser melting. J. Dent. 2012, 40, 453–457. [Google Scholar] [CrossRef]
- Wei, W.; Zhou, Y.; Liu, W.; Li, N.; Yan, J.; Li, H. Microstructural Characterization, Mechanical Properties, and Corrosion Resistance of Dental Co-Cr-Mo-W Alloys Manufactured by Selective Laser Melting. J. Mater. Eng. Perform. 2018, 27, 5312–5320. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, D.; Liu, X.; Zhang, D.; Qu, S.; Ma, J.; London, G.; Shen, Z.; Liu, W. 3D-imaging of selective laser melting defects in a Co–Cr–Mo alloy by synchrotron radiation micro-CT. Acta Mater. 2015, 98, 1–16. [Google Scholar] [CrossRef]
- Wijesinghe, T.S.L.; Blackwood, D.J. Real time pit initiation studies on stainless steels: The effect of sulphide inclusions. Corros. Sci. 2007, 49, 1755–1764. [Google Scholar] [CrossRef]
- Hara, N.; Sugawara, Y.; Muto, I. The role of MnS inclusions and passive films in the initiation of pitting corrosion of stainless steels. ECS Meet. Abstr. 2012. [Google Scholar] [CrossRef]
- Duan, Z.; Man, C.; Dong, C.; Cui, Z.; Kong, D.; Wang, L.; Wang, X. Pitting behavior of SLM 316L stainless steel exposed to chloride environments with different aggressiveness: Pitting mechanism induced by gas pores. Corros. Sci. 2020, 167, 108520. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Liu, T.; Kong, D.; Wang, D.; Li, X. The enhancement of microstructure on the passive and pitting behaviors of selective laser melting 316L SS in simulated body fluid. Appl. Surf. Sci. 2019, 467-468, 193–205. [Google Scholar] [CrossRef]
- Schaller, R.F.; Mishra, A.; Rodelas, J.M.; Taylor, J.M.; Schindelholz, E.J. The Role of Microstructure and surface finish on the corrosion of selective laser melted 304L. J. Electrochem. Soc. 2018, 165, C234–C242. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Liang, J.; Xiao, K.; Yu, Q.; Li, X. Characterization of the passive film and corrosion of martensitic AM355 stainless steel. Anal. Lett. 2016, 50, 1091–1111. [Google Scholar] [CrossRef]
