Corrosion Behavior of Extruded AM60-AlN Metal Matrix Nanocomposite and AM60 Alloy Exposed to Simulated Acid Rain Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and SAR Model Solution
2.2. Immersion Test and Analysis of Surface
2.3. Electrochemical Characterization
3. Results and Discussion
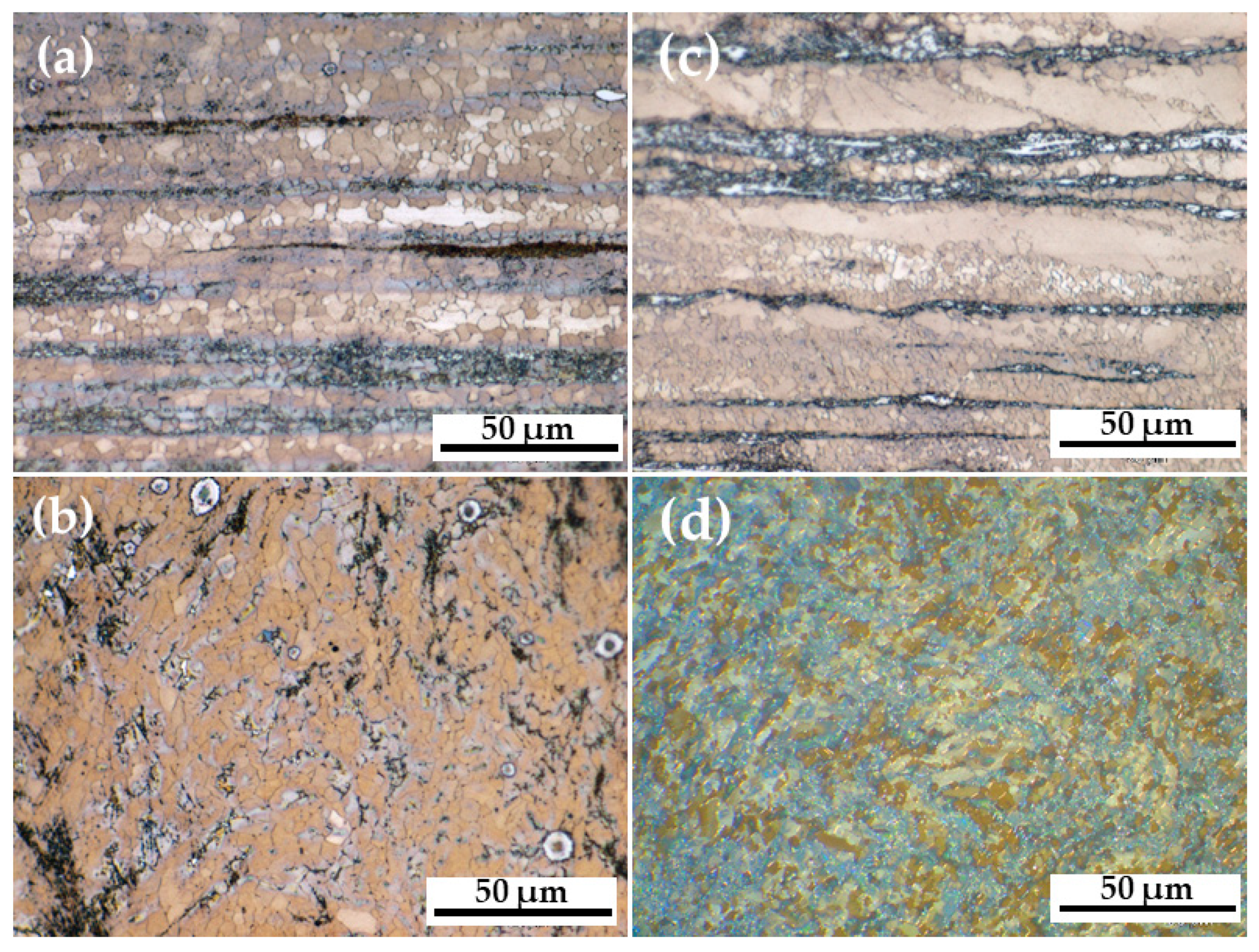
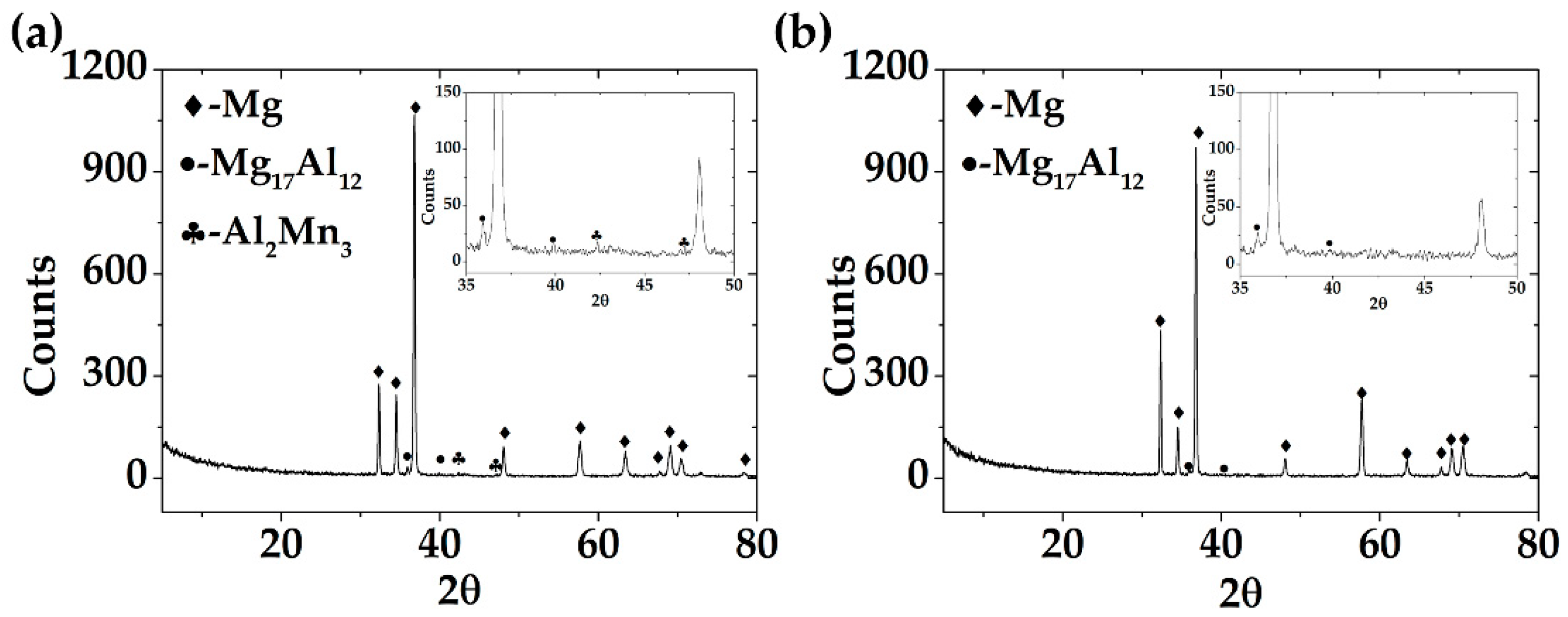
3.1. Microstructure of AM60-AlN and AM60
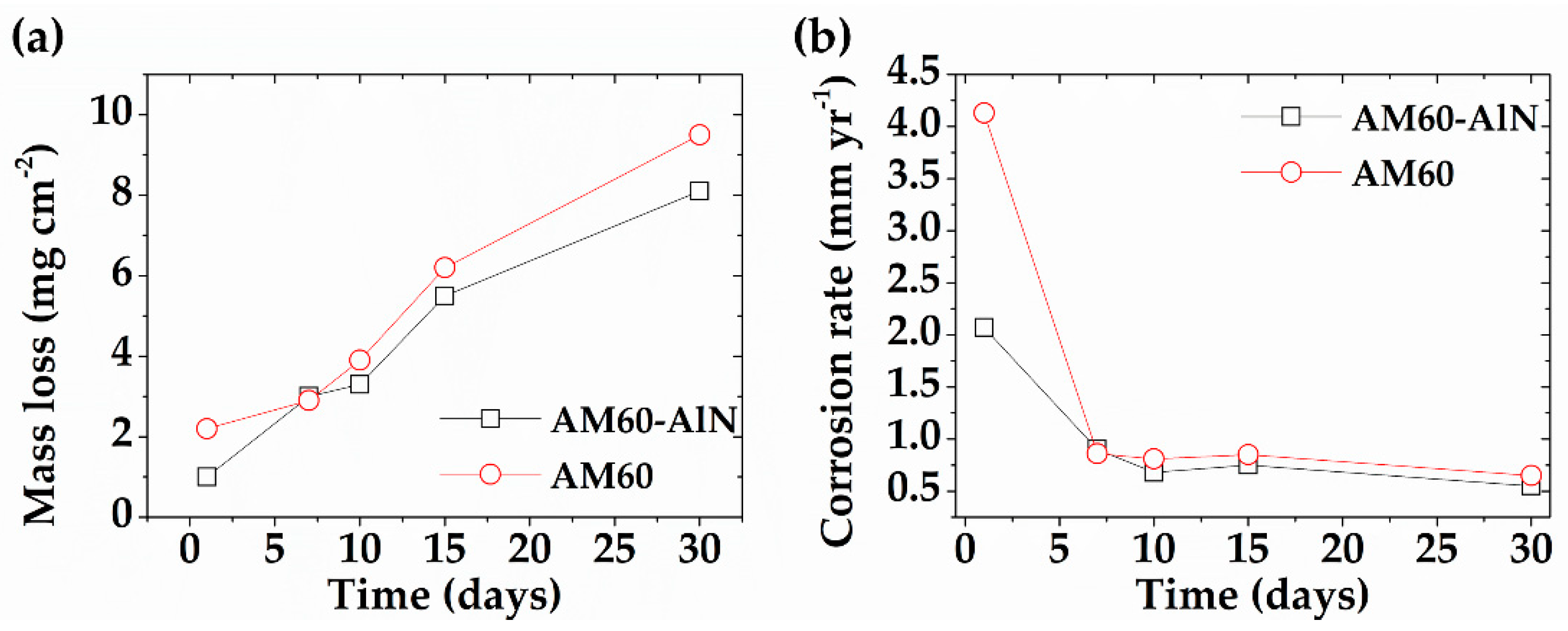
3.2. Test Solution Monitoring and Mass Loss Measurement
