Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure
Abstract
:1. Introduction
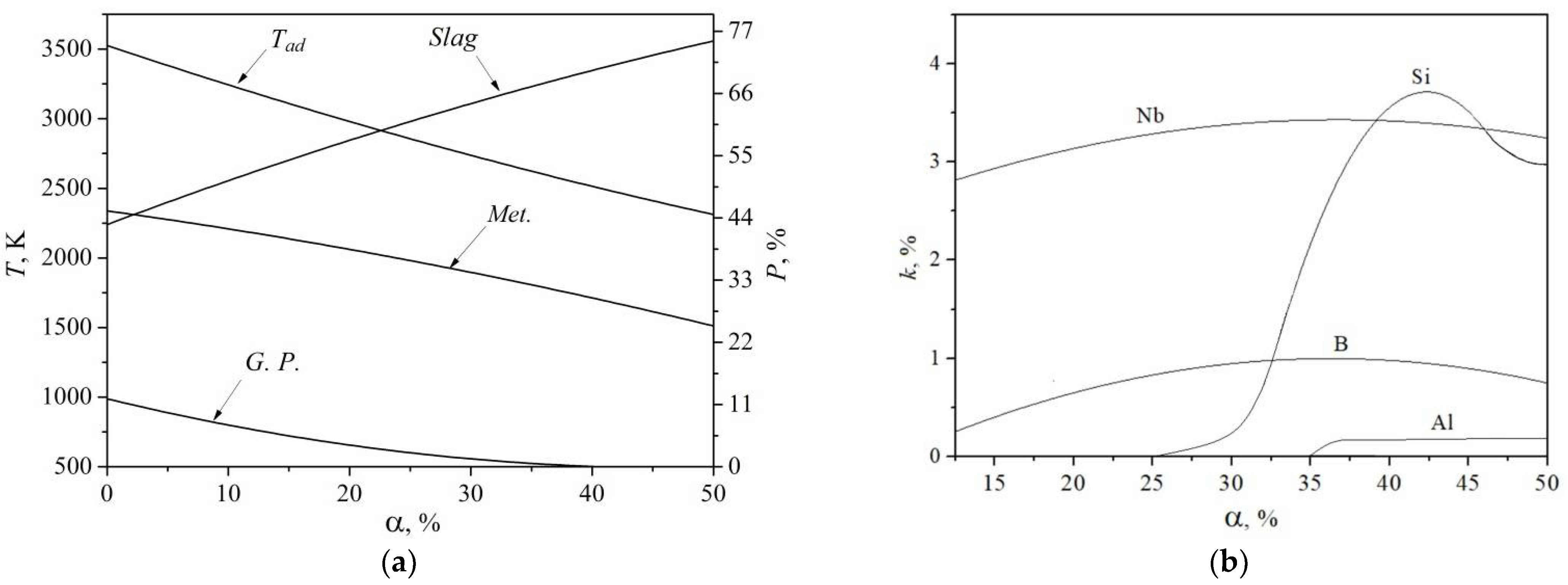
2. Thermodynamic Calculation
3. Experimental
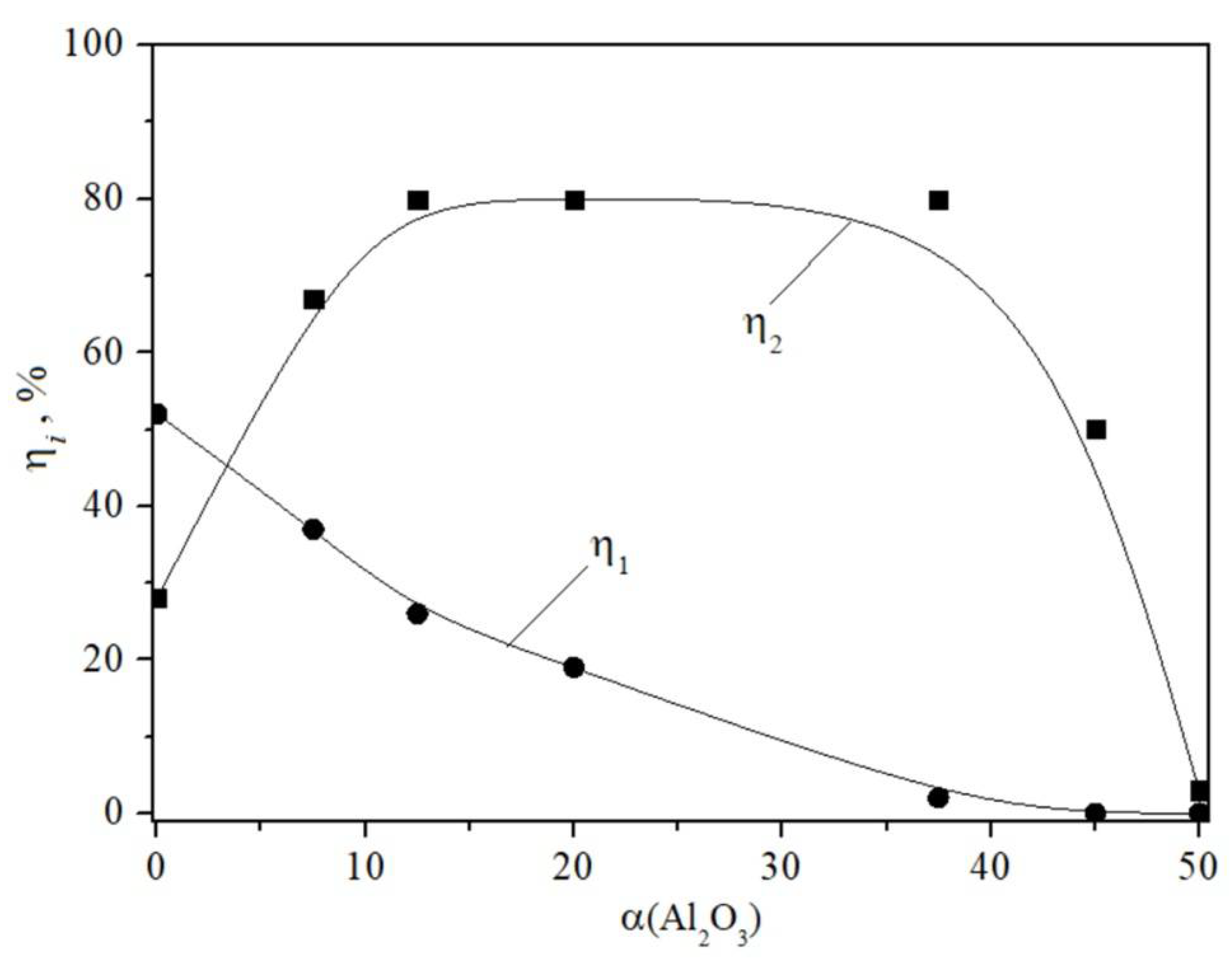
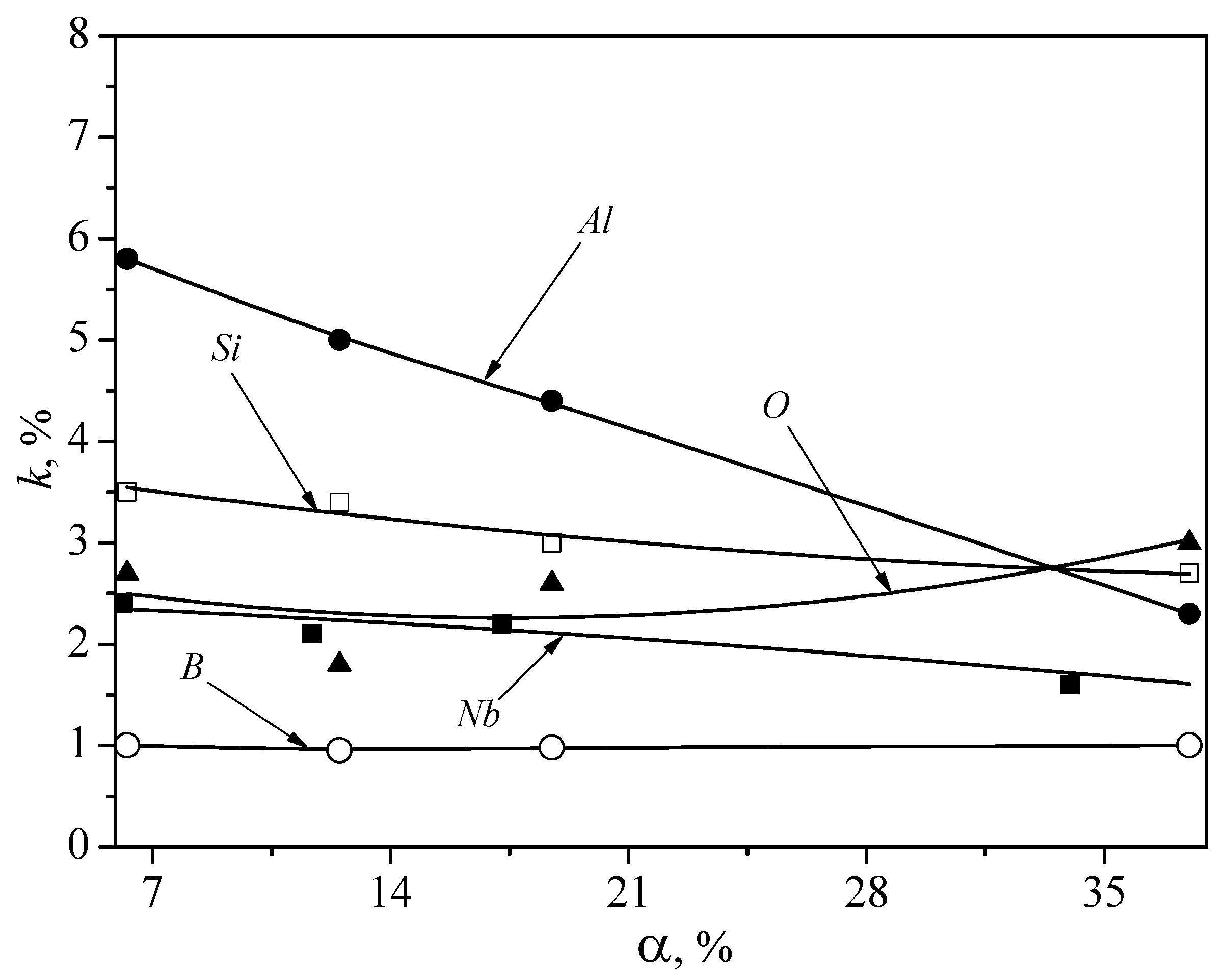
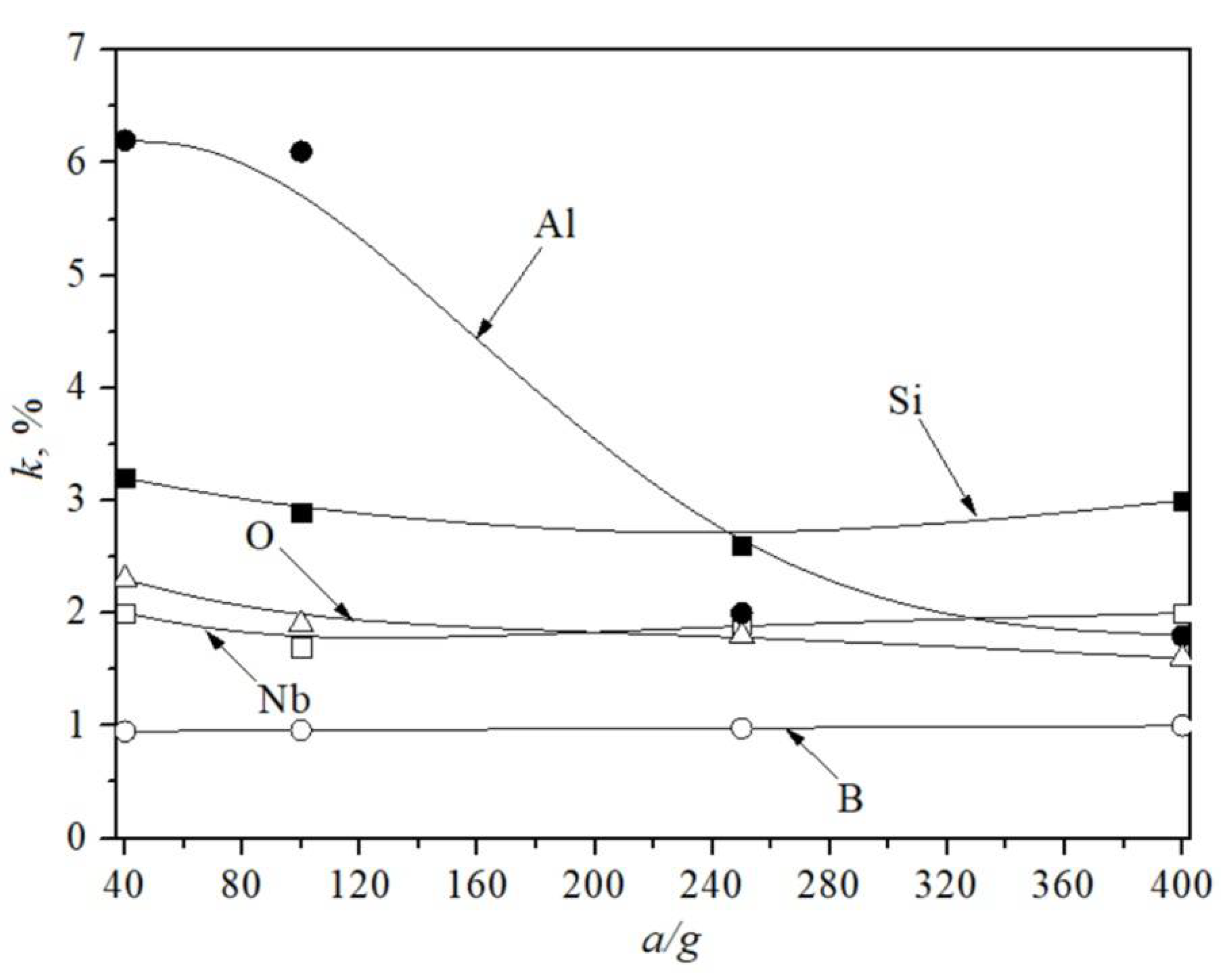
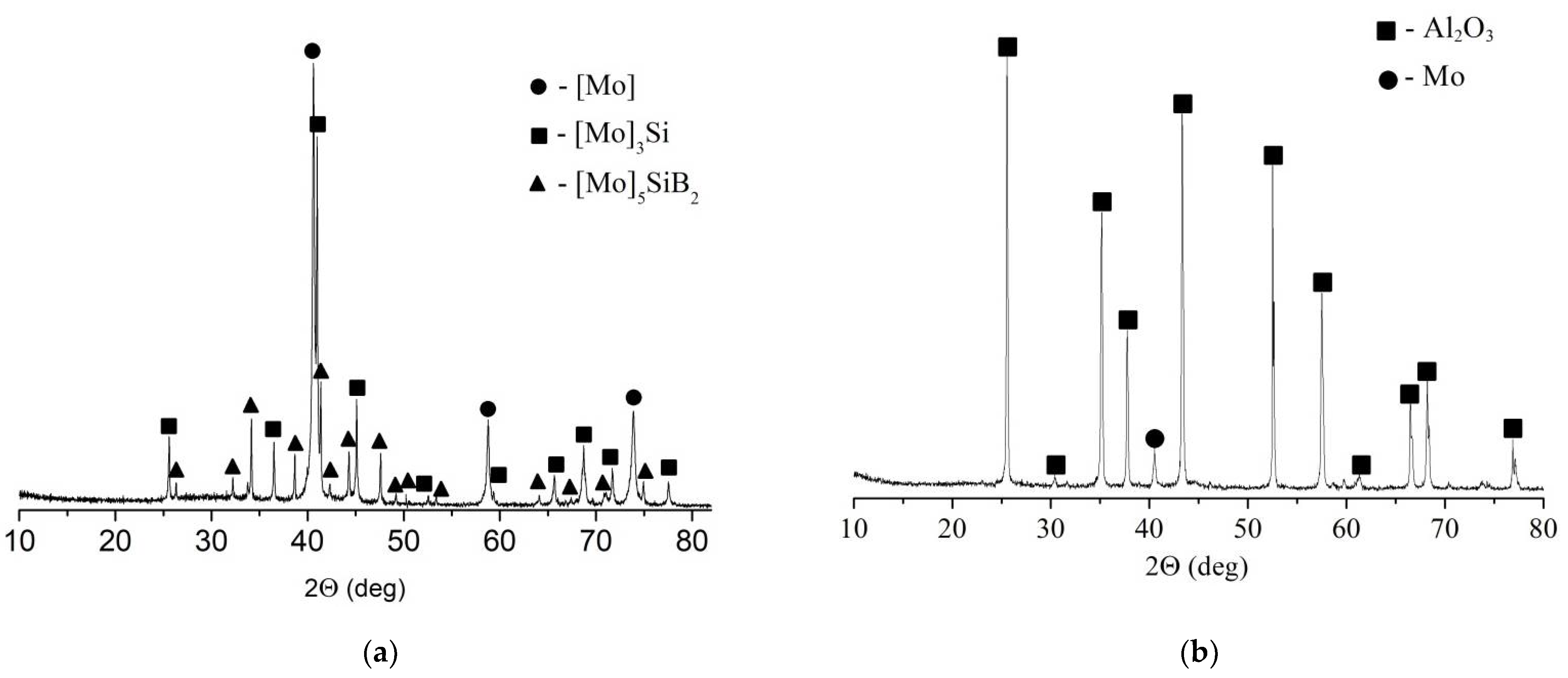
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jéhanno, P.; Heilmaier, M.; Saage, H.; Böning, M.; Kestler, H.; Freudenberger, J.; Drawin, S. Assessment of the high temperature deformation behavior of molybdenum silicide alloys. Mater. Sci. Eng. A 2007, 463, 216–223. [Google Scholar] [CrossRef]