- Mischler, S.; Vogel, A.; Mathieu, H.J.; Landolt, D. ChemInform abstract: Chemical composition of the passive film on Fe-24Cr and Fe-24Cr-11Mo studied by AES, XPS, and SIMS. ChemInform 2010, 22. [Google Scholar] [CrossRef]
- Bhasker-Ranganath, S.; Wick, C.; Ramachandran, B. Computational insights into the molecular mechanisms for chromium passivation of stainless-steel surfaces. Mater. Today Chem. 2020, 17, 100298. [Google Scholar] [CrossRef]
- Lv, J.; Meng, Y.; Miura, H.; Liang, T. The effect of surface enriched chromium and grain refinement by ball milling on corrosion resistance of 316L stainless steel. Mater. Res. Bull. 2017, 91, 91–97. [Google Scholar] [CrossRef]
- Olsson, C.-O.; Landolt, D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, T.; Liu, J.; Zhang, X.; Shen, B.; Hu, W.; Liu, L. Effects of pack chromizing on the microstructure and anticorrosion properties of 316L stainless steel. Surf. Coat. Technol. 2019, 366, 86–96. [Google Scholar] [CrossRef]
- Nath, S.D.; Clinning, E.; Gupta, G.; Wuelfrath-Poirier, V.; L’Espérance, G.; Gulsoy, O.; Kearns, M.; Atre, S.V. Effects of Nb and Mo on the microstructure and properties of 420 stainless steel processed by laser-powder bed fusion. Addit. Manuf. 2019, 28, 682–691. [Google Scholar] [CrossRef]
- Saidi, D.; Zaid, B.; Souami, N.; Saoula, N.; Siad, M.; Ahmed, A.S.; Bibérian, J. AES depth profiles in Mo-coated 304L stainless steel achieved by RF-magnetron sputtering and influence of Mo on the corrosion in 3.5% NaCl solution. J. Alloys Compd. 2015, 645, 45–50. [Google Scholar] [CrossRef]
- Lv, J.; Liang, T.; Wang, C. Surface enriched molybdenum enhancing the corrosion resistance of 316L stainless steel. Mater. Lett. 2016, 171, 38–41. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Chen, D.; Liang, P. Molybdenum addition enhancing the corrosion behaviors of 316L stainless steel in the simulated cathodic environment of proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2015, 40, 5947–5957. [Google Scholar] [CrossRef]
- Tobler, W.; Virtanen, S. Effect of Mo species on metastable pitting of Fe18Cr alloys—A current transient analysis. Corros. Sci. 2006, 48, 1585–1607. [Google Scholar] [CrossRef]