3.3. Surface Characterization after Exposure to SAR Solution
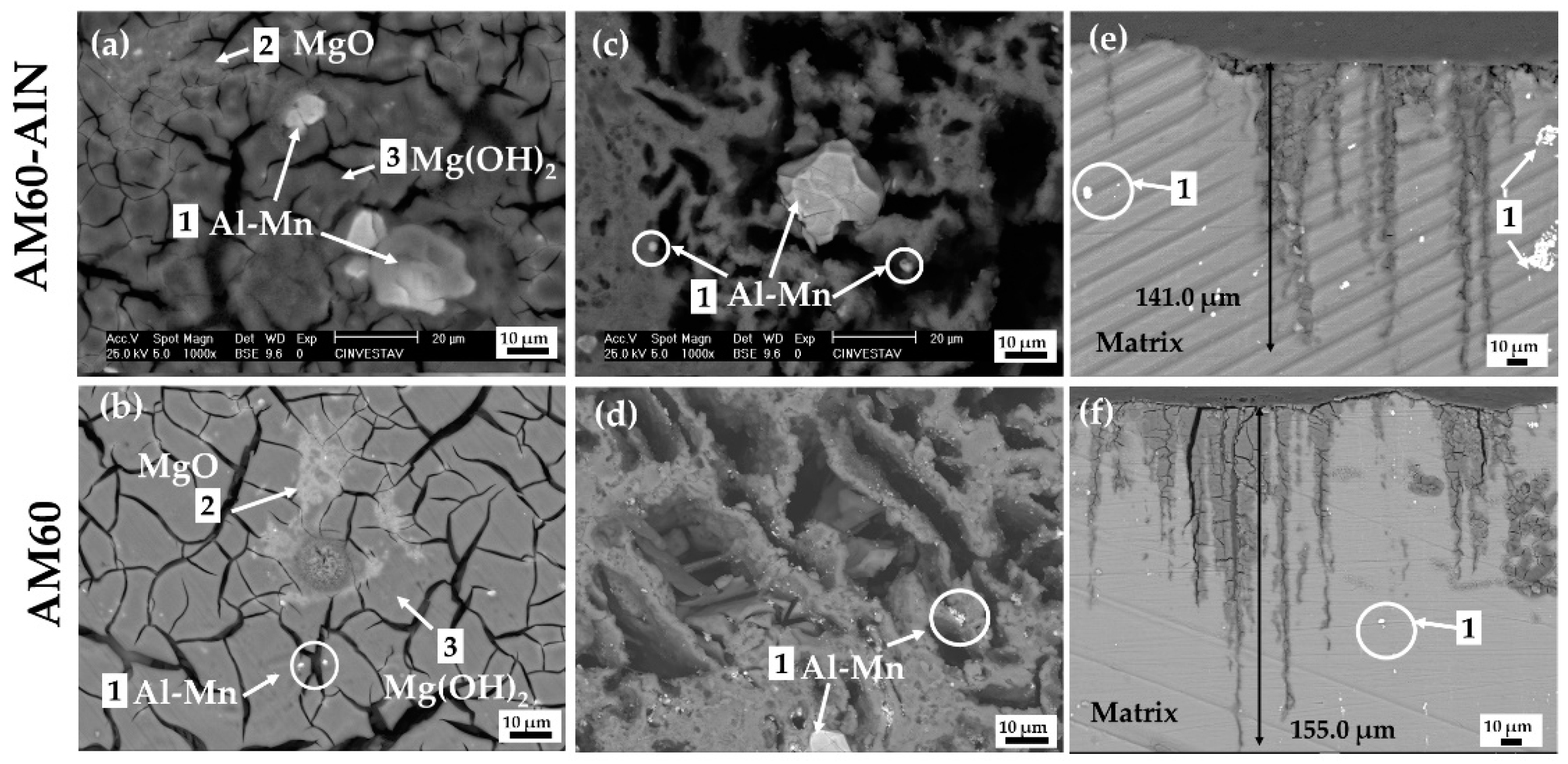
3.3.1. SEM-EDS Analysis
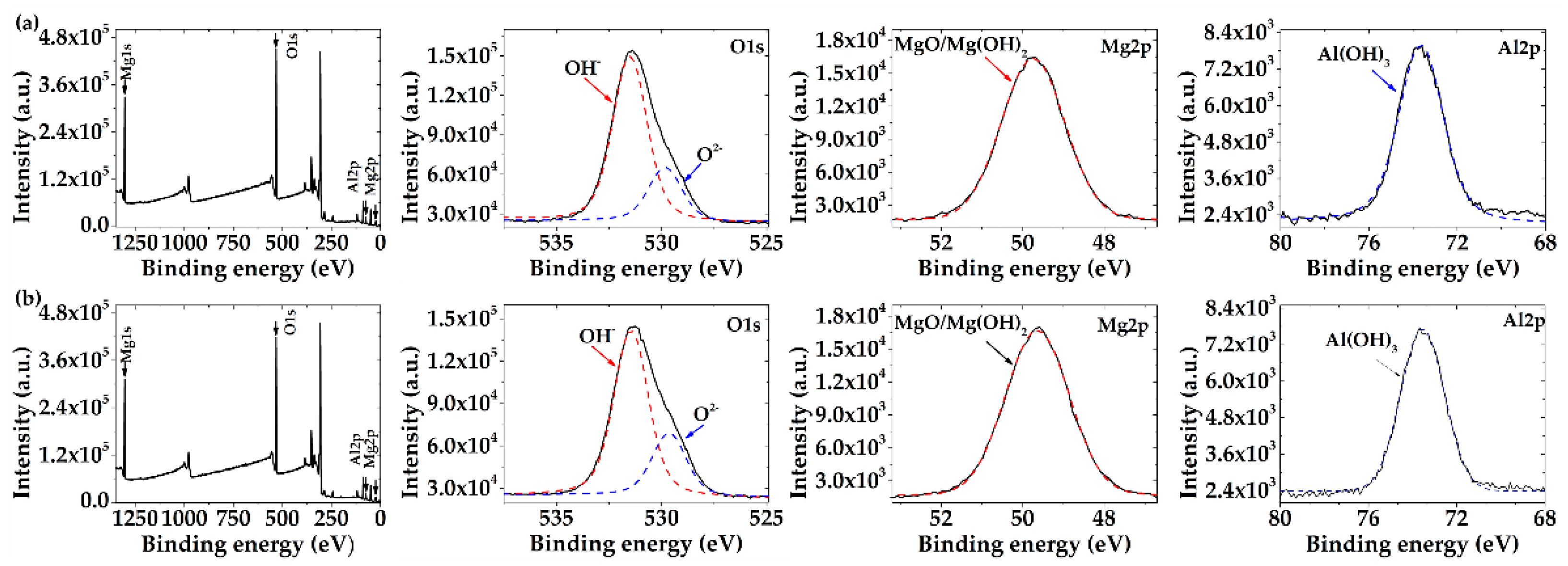
3.3.2. XPS Analysis
3.4. Electrochemical Measurements
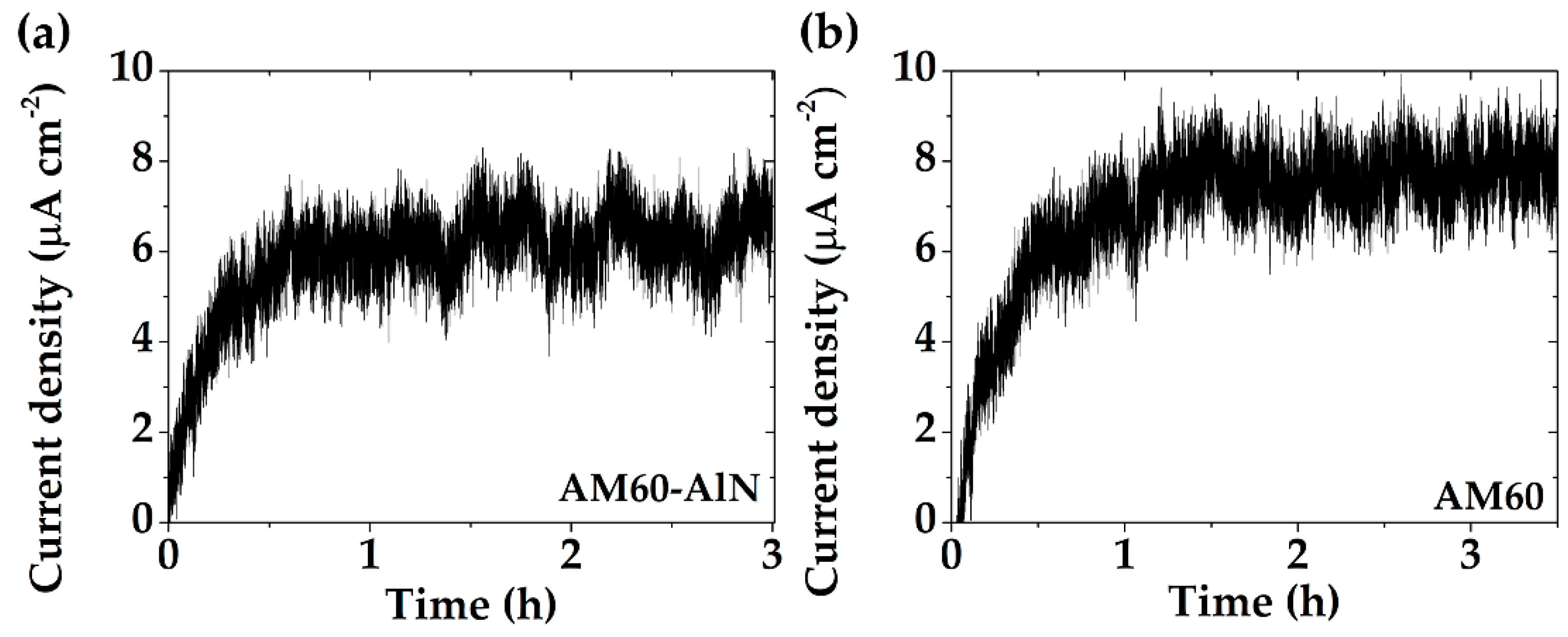
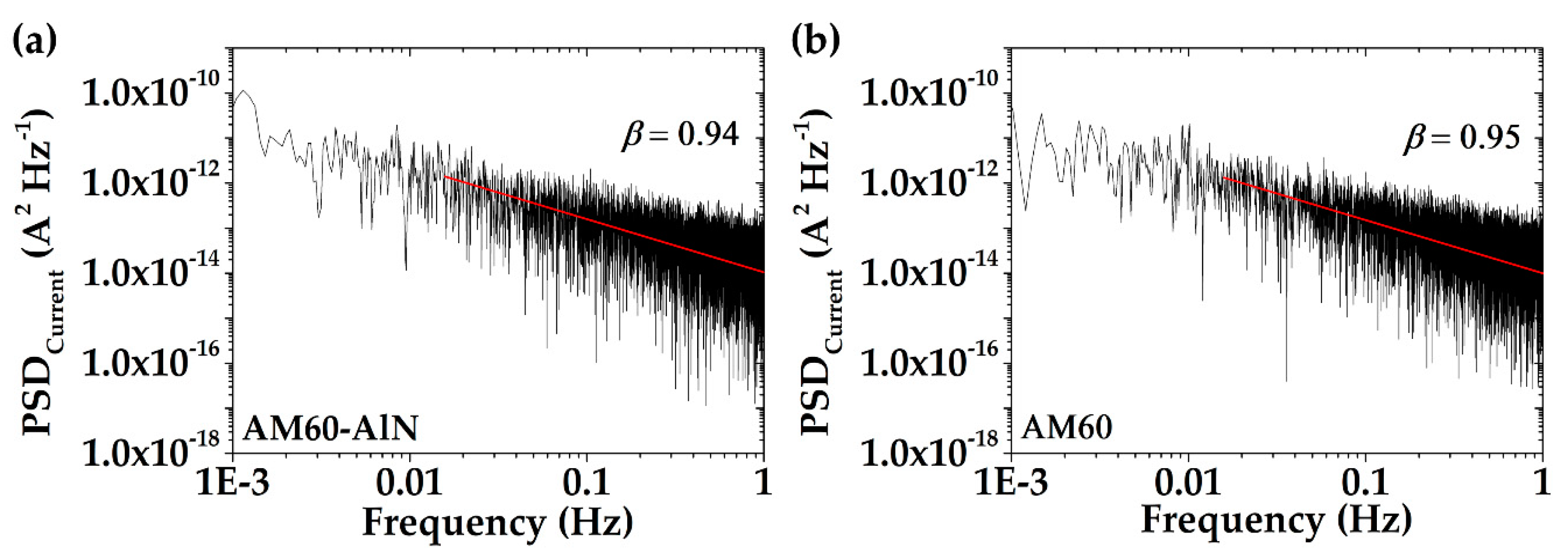
3.4.1. Electrochemical Noise (EN) Analysis
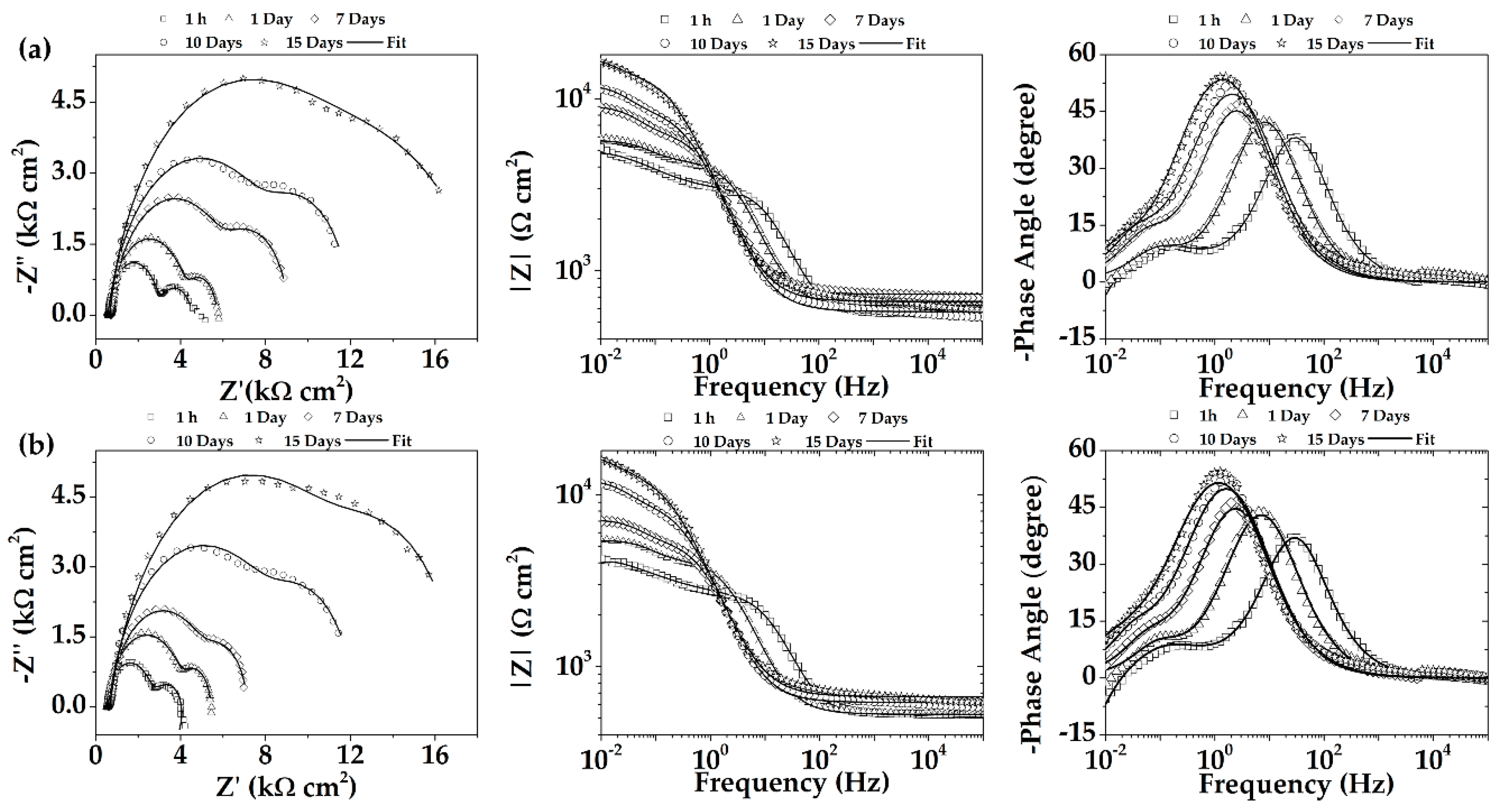
3.4.2. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
- The incorporation of 1.0% wt.% AlN aluminum nitride nanoparticles (≈ 80 nm average diameter) in the AM60 matrix favored a grain size reduction of 12%, as revealed by the optical images, as well as a slight 7% increase in the Vickers hardness. The AlN particles were “attached” to the phase of Mn-rich Al-Mn intermetallic particles, forming a cluster, according to the SEM images.
- The immersion test revealed that the increase in alkaline values of SAR solution was lower during the exposure of the AlN nanocomposite than that of the AM60 alloy. This fact correlates with the 24% lower concentration of the released Mg-ions from the AM60-AlN surface. However, the pH increase suggests that Al de-alloying may occur, as well as Al(OH)3 formation as a corrosion product, as suggested by XPS analysis.
- The formed corrosion layers showed cracks at the end of the exposure and localized attacks near Al-Mn intermetallic particles, acting as efficient cathodic sites. The cross-sectional images revealed a higher intensity of attacks on the AM60 alloy surface without reinforcement.
