Hydrogen Effects in Equiatomic CrFeNiMn Alloy Fabricated by Laser Powder Bed Fusion
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Defect Structure
3.2. Hydrogen Uptake Ability
3.3. Analysis of TDS-Tested Specimens
3.4. Analysis of TEM Specimen
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Fujimoto, S.; Kambara, Y.; Kawamura, M.; Chen, Z.M.T.; Matsunoshita, H.; Tanaka, K.; Inui, H.; George, E.P. Size effect, critical resolved shear stress, stacking fault energy, and solid solution strengthening in the CrMnFeCoNi high-entropy alloy. Sci. Rep. 2016, 6, 35863. [Google Scholar] [CrossRef]
- Tasan, C.C.; Deng, Y.; Pradeep, K.G.; Yao, M.J.; Springer, H.; Raabe, D. Composition dependence of phase stability, deformation mechanisms, and mechanical properties of the CoCrFeMnNi High-entropy alloy system. JOM 2014, 66, 1993–2001. [Google Scholar] [CrossRef]
- Wu, H. Excellent mechanical properties of in-situ TiC/FeCrNiCuV0.1 high entropy alloy matrix composites. Mater. Lett. 2019, 4, 257. [Google Scholar] [CrossRef]
- Lu, C.; Niu, L.; Chen, N.; Jin, K.; Yang, T.; Xiu, P.; Zhang, Y.; Gao, F.; Bei, H.; Shi, S.; et al. Enhancing radiation tolerance by controlling defect mobility and migration pathways in multicomponent single-phase alloys. Nat. Commun. 2016, 7, 13564. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bei, H. Microstructures and mechanical properties of compositionally complex Co-free FeNiMnCr18 FCC solid solution alloy. Mater. Sci. Eng. A 2015, 640, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.A.P.K.; Li, C.; Leonard, K.J.; Bei, H.; Zinkle, S.J. Microstructural stability and mechanical behavior of FeNiMnCr high entropy alloy under ion irradiation. Acta Mater. 2016, 113, 230–244. [Google Scholar] [CrossRef] [Green Version]
- Eißmann, N.; Klöden, B.; Weißgärber, T.; Kieback, B. High-entropy alloy CoCrFeMnNi produced by powder metallurgy. Powder Metall. 2017, 60, 184–197. [Google Scholar] [CrossRef]
- Liu, L.; Ding, Q.; Zhong, Y.; Zou, J.; Wu, J.; Chiu, Y.-L.; Li, J.; Zhang, Z.; Yu, Q.; Shen, Z. Dislocation network in additive manufactured steel breaks strength–ductility trade-off. Mater. Today 2018, 21, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Ge, Y.; Lehtonen, J.; Hannula, S.-P. Hierarchical microstructure of laser powder bed fusion produced face-centered-cubic-structured equiatomic CrFeNiMn multicomponent alloy. Materials 2020, 13, 4498. [Google Scholar] [CrossRef]
- Donik, Č.; Kraner, J.; Paulin, I.; Godec, M. Influence of the energy density for selective laser melting on the microstructure and mechanical properties of stainless steel. Metals 2020, 10, 919. [Google Scholar] [CrossRef]
- Konovalov, S.; Osintsev, K.; Golubeva, A.; Smelov, V.; Ivanov, Y.; Chen, X.; Komissarova, I. Surface modification of Ti-based alloy by selective laser melting of Ni-based superalloy powder. J. Mater. Res. Technol. 2020, 9, 8796–8807. [Google Scholar] [CrossRef]
- Do, D.K.; Li, P. The Effect of laser energy input on the microstructure, physical and mechanical properties of Ti-6Al-4V alloys by selective laser melting. Virtual Phys. Prototyp. 2016, 11, 41–47. [Google Scholar] [CrossRef]
- Chang, K.-C.; Zhao, J.-R.; Hung, F.-Y. Microstructure, mechanical properties, and fatigue fracture characteristics of high-fracture-resistance selective laser melting Al-Ni-Cu alloys. Metals 2021, 11, 87. [Google Scholar] [CrossRef]