- Kawakita, J.; Kuroda, S.; Fukushima, T.; Kodama, T. Improvement of corrosion resistance of high-velocity oxyfuel-sprayed stainless steel coatings by addition of molybdenum. J. Therm. Spray Technol. 2005, 14, 224–230. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Zhang, B.; Li, Y.; Wang, F. An investigation on the continuous and uniform thin membrane passive film formed on sputtered nanocrystalline stainless steel. Corros. Sci. 2016, 104, 71–83. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Cui, Z.; Xiao, K.; Yu, Q.; Li, X. Characterization of the outer layer nanostructure in the electrochemical response of stainless steel in aqueous sodium hydroxide. Anal. Lett. 2018, 51, 1384–1399. [Google Scholar] [CrossRef]
- Gaberšček, M.; Pejovnik, S. Impedance spectroscopy as a technique for studying the spontaneous passivation of metals in electrolytes. Electrochim. Acta 1996, 41, 1137–1142. [Google Scholar] [CrossRef]
- Sun, M.; Xiao, K.; Dong, C.; Li, X.; Zhong, P. Effect of stress on electrochemical characteristics of pre-cracked ultrahigh strength stainless steel in acid sodium sulphate solution. Corros. Sci. 2014, 89, 137–145. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Zhong, Y.; Shen, Z. Hardened austenite steel with columnar sub-grain structure formed by laser melting. Mater. Sci. Eng. A 2015, 625, 221–229. [Google Scholar] [CrossRef]
- Belfrouh, A.; Masson, C.; Vouagner, D.; De Becdelievre, A.; Prakash, N.; Audouard, J. The cumulative effect of alloying elements N, W, Mo and Cu on the corrosion behaviour of 17Cr-13Ni stainless steel in 2N H2SO4. Corros. Sci. 1996, 38, 1639–1648. [Google Scholar] [CrossRef]
- Tomashov, N.D.; Chernova, G.P.; Marcova, O.N. Effect of supplementary alloying elements on pitting corrosion susceptibility of 18Cr-14Ni stainless steel. Corrosion 1964, 20, 166t–173t. [Google Scholar] [CrossRef]
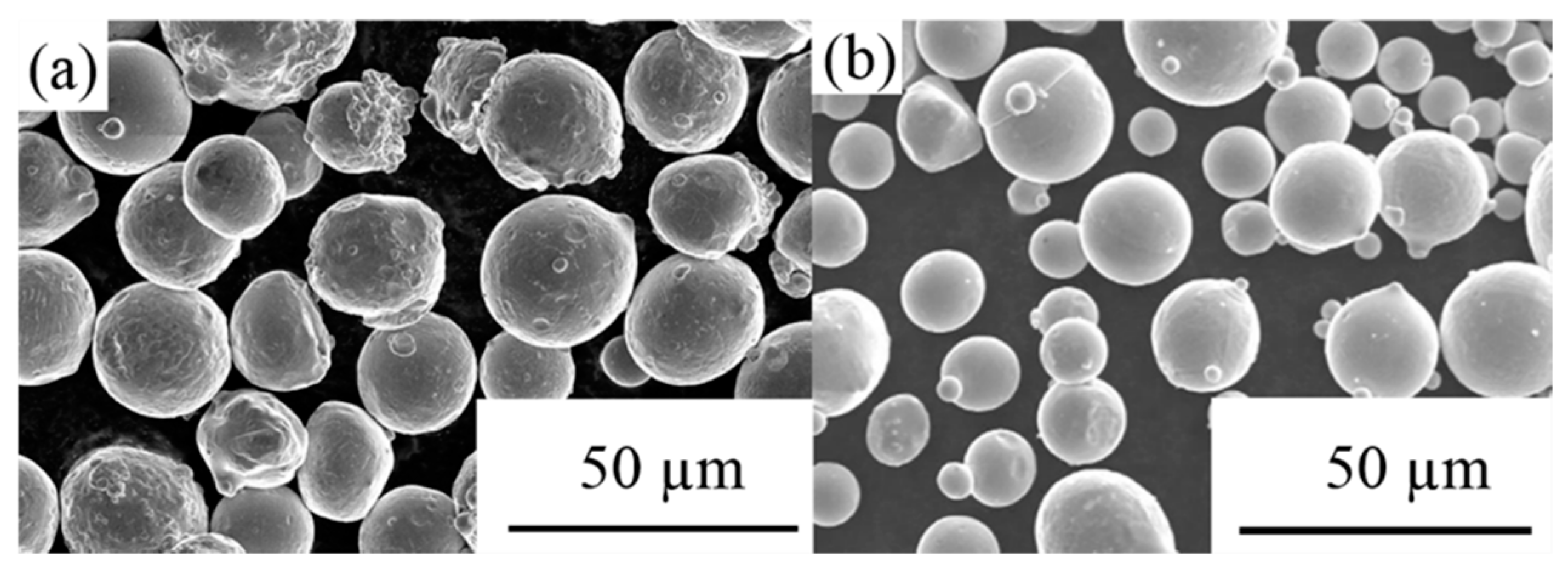
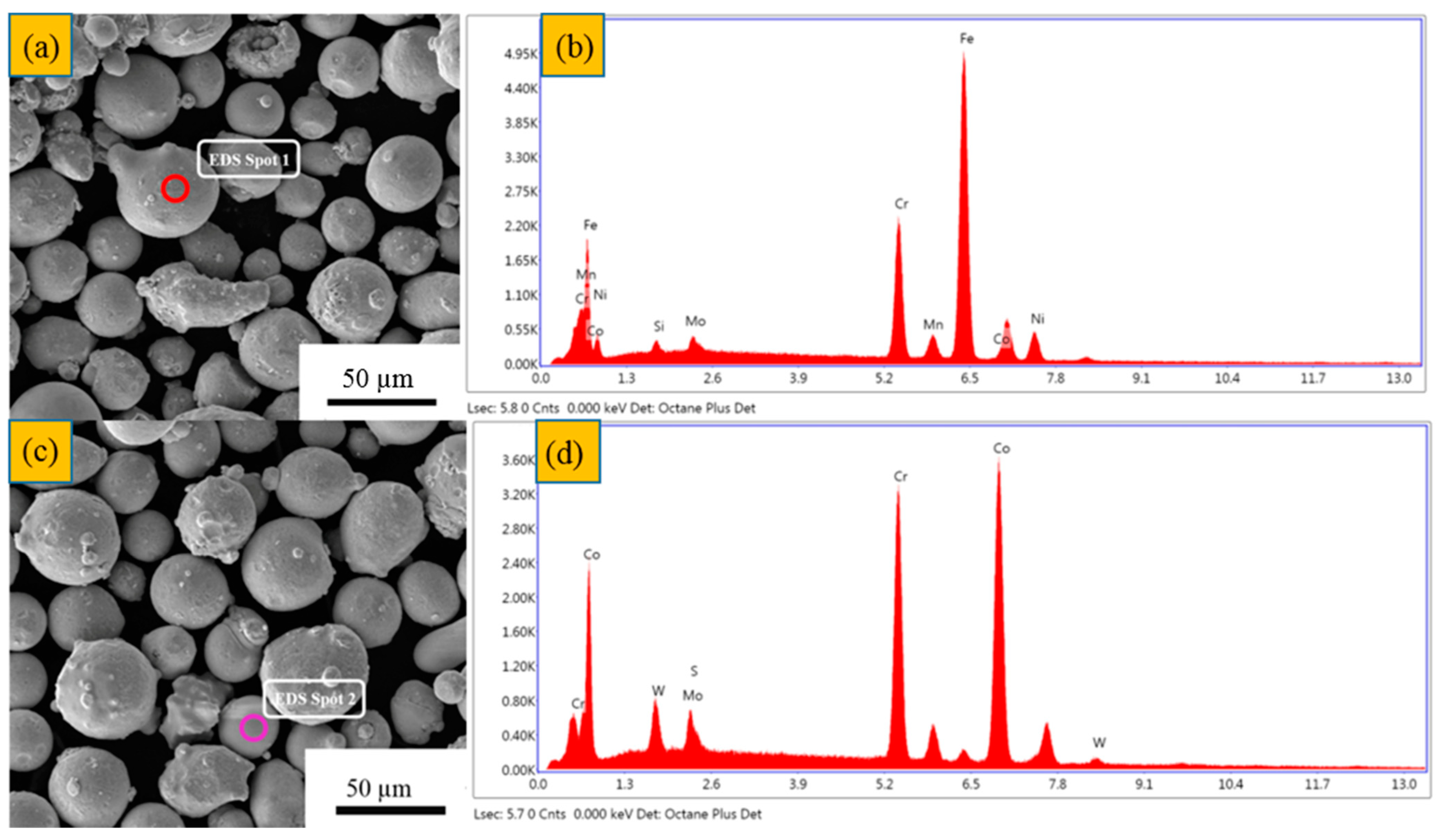