- The β exponents extracted from the PSD plots of the corrosion current fluctuations classified the corrosion of the studied materials as fractional Gaussian noise (fGn) of the stationary persistent localized process.
- XPS analysis suggests that the main corrosion products were MgO and Mg(OH)2, as well as a lower content of Al(OH)3 that may delay the advance of the AM60-AlN corrosion process.
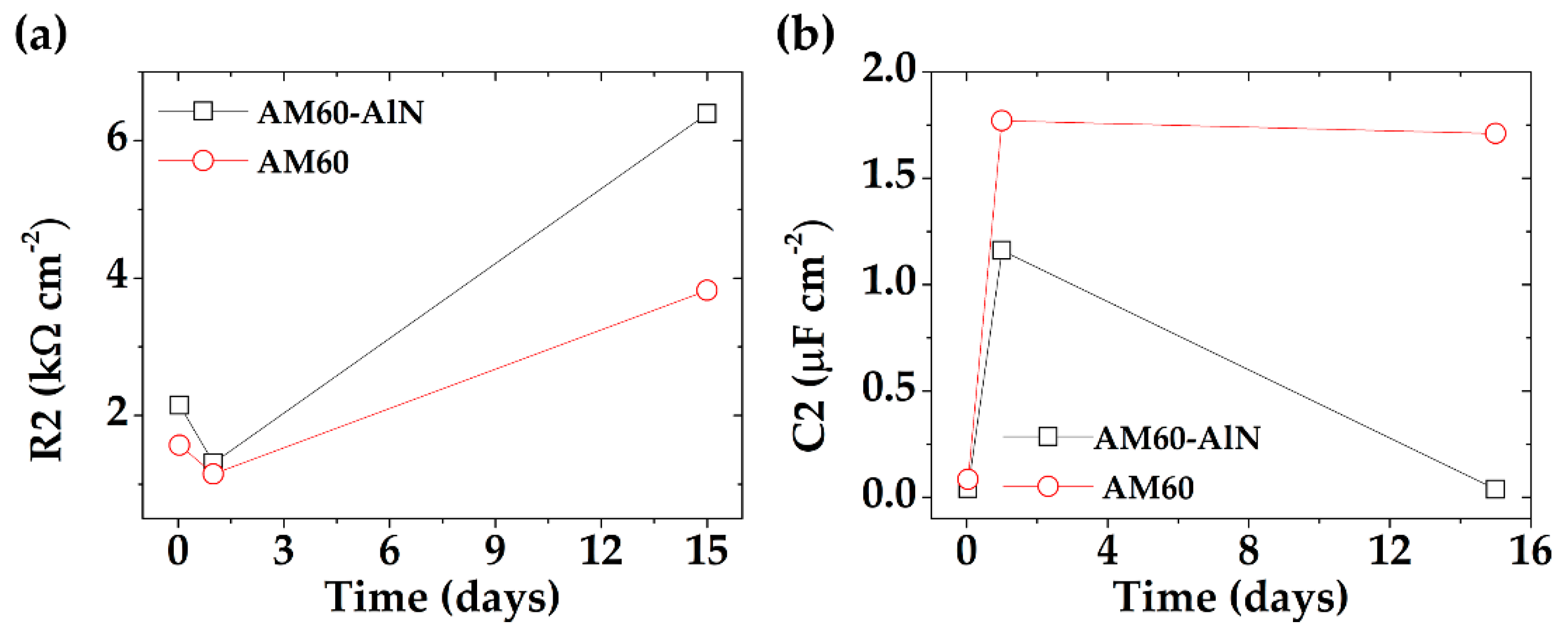
- The EIS Nyquist plots and the parameters of the adjusted equivalent circuits indicated that the charge transfer resistance (R2) and capacitance values, characteristic of the double layer in the presence of corrosion products, were higher in the presence of AlN nanoparticles. This may favor the formation of a more dense and efficient protective corrosion layer because of a slight grain refinement. The tendency of the increase in R2 values for the AM60-AlN nanocomposite coincided with that of Rn values obtained from EN measurements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avedesian, M.M.; Baker, H. Metallurgy and alloys. In ASM Speciality Handbook: Magnesium and Magnesium Alloys; Avedesian, M.M., Baker, H., Eds.; ASM International: Russel Towship, OH, USA, 1999; pp. 3–51. [Google Scholar]
- Kim, S.G.; Inoue, A.; Masumoto, T. Increase of mechanical strength of a Mg85Zn12Ce3 amorphous alloy by dispersion of ultrafine hcp-Mg particles. Mater. Trans. JIM 1991, 32, 875–878. [Google Scholar] [CrossRef] [Green Version]
- Polmear, I.; StJohn, D.; Nie, J.-F.; Qian, M. Magnesium alloys. In Light Alloys: Metallurgy of the Light Metals, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 287–367. [Google Scholar]
- Kainer, K.U. The current state of technology and potential for futher development of magnesium applications. In Magnesium—Alloys and Technology; Kainer, K.U., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 1–22. [Google Scholar]
- Tokarska, K.B.; Gillett, N.P.; Weaver, A.J.; Arora, V.K.; Eby, M. The climate response to five trillion tonnes of carbon. Nat. Clim. Chang. 2016, 6, 851–856. [Google Scholar] [CrossRef]
- Cheah, L.; Evans, C.; Bandivadekar, A.; Heywood, J. Factor of two: Halving the fuel consumption of new US automobiles by 2035. In Reducing Climate Impacts in the Transportation Sector, 1st ed.; Daniel, S., James, S.C., Eds.; Springer: Berlin, Germany, 2008; pp. 49–71. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, X. The present situation and the development trend of new materials used in automobile lightweight. Appl. Mech. Mater. 2012, 189, 58–62. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J. Corrosion studies of rapidly solidified magnesium alloys. J. Electrochem. Soc. 1990, 137, 414–421. [Google Scholar] [CrossRef]
- Loose, W.S. Corrosion and protection of magnesium. In Metals Handbook; Pindgeon, L.M., Mathes, J.C., Woldmen, N.E., Eds.; ASM International: Russel Towship, OH, USA, 1946; pp. 173–260. [Google Scholar]
- Cheng, Y.-L.; Qin, T.-W.; Wang, H.-M.; Zhang, Z. Comparison of corrosion behaviors of AZ31, AZ91, AM60 and ZK60 magnesium alloys. Trans. Nonferrous Met. Soc. China 2019, 19, 517–524. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J. Corrosion of magnesium. Inter. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Song, G.L. Corrosion electrochemistry of magnesium (Mg) and its alloys. In Corrosion of Magnesium Alloys, 1st ed.; Song, G.L., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–65. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Hu, T.T.; Xiang, B.; Liao, S.G.; Huang, W.Z. Corrosion of AM60B magnesium alloy in simulated acid rain. Anti Corros. Method. Mater. 2010, 57, 244–248. [Google Scholar] [CrossRef]
- LeBozec, N.; Jonsson, M.; Thierry, D. Atmospheric corrosion of magnesium alloys: Influence of temperature, relative humidity, and chloride deposition. Corrosion 2004, 60, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Danaie, M.; Asmussen, R.M.; Jakupi, P.; Shoesmith, D.W.; Botton, G.A. The role of aluminum distribution on the local corrosion resistance of the microstructure in a sand-cast AM50 alloy. Corros. Sci. 2013, 77, 151–163. [Google Scholar] [CrossRef]
- Jönsson, M. Atmospheric Corrosion of Magnesium Alloys: Influence of Microstructure and Environment. Ph.D. Thesis, KTH, School of Chemical Science and Engineering, Stockholm, Sweden, 7 December 2007. [Google Scholar]
- Yang, L.-J.; Li, Y.-F.; Wei, Y.-H.; Hou, L.-F.; Li, Y.-G.; Tian, Y. Atmospheric corrosion of field-exposed AZ91D Mg alloys in a polluted environment. Corros. Sci. 2010, 52, 2188–2196. [Google Scholar] [CrossRef]
- Liu, F.; Song, Y.; Shan, D.; Han, E.-H. Corrosion Mechanism of AM50 Magnesium Alloy in Simulated Acid Rain. Recent Pat. Corros. Sci. 2013, 3, 47–57. [Google Scholar] [CrossRef]
- Cui, L.; Liu, Z.; Hu, P.; Shao, J.; Li, X.; Du, C.; Jiang, B. The corrosion behavior of AZ91D magnesium alloy in simulated haze aqueous solution. Materials 2018, 11, 970. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Pandey, K.M. Magnesium Metal Matrix Composites—A Review. Rev. Adv. Mater. Sci. 2015, 42, 58–67. [Google Scholar]
- Dash, D.; Samanta, S.; Rai, R. Study on fabrication of magnesium based metal matrix composites and its improvement in mechanical and tribological properties—A Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 012133–012140. [Google Scholar] [CrossRef]
- Chawla, K.K. Metal Matrix matrix composite. In Composite Materials: Science and Engeering, 3rd ed.; Chawla, K.K., Ed.; Springer: Birmingham, AL, USA, 2012; pp. 197–248. [Google Scholar] [CrossRef]