- Brif, Y.; Thomas, M.; Todd, I. The use of high-entropy alloys in additive manufacturing. Scr. Mater. 2015, 99, 93–96. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Zhong, Y.; Shen, Z.J. Hardened austenite steel with columnar sub-grain structure formed by laser melting. Mater. Sci. Eng. A 2015, 625, 221–229. [Google Scholar] [CrossRef]
- Turnbull, A.; Hutchings, R.B.; Ferriss, D.H. Modelling of thermal desorption of hydrogen from metals. Mater. Sci. Eng. A 1997, 238, 317–328. [Google Scholar] [CrossRef]
- Yagodzinskyy, Y.; Saukkonen, T.; Kilpeläinen, S.; Tuomisto, F.; Hänninen, H. Effect of hydrogen on plastic strain localization in single crystals of austenitic stainless steel. Scr. Mater. 2010, 62, 155–158. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Raabe, D. Hydrogen enhances strength and ductility of an equiatomic high-entropy alloy. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nygren, K.E.; Bertsch, K.M.; Wang, S.; Bei, H.; Nagao, A.; Robertson, I.M. Hydrogen embrittlement in compositionally complex FeNiCoCrMn FCC solid solution alloy. Curr. Opin. Solid State Mater. Sci. 2017. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Suh, J.-Y.; Lee, K.-A. Effect of gaseous hydrogen embrittlement on the mechanical properties of additively manufactured CrMnFeCoNi high-entropy alloy strengthened by in-situ formed oxide. Mater. Sci. Eng. A 2020, 796, 140039. [Google Scholar] [CrossRef]
- Lee, D.-H.; Sun, B.; Lee, S.; Ponge, D.; Jägle, E.A.; Raabe, D. Comparative study of hydrogen embrittlement resistance between additively and conventionally manufactured 304L austenitic stainless steels. Mater. Sci. Eng. A 2021, 803, 140499. [Google Scholar] [CrossRef]
- Silverstein, R.; Eliezer, D. Mechanisms of hydrogen trapping in austenitic, duplex, and super martensitic stainless steels. J. Alloys Compd. 2017, 720, 451–459. [Google Scholar] [CrossRef]
- Fukai, Y.; Mizutani, M.; Yokota, S.; Kanazawa, M.; Miura, Y.; Watanabe, T. Superabundant vacancy–hydrogen clusters in electrodeposited Ni and Cu. J. Alloys Compd. 2003, 4, 270–273. [Google Scholar] [CrossRef]
- Tuomisto, F.; Makkonen, I. Defect identification in semiconductors with positron annihilation: Experiment and theory. Rev. Mod. Phys. 2013, 85, 1583–1631. [Google Scholar] [CrossRef] [Green Version]
- Lu, E.; Makkonen, I.; Mizohata, K.; Li, Z.; Räisänen, J.; Tuomisto, F. Effect of interstitial carbon on the evolution of early-stage irradiation damage in equi-atomic FeMnNiCoCr high-entropy alloys. Appl. Phys. 2020, 8. [Google Scholar] [CrossRef]
- Lehtonen, J.; Ge, Y.; Ciftci, N.; Heczko, O.; Uhlenwinkel, V.; Hannula, S.-P. Phase structures of gas atomized equiatomic CrFeNiMn high entropy alloy powder. J. Alloys Compd. 2020, 827, 154142. [Google Scholar] [CrossRef]
- Lu, E.; Cao, X.; Jin, S.; Zhang, P.; Zhang, C.; Yang, J.; Wu, Y.; Guo, L.; Wang, B. Investigation of vacancy-type defects in helium irradiated FeCrNi alloy by slow positron beam. J. Nucl. Mater. 2015, 458, 240–244. [Google Scholar] [CrossRef]
- Morris, M.A.; George, O.; Morris, D.G. Vacancies, vacancy aggregates and hardening in FeAl. Mater. Sci. Eng. A 1998, 258, 99–107. [Google Scholar] [CrossRef]
- Ryu, J.H.; Chun, Y.S.; Lee, C.S.; Bhadeshia, H.K.D.H.; Suh, D.W. Effect of deformation on hydrogen trapping and effusion in TRIP-assisted steel. Acta Mater. 2012, 60, 4085–4092. [Google Scholar] [CrossRef]
- Kirchheim, R.; Pundt, A. Hydrogen in metals. In Physical Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 2597–2705. ISBN 978-0-444-53770-6. [Google Scholar]