- Jéhanno, P.; Heilmaier, M.; Kestler, H.; Böning, M.; Venskutonis, A.; Bewlay, B.P.; Jackson, M.R. Assessment of a powder metallurgical processing route for refractory metal silicide alloys. Metall. Mater. Trans. 2005, 36A, 515–523. [Google Scholar] [CrossRef]
- Berczik, D.M. Method for enhancing the oxidation resistance of a molybdenum alloy and a method of making a molybdenum alloy. U.S. Patent 5595616, 21 January 1997. [Google Scholar]
- Meyer, M.K.; Thom, A.J.; Akinc, M. Oxide scale formation and isothermal oxidation behavior of Mo–Si–B intermetallics at 600–1000 °C. Intermetallics 1999, 7, 153–162. [Google Scholar] [CrossRef]
- Schneibel, J.H.; Kruzic, J.J.; Ritchie, R.O. Mo-Si-B Alloy Development. In Proceedings of the 17th Annual Conference on Fossil Energy Materials, Baltimore, MD, USA, 22–24 April 2003. [Google Scholar]
- Takata, N.; Sekido, N.; Takeyama, M.; Perepezko, J.H.; Follett-Figueroa, M.; Zhang, C. Solidification of BCC/T1/T2 three-phase microstructure in Mo–Nb–Si–B alloys. Intermetallics 2016, 72, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Byan, J.M.; Bang, S.-R.; Kim, S.H.; Choi, W.J.; Kim, Y.D. Mechanical properties of Mo–Nb–Si–B quaternary alloys fabricated by powder metallurgical method. Int. J. Refract. Met. Hard Mater. 2017, 65, 14–18. [Google Scholar] [CrossRef]
- Drawin, S.; Justin, J.F. Advanced lightweight silicide and nitride based materials for turbo-engine applications. AerospaceLab 2011, 3, 1–13. [Google Scholar]
- Vdovin, Y.S.; Andreev, D.E.; Yukhvid, V.I. Mo-Based composites reinforced with Nb, Si, and B by metallothermic SHS under artificial gravity. Int. J. Self-Propag. High.-Temp. Synth. 2019, 28, 274–275. [Google Scholar] [CrossRef]