- Haghshenas, M. Metal-matrix composites. Ref. Mod. Mater. Sci. Mater. Eng. 2016, 03950–03953. [Google Scholar] [CrossRef]
- Nie, K.B.; Deng, K.X.; Wang, X.J.; Gan, W.M.; Xu, F.J.; Wu, K.; Zheng, M.Y. Microstructures and mechanical properties of SiCp/AZ91 magnesium matrix nanocomposites processed by multidirectional forging. J. Alloys Comp. 2015, 622, 1018–1026. [Google Scholar] [CrossRef]
- Jiang, Q.C.; Wang, H.Y.; Ma, B.X.; Wang, Y.; Zhao, F. Fabrication of B4C particulate reinforced magnesium matrix composite by powder metallurgy. J. Alloys Comp. 2005, 386, 177–181. [Google Scholar] [CrossRef]
- Moll, F.; Kainer, K.U. Particle-Reinforced Magnesium Alloys. In Magnesium—Alloys and Technology; Kainer, K.U., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 197–217. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Development of high performance magnesium nano-composites using nano-Al2O3 as reinforcement. Mater. Sci. Eng. A 2005, 392, 163–168. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Properties and deformation behaviour of Mg-Y2O3 nanocomposites. Acta Mater. 2007, 55, 5115–5121. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Nai, M.H.; Almajid, A.; Gupta, M. Development of high performance Mg-TiO2 nanocomposites targeting for biomedical/structural applications. Mater Des. 2015, 65, 104–114. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Seetharaman, S.; Gupta, M. Enhancing overall tensile and compressive response of pure Mg using nano-TiB2 particulates. Mater. Character. 2014, 94, 178–188. [Google Scholar] [CrossRef]
- Paramsothy, M.; Chan, J.; Kwok, R.; Gupta, M. Nitride nanoparticle addition to beneficially reinforce hybrid magnesium alloys. Metall. Mater. Trans. A 2013, 44, 1123–1138. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Habibi, M.K.; Jayalakshmi, S.; Jia Ai, K.; Almajida, A.; Gupta, M. Nano-AlN particle reinforced Mg composites: Microstructural and mechanical properties. Mater. Sci. Technol. 2015, 31, 1122–1131. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Nai, M.H.; Almajid, A.; Gupta, M. Reinforcing low-volume fraction nano-TiN particulates to monolithical, pure Mg for enhanced tensile and compressive response. Materials 2016, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Choi, H.; Oportus, J.; Konishi, H. Li, X. Study on tensile properties and microstructure of cast AZ91D/AlN nanocomposites. Mater. Sci. Eng. A 2008, 494, 127–131. [Google Scholar] [CrossRef]
- Khrustalyov, A.P.; Garkushin, G.V.; Zhukov, I.A.; Razorenov, S.V.; Vorozhtsov, A.B. Quasi-static and plate impact loading of cast magnesium alloy ML5 reinforced with aluminum nitride nanoparticles. Metals 2019, 9, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zander, D.; Jiang, B.; Huang, Y.; Gavras, S.; Kainer, K.U.; Dieringa, H. Effects of heat treatment on the microstructural evolution and creep resistance of Elektron21 alloy and its nanocomposite. Mater. Sci. Eng. A 2020, 789, 139669–139682. [Google Scholar] [CrossRef]
- Yang, H.; Zander, D.; Huang, Y.; Kainer, K.U.; Dieringa, H. Individual/synergistic effects of Al and AlN on the microstructural evolution and creep resistance of Elektron21 alloy. Mater. Sci. Eng. A 2020, 777, 139072–139086. [Google Scholar] [CrossRef]
- Yang, H.; Huang, Y.; Tolnai, D.; Kainer, K.U.; Dieringa, H. Influences of Al and high shearing dispersion technique on the microstructure and creep resistance of Mg-2.85-Nd-0.92-Gd-0.41-Zr-0.29Zn alloy. Mater. Sci. Eng. A 2019, 764, 138215–138225. [Google Scholar] [CrossRef]
- Yang, H.; Huang, Y.; Gavras, S.; Kainer, K.U.; Hort, N.; Dieringa, H. Influences of AlN/Al Nanoparticles on the Creep Properties of Elektron21 Prepared by High Shear Dispersion Technology. JOM 2019, 71, 2245–2252. [Google Scholar] [CrossRef]
- Yang, H.; Huang, Y.; Song, B.; Kainer, K.U.; Dieringa, H. Enhancing the creep resistance of AlN/Al nanoparticles reinforced Mg-2.85Nd-0.92Gd-0.41Zr-0.29Zn alloy by a high shear dispersion technique. Mater. Sci. Eng. A 2019, 755, 18–27. [Google Scholar] [CrossRef]
- Giannopoulou, D.; Dieringa, H.; Bohlen, J. Influence of AlN nanoparticle addition on microstructure and mechanical properties of extruded pure magnesium and an aluminum-free Mg-Zn-Y alloy. Metals 2019, 9, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Dieringa, H. Properties of magnesium alloys reinforced with nanoparticles and carbon nanotubes: A review. J. Matr. Sci. 2011, 46, 289–306. [Google Scholar] [CrossRef]
- Wong, W.L.E.; Gupta, M. Enhancing thermal stability, modulus and ductility of magnesium using molybdenum as reinforcement. Adv. Eng. Mater. 2005, 7, 250–256. [Google Scholar] [CrossRef]
- Hassan, S.F.; Ho, K.F.; Gupta, M. Increasing elastic modulus, strength and CTE of AZ91 by reinforcing pure magnesium with elemental copper. Mater. Lett. 2004, 58, 2143–2146. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Development of ductile magnesium composite materials using titanium as reinforcement. J. Alloys Compd. 2002, 345, 246–251. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Development of a novel magnesium/nickel composite with improved mechanical properties. J. Alloys Compd. 2002, 335, L10–L15. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal matrix composites reinforced by nano-particles—A review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Malaki, M.; Xu, W.; Kasar, A.K.; Menezes, P.L.; Dieringa, H.; Varma, R.S.; Gupta, M. Advanced metal matrix nanocomposites. Metals 2019, 9, 330–368. [Google Scholar] [CrossRef] [Green Version]
- Nie, K.B.; Wang, X.J.; Deng, K.K.; Hu, X.S.; Wu, K. Magnesium matrix composite reinforced by nanoparticles—A review. J. Magnes. Alloys 2021, 15, 57–77. [Google Scholar] [CrossRef]