- Zhao, Y.; Lee, D.-H.; Seok, M.-Y.; Lee, J.-A.; Phaniraj, M.P.; Suh, J.-Y.; Ha, H.-Y.; Kim, J.-Y.; Ramamurty, U.; Jang, J. Resistance of CoCrFeMnNi high-entropy alloy to gaseous hydrogen embrittlement. Scr. Mater. 2017, 135, 54–58. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. (Eds.) High-Entropy Alloys; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-27011-1. [Google Scholar]
- Zhao, Y.; Park, J.-M.; Lee, D.-H.; Song, E.J.; Suh, J.-Y.; Ramamurty, U.; Jang, J. Influences of hydrogen charging method on the hydrogen distribution and nanomechanical properties of face-centered cubic high-entropy alloy: A comparative study. Scr. Mater. 2019, 168, 76–80. [Google Scholar] [CrossRef]
- Baek, S.-W.; Song, E.J.; Kim, J.H.; Lee, Y.-H.; Ryu, K.S.; Kim, S.-W. Hydrogen susceptibility of nano-sized oxide dispersed austenitic steel for fusion reactor. Fusion Eng. Des. 2017, 121, 105–110. [Google Scholar] [CrossRef]
- Lin, J.; Chen, F.; Liu, F.; Xu, D.; Gao, J.; Tang, X. Hydrogen permeation behavior and hydrogen-induced defects in 316L stainless steels manufactured by additive manufacturing. Mater. Chem. Phys. 2020, 250, 123038. [Google Scholar] [CrossRef]
- Park, J.-M.; Zhao, Y.; Voisin, T.; Lee, D.-H.; Komazaki, S.; Ko, Y.; Kim, D.-I.; Suh, J.-Y.; Han, H.N.; Wang, Y.M.; et al. Hydrogen uptake and its influence in selective laser melted austenitic stainless steel: A nanoindentation study. Scr. Mater. 2021, 194, 113718. [Google Scholar] [CrossRef]
- Macadre, A.; Masumura, T.; Manabe, R.; Tsuchiyama, T.; Takaki, S. Effect of nitrogen-addition on the absorption and diffusivity of hydrogen in a stable austenitic stainless steel. Int. J. Hydrogen Energy 2019, 44, 1263–1271. [Google Scholar] [CrossRef]
- Hoelzel, M.; Rajevac, V.; Danilkin, S.A.; Udovic, T.J.; Wipf, H.; Fuess, H. Lattice dynamics of high-pressure hydrogenated austenitic stainless steels. J. Phys. Condens. Matter 2005, 17, 3537–3546. [Google Scholar] [CrossRef]
- Narita, N.; Altstetter, C.J.; Birnbaum, H.K. Hydrogen-related phase transformations in austenitic stainless steels. Metall. Trans. A 1982, 13, 1355–1365. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Zhang, L.; Luo, H.; Li, R.; Wang, L.; Man, C.; Li, X. Superior resistance to hydrogen damage for selective laser melted 316L stainless steel in a proton exchange membrane fuel cell environment. Corros. Sci. 2020, 166, 108425. [Google Scholar] [CrossRef]
- Whiteman, M.B.; Troiano, A.R. the influence of hydrogen on the stacking fault energy of an austenitic stainless steel. Phys. Status Solid. B 1964, 7, K109–K110. [Google Scholar] [CrossRef]
- Eliezer, D.; Chakrapani, D.G.; Altstetter, C.J.; Pugh, E.N. The influence of austenite stability on the hydrogen embrittlement and stress- corrosion cracking of stainless steel. Metall. Trans. A 1979, 10, 935–941. [Google Scholar] [CrossRef]
- Rozenak, P.; Eliezer, D. Phase changes related to hydrogen-induced cracking in austenitic stainless steel. Acta Metall. 1987, 35, 2329–2340. [Google Scholar] [CrossRef]
- Zevin, L.S.; Rozenak, P.; Eliezer, D. Quantitative X-ray phase analysis of surface layers. J. Appl. Crystallogr. 1984, 17, 18–21. [Google Scholar] [CrossRef]
- Eliezer, D.; Tal-Gutelmacher, E.; Boellinghaus, T. Hydrogen Embrittlement in Hydride- and Non Hydride-Forming Systens–Microstructural/Phase Changes and Cracking Mechanisms. In Proceedings of the 11th International Conference on Fracture, Turin, Italy, 20–25 March 2005; Available online: http://www.gruppofrattura.it/ocs/index.php/ICF/ICF11/paper/view/10870/10199 (accessed on 19 March 2016).