| Element (wt%) | Cr | Co | Mo | W | Mn | C | P | Ni | Fe | Si | S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 316L | 17.1 | - | 2.3 | - | 1.13 | 0.018 | 0.02 | 10.6 | Bal. | 0.63 | 0.009 |
| Co-Cr-Mo-W | 24.12 | Bal. | 4.95 | 5.45 | - | - | - | - | - | 0.95 | - |
| Laser Power (w) | Scanning Speed (mm/s) | Laser Energy Density (J/mm3) | Relative Density of 316L (%) | Relative Density of 316L + 10 Co-Cr-Mo-W (%) |
|---|---|---|---|---|
| 170 | 590 | 80 | 97.01 ± 1.16 | 97.20 ± 0.84 |
| 200 | 694 | 80 | 98.29 ± 0.88 | 96.55 ± 1.63 |
| 230 | 800 | 80 | 97.39 ± 0.93 | 98.30 ± 0.63 |
| 170 | 680 | 69 | 97.11 ± 1.10 | 99.15 ± 0.43 |
| 200 | 800 | 69 | 99.04 ± 0.69 | 97.85 ± 1.50 |
| 230 | 920 | 69 | 98.73 ± 0.50 | 97.49 ± 1.53 |
| 170 | 787 | 60 | 97.14 ± 0.89 | 97.40 ± 0.96 |
| 200 | 926 | 60 | 96.57 ± 1.32 | 97.69 ± 1.11 |
| 230 | 1065 | 60 | 98.22 ± 0.47 | 97.81 ± 1.00 |
| Sample | 2θ (°) | Intensity (cts) | FWHM (rad) | 2θ (°) | Intensity (cts) | FWHM (rad) | 2θ (°) | Intensity (cts) | FWHM (rad) |
|---|---|---|---|---|---|---|---|---|---|
| 316L SS | 43.660 | 3366 | 0.502 | 50.640 | 2120 | 0.494 | 74.601 | 4387 | 0.426 |
| 316L+10Co-Cr-Mo-W | 43.582 | 3019 | 0.439 | 50.679 | 1966 | 0.458 | 74.639 | 5333 | 0.381 |
| Sample | Rct (KΩ·cm2) | Rs (KΩ·cm2) | Q × 10−5, Y0 (S·Secn/cm2) | n |
|---|---|---|---|---|
| 316L SS | 205.6 | 4.279 | 4.524 | 0.906 |
| 316L + 10Co-Cr-Mo-W | 1077 | 3.836 | 3.489 | 0.896 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wang, T.; Li, P.; Wang, S.; Wang, L. Selective Laser Melting of 316L Stainless Steel: Influence of Co-Cr-Mo-W Addition on Corrosion Resistance. Metals 2021, 11, 597. https://doi.org/10.3390/met11040597
Li B, Wang T, Li P, Wang S, Wang L. Selective Laser Melting of 316L Stainless Steel: Influence of Co-Cr-Mo-W Addition on Corrosion Resistance. Metals. 2021; 11(4):597. https://doi.org/10.3390/met11040597
Chicago/Turabian StyleLi, Bolin, Tingting Wang, Peizhen Li, Shenghai Wang, and Li Wang. 2021. "Selective Laser Melting of 316L Stainless Steel: Influence of Co-Cr-Mo-W Addition on Corrosion Resistance" Metals 11, no. 4: 597. https://doi.org/10.3390/met11040597