- Dieringa, H. Processing of magnesium-based metal matrix nanocomposites by ultrasound-assisted particle dispersion: A review. Metals 2018, 8, 431–447. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Soren, S. Selection of reinforcement for Al/Mg alloy metal matrix composites. Mater. Today Proc. 2020, 21, 1605–1609. [Google Scholar] [CrossRef]
- Kumari, S.S.; Pillai, U.; Pai, B. Synthesis and characterization of in situ Al–AlN composite by nitrogen gas bubbling method. J. Alloy. Comp. 2011, 509, 2503–2509. [Google Scholar] [CrossRef]
- Bruls, R.J.; Hintzen, H.T.; De With, G.; Metselaar, R. The temperature dependence of the Young’s modulus of MgSiN2, AlN and Si3N4. J. Eur. Ceram. Soc. 2001, 21, 263–268. [Google Scholar] [CrossRef]
- Toutanji, H.A.; Friel, D.; El-Korchi, T.; Katz, R.N.; Wechsler, G.; Rafaniello, W. Room temperature tensile and flexural strength of ceramics in AlN-SiC system. J. Eur. Ceram. Soc. 1995, 15, 425–434. [Google Scholar] [CrossRef]
- Chen, J.; Bao, C.-G.; Wang, Y.; Liu, J.-L.; Suryanarayana, C. Microstructure and lattice parameters of AlN particle-reinforced magnesium matrix composites fabricated by powder metallurgy. Acta Metall. Sin. 2015, 28, 1354–1363. [Google Scholar] [CrossRef] [Green Version]
- Bedolla, E.; Lemus-Ruiz, J.; Contreras, A. Synthesis and characterization of Mg-AZ91/AlN composites. Mater. Des. 2012, 38, 91–98. [Google Scholar] [CrossRef]
- Fu, H.M.; Zhang, M.-X.; Qiu, D.; Kelly, P.M.; Taylor, J.A. Grain refinement by AlN particles in Mg-Al based alloys. J. Alloys Comp. 2009, 478, 809–812. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dahle, A.K.; StJohn, D.H. Grain refinement of magnesium. In Essential Readings in Magnesium Technology, 1st ed.; Mathaudhu., S.N., Luo, A.A., Neelameggham, N.R., Nyberg, E.A., Sillekens, W.H., Eds.; Springer: Berlin, Germany, 2016; pp. 247–254. [Google Scholar] [CrossRef]
- Dieringa, H.; Katsarou, L.; Buzolin, R.; Szakács, G.; Horstmann, M.; Wolff, M.; Mendis, C.; Vorozhtsov, S.; StJohn, D. Ultrasound assisted casting of an AM60 based metal matrix nanocomposite, its properties, and recyclability. Metals 2017, 7, 388. [Google Scholar] [CrossRef] [Green Version]
- Saboori, A.; Padovano, E.; Pavese, M.; Badini, C. Novel magnesium Elektron21-AlN nanocomposites produced by ultrasound-assisted casting; microstructure, thermal and electrical conductivity. Materials 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, M.I.; Glazkova, E.A.; Lozhkomoev, A.S.; Svarovskaya, N.V.; Bakina, O.V.; Pervikov, A.V.; Psakhie, S.G. Synthesis of Al nanoparticles and Al/AlN composite nanoparticles by electrical explosion of aluminum wires in argon and nitrogen. Powder Technol. 2016, 295, 307–314. [Google Scholar] [CrossRef]
- Lerner, M.; Vorozhtsov, A.; Guseinov, S.; Storozhenko, P. Metal nanopowders production. In Metal Nanopowders: Production, Characterization, and Energetic Applications, 1st ed.; Gromov, A.A., Teipel, U., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2014; pp. 79–106. [Google Scholar]
- Feng, L.; Song, Y.-W.; Shan, D.-Y.; Han, E.-H. Corrosion behavior of AZ31 magnesium alloy in simulated acid rain solution. Tran. Nonferrous Metals Soc. China 2010, 20, s638–s642. [Google Scholar] [CrossRef]
- ASTM-NACE/ASTM G31-12a. Standard Guide for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar] [CrossRef]
- ASTM G1-03. Standard Practice for Preparing, Cleaning and Evaluating Corrosion Test Specimens; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- ASTM G199-09. Standard Guide for Electrochemical Noise Measurement; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- Dawson, J.L. Electrochemical noise measurement: The definitive in-situ technique for corrosion applications. In Electrochemical Noise Measurement for Corrosion Applications; Kearns, J., Scully, J., Roberge, P., Reichert, D., Dawson, J.L., Eds.; ASTM STP 1277; ASTM International: West Conshohocken, PA, USA, 1996; pp. 3–35. [Google Scholar] [CrossRef]
- Xia, D.-H.; Song, S.-Z.; Behnamian, Y. Detection of corrosion degradation using electrochemical noise (EN): Review of signal processing methods for identifying corrosion forms. Corros. Eng. Sci. Techn. 2016, 51, 527–544. [Google Scholar] [CrossRef]
- Somekawa, H.; Mukai, T. Effect of grain refinement on fracture toughness in extruded pure magnesium. Scr. Mater. 2005, 53, 1059–1064. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, Y.S.; Lee, J.H. Effect of grain refinement of magnesium alloy AZ31 by severe plastic deformation on material characteristics. J. Mater. Process. Techn. 2008, 201, 436–440. [Google Scholar] [CrossRef]
- Mukai, T.; Yamanoi, M.; Watanabe, H.; Ishikawa, K.; Higashi, K. Effect of grain refinement on tensile ductility in ZK60 magnesium alloy under dynamic loading. Mater. Trans. 2001, 42, 1177–1181. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.; Qian, M.; StJohn, D.H. Effect of iron on grain refinement of high-purity Mg-Al alloys. Scr. Mater. 2004, 51, 125–129. [Google Scholar] [CrossRef]
- ASM International. Binary alloy diagrams. In ASM Handbook. Alloy Phase Diagrams, 10th ed.; ASM International: Materials Park, OH, USA, 1992; Volume 3, p. 48. [Google Scholar]
- Bowen, P.; Highfield, J.G.; Mocellin, A.; Ring, T.A. Degradation of aluminum nitride powder in an aqueous environment. J. Am. Ceram. Soc. 1990, 73, 724–728. [Google Scholar] [CrossRef]
- Haynes, W.M. Properties of the elements and inorganic compounds. In CRC Handbook of Chemistry and Physics, 97th ed.; Haynes, W.M., Ed.; CRC Press Technology: Boca Raton, FL, USA, 2017; pp. 4–71. [Google Scholar]