- Hannula, S.-P. The effect of pre-existing epsilonmartensite on the hydrogen induced fracture of austenitic stainless steel. Scr. Metall. 1983, 17, 509–513. [Google Scholar] [CrossRef]
- Baranowskip, B.; Flanagans, T.B. The volume increase of Fcc metals and alloys due to interstitial hydrogen over a wide range of hydrogen contents. J. Phys. F Met. Phys. 1971, 1, 5. [Google Scholar]
- von Pezold, J.; Lymperakis, L.; Neugebeauer, J. Hydrogen-enhanced local plasticity at dilute bulk H concentrations: The role of H–H interactions and the formation of local hydrides. Acta Mater. 2011, 59, 2969–2980. [Google Scholar] [CrossRef]
- Mine, Y.; Kimoto, T. Hydrogen uptake in austenitic stainless steels by exposure to gaseous hydrogen and its effect on tensile deformation. Corros. Sci. 2011, 53, 2619–2629. [Google Scholar] [CrossRef]


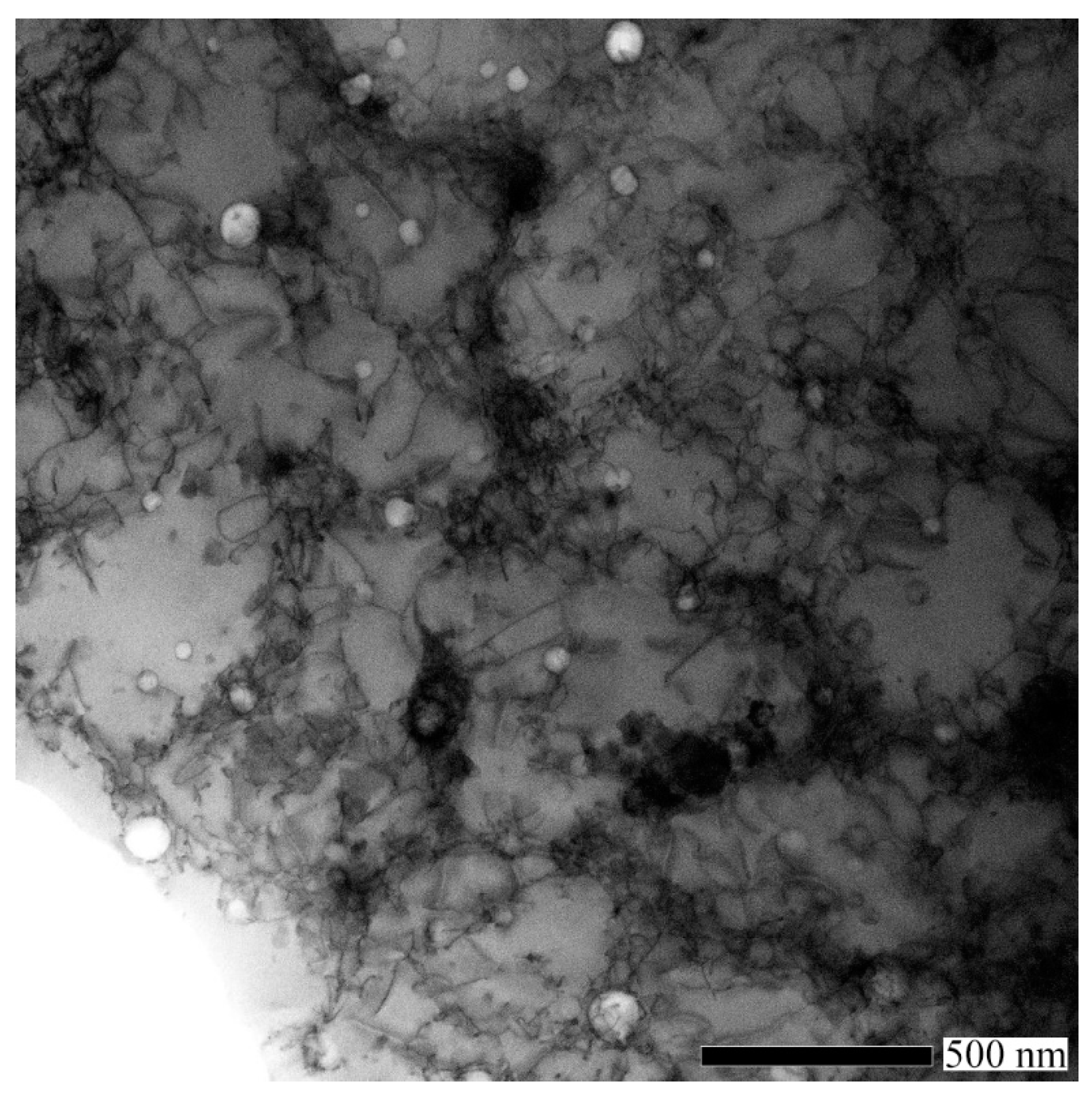
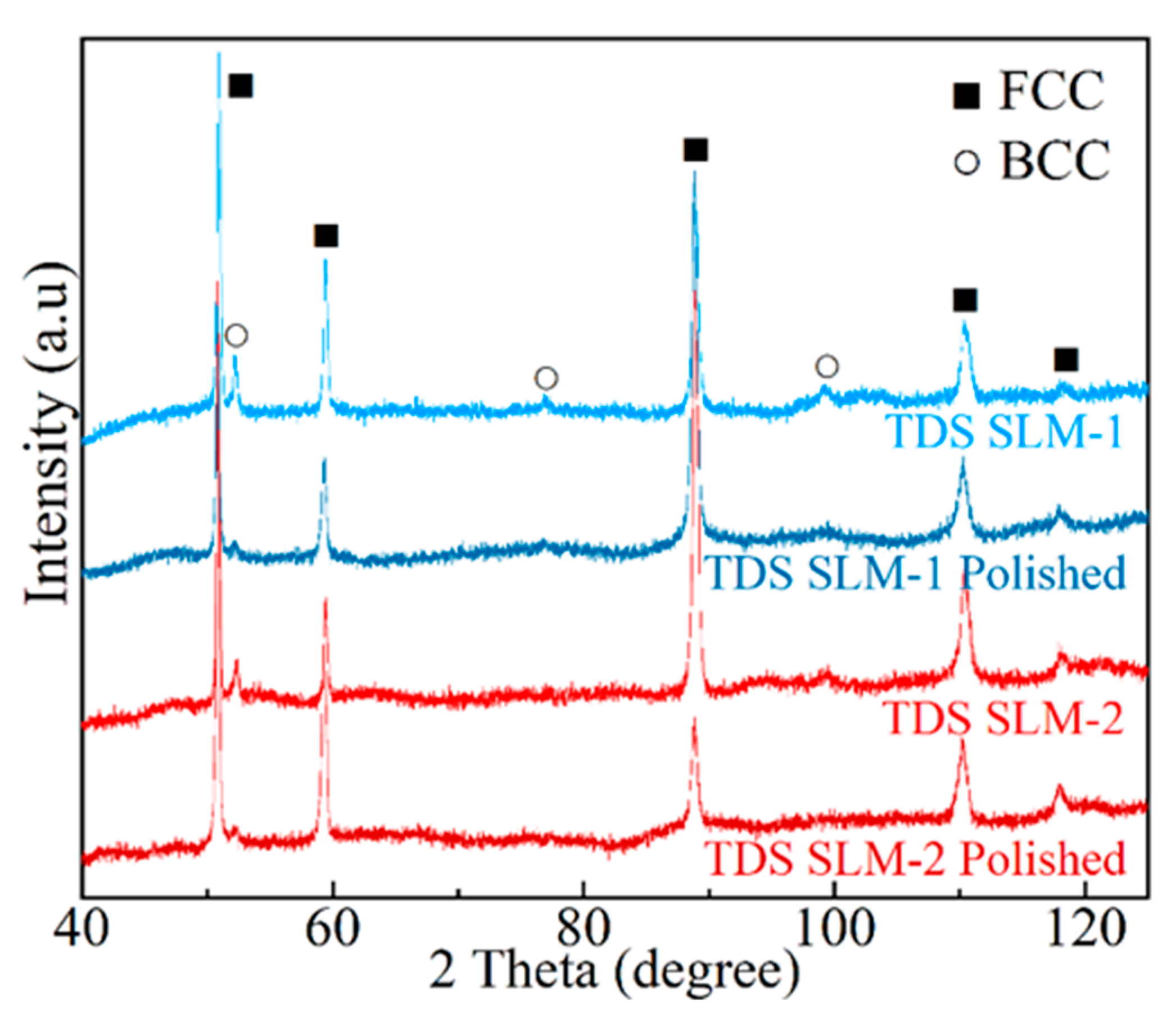



| Sample | Cr | Mn | Fe | Ni | Porosity | Average Cell Size (nm) |
|---|---|---|---|---|---|---|
| SLM-1 | 25.5 | 24.9 | 24.8 | 24.8 | 2.0 | 390 |
| TDS SLM-1 | 25.7 | 24.7 | 25.0 | 24.6 | - | - |
| TDS SLM-1 Polished | 25.1 | 25.0 | 25.2 | 24.7 | - | - |
| SLM-2 | 25.8 | 24.0 | 25.3 | 24.9 | 2.1 | 520 |
| TDS SLM-2 | 26.1 | 23.4 | 25.5 | 25.0 | - | - |
| TDS SLM-2 Polished | 25.3 | 23.9 | 25.6 | 25.2 | - | - |
| PECS | 26.0 | 24.2 | 25.3 | 24.5 | 2.4 | - |
| TDS PECS | 26.4 | 23.6 | 25.0 | 25.0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yagodzinskyy, Y.; Ge, Y.; Lu, E.; Lehtonen, J.; Kollo, L.; Hannula, S.-P. Hydrogen Effects in Equiatomic CrFeNiMn Alloy Fabricated by Laser Powder Bed Fusion. Metals 2021, 11, 872. https://doi.org/10.3390/met11060872
Yang X, Yagodzinskyy Y, Ge Y, Lu E, Lehtonen J, Kollo L, Hannula S-P. Hydrogen Effects in Equiatomic CrFeNiMn Alloy Fabricated by Laser Powder Bed Fusion. Metals. 2021; 11(6):872. https://doi.org/10.3390/met11060872
Chicago/Turabian StyleYang, Xuan, Yuriy Yagodzinskyy, Yanling Ge, Eryang Lu, Joonas Lehtonen, Lauri Kollo, and Simo-Pekka Hannula. 2021. "Hydrogen Effects in Equiatomic CrFeNiMn Alloy Fabricated by Laser Powder Bed Fusion" Metals 11, no. 6: 872. https://doi.org/10.3390/met11060872
APA StyleYang, X., Yagodzinskyy, Y., Ge, Y., Lu, E., Lehtonen, J., Kollo, L., & Hannula, S.-P. (2021). Hydrogen Effects in Equiatomic CrFeNiMn Alloy Fabricated by Laser Powder Bed Fusion. Metals, 11(6), 872. https://doi.org/10.3390/met11060872