- Shiryaev, A. Thermodynamics of SHS processes: An advanced approach. Int. J. Self-Propag. High.-Temp. Synth. 1995, 4, 351–362. [Google Scholar]
- Yukhvid, V.I.; Alymov, M.I.; Sanin, V.N.; Andreev, D.E.; Sachkova, N.V. Synthesis of composition materials based on niobium silicides by metallothermic SHS. Inorg. Mater. 2015, 51, 1347–1354. [Google Scholar] [CrossRef]
- Lim, K.S.; Kim, Y.S.; Hong, S.H.; Song, G.; Kim, K.B. Influence of N2 gas flow ratio and working pressure on amorphous Mo–Si–N coating during magnetron sputtering. Coatings 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]


| Mo | Si | Nb | B | MoO3 | Nb2O5 | Al | |
|---|---|---|---|---|---|---|---|
| Green mixture | – | 1.5 | – | 0.5 | 68.9 | 2.4 | 26.7 |
| Combustion product | 92.5 | 3.0 | 3.4 | 1.0 | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreev, D.; Vdovin, Y.; Yukhvid, V.; Golosova, O. Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure. Metals 2021, 11, 803. https://doi.org/10.3390/met11050803
Andreev D, Vdovin Y, Yukhvid V, Golosova O. Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure. Metals. 2021; 11(5):803. https://doi.org/10.3390/met11050803
Chicago/Turabian StyleAndreev, Dmitrii, Yurii Vdovin, Vladimir Yukhvid, and Olga Golosova. 2021. "Mo–Nb–Si–B Alloy: Synthesis, Composition, and Structure" Metals 11, no. 5: 803. https://doi.org/10.3390/met11050803