- Svedberg, L.M.; Arndt, K.C.; Cima, M.J. Corrosion of aluminum nitride (AlN) in aqueous cleaning solutions. J. Am. Ceram. Soc. 2000, 83, 41–46. [Google Scholar] [CrossRef]
- Krnel, K.; Kosmač, T. Reactivity of aluminum nitride powder in dilute inorganic acids. J. Am. Ceram. Soc. 2000, 83, 1375–1378. [Google Scholar] [CrossRef]
- Lunder, O.; Nordien, J.H.; Nisancioglu, K. Corrosion resistance of cast Mg-Al alloys. Corross Rev. 1997, 15, 439–470. [Google Scholar] [CrossRef]
- Davoodi, A.; Pan, J.; Leygraf, C.; Norgren, S. The role of intermetallic particles in localized corrosion of aluminium alloy studied by SKPFM and integrated AFM/SECM. J. Electrochem. Soc. 2008, 155, C211–C218. [Google Scholar] [CrossRef] [Green Version]
- Pawar, S.; Zhou, X.; Thompson, G.E.; Scamans, G.; Fan, Z. The role of intermetallics on corrosion initiation of twin roll cast AZ31 Mg Alloy. J. Electrochem. Soc. 2015, 162, C442–C448. [Google Scholar] [CrossRef]
- Asmussen, R.M.; Binns, W.J.; Parfov-Nia, R.; Jakupi, P.; Shoesmith, D.W. The stability of aluminium-manganese intermetallic phases under the microgalvanic coupling conditions. Mater. Corros. 2016, 67, 39–50. [Google Scholar] [CrossRef]
- Santamaria, M.; Di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Fournier, V.; Marcus, P.; Olefjord, I. Oxidation of magnesium. Surf. Inter. Anal. 2002, 34, 494–497. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Physical Electronics Inc.: Chanhassen, MN, USA, 1992. [Google Scholar]
- Liao, J.; Hotta, M. Corrosion products of field-exposed Mg-Al series magnesium alloys. Corros. Sci. 2016, 112, 276–288. [Google Scholar] [CrossRef]
- Cottis, R.A. Interpretation of electrochemical noise data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]
- Bertocci, U.; Gabrielli, C.; Huet, F.; Keddam, M. Noise resistance applied to corrosion measurements I. theoretical analysis. J. Electrochem. Soc. 1997, 144, 31–37. [Google Scholar] [CrossRef]
- Bertocci, U.; Gabrielli, C.; Huet, F.; Keddam, M.; Rousseau, P. Noise resistance applied to corrosion measurements ii. experimental tests. J. Electrochem. Soc. 1997, 144, 37–43. [Google Scholar] [CrossRef]
- Sanchez-Amaya, J.M.; Cottis, R.A.; Botana, F.J. Shot noise and statistical parameters for the estimation of corrosion mechanisms. Corros Sci. 2005, 47, 3280–3299. [Google Scholar] [CrossRef]
- Acosta, G.; Mena-Morcillo, E.; Veleva, L. Electrochemical assesment of the Mg-Zn-Ca alloy degradation in Hanks’ physiological solution. Rev. Metal. 2020, 56, e181. [Google Scholar] [CrossRef]
- Eke, A.; Herman, P.; Bassingthwaighte, J.; Raymond, G.; Percival, D.; Cannon, M.; Balla, I.; Ikrényi, C. Physiological time series: Distinguishing fractal noises from motions. Pflügers Archiv. 2000, 439, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Delignieres, D.; Ramdani, S.; Lemoine, L.; Torre, K.; Fortes, M.; Ninot, G. Fractal analyses for ‘short’ time series: A re-assessment of classical methods. J. Math. Psychol. 2006, 50, 525–544. [Google Scholar] [CrossRef]
- Malamud, B.D.; Turcotte, D.L. Self-affine time series: Measures of weak and strong persistence. J. Stat. Plan. Inference 1999, 80, 173–196. [Google Scholar] [CrossRef]
- Dinodi, N.; Shetty, A.N. Alkyl carboxylates as efficient and green inhibitors of magnesium alloy ZE41 corrosion in aqueous salt solution. Corros. Sci. 2014, 85, 411–427. [Google Scholar] [CrossRef]
- Coy, A.E.; Viejo, F.; Garcia-Garcia, F.J.; Liu, Z.; Skeldon, P.; Thompson, G.E. Effect of excimer laser surface melting on the microstructure and corrosion performance of the die cast AZ91D magnesium alloy. Corros. Sci. 2010, 52, 387–397. [Google Scholar] [CrossRef]
- Galicia, G.; Pébère, N.; Tribollet, B.; Vivier, V. Local and global electrochemical impedances applied to the corrosion behaviour of an AZ91 magnesium alloy. Corros. Sci. 2009, 51, 1789–1794. [Google Scholar] [CrossRef] [Green Version]
- Ardelean, H.; Frateur, I.; Zanna, S.; Atrens, A.; Marcus, P. Corrosion protection of AZ91 magnesium alloy by anodizing in niobium and zirconium-containing electrolytes. Corros. Sci. 2009, 51, 3030–3038. [Google Scholar] [CrossRef]
- Baril, G.; Pebere, N. The corrosion of pure magnesium in aerated and deaerated sodium sulphate solutions. Corros. Sci. 2001, 43, 471–484. [Google Scholar] [CrossRef]
- Baril, G.; Blanc, C.; Pébère, N. AC impedance spectroscopy in characterizing time-dependent corrosion of AZ91 and AM50 magnesium alloys characterization with respect to their microstructures. J. Electrochem. Soc. 2001, 148, B489–B496. [Google Scholar] [CrossRef]
- Baril, G.; Galicia, G.; Deslouis, C.; Pébère, N.; Tribollet, B.; Vivier, V. An impedance investigation of the mechanism of pure magnesium corrosion in sodium sulfate solutions. J. Electrochem. Soc. 2006, 154, C108–C113. [Google Scholar] [CrossRef] [Green Version]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
- Feliu, S., Jr. Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: Brief review and challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Souza, J.S.D.; Oliveira, L.A.D.; Sayeg, I.J.; Antunes, R.A. Electrochemical study of the AISI 409 ferritic stainless steel: Passive film stability and pitting nucleation and growth. Mater. Res. 2017, 20, 1669–1680. [Google Scholar] [CrossRef] [Green Version]
- Bacelis, Á.; Veleva, L.; Feliu, S.; Cabrini, M.; Lorenzi, S. Corrosion Activity of Carbon Steel B450C and Low Chromium Ferritic Stainless Steel 430 in Cement Extract Solution. Buildings 2021, 11, 220. [Google Scholar] [CrossRef]



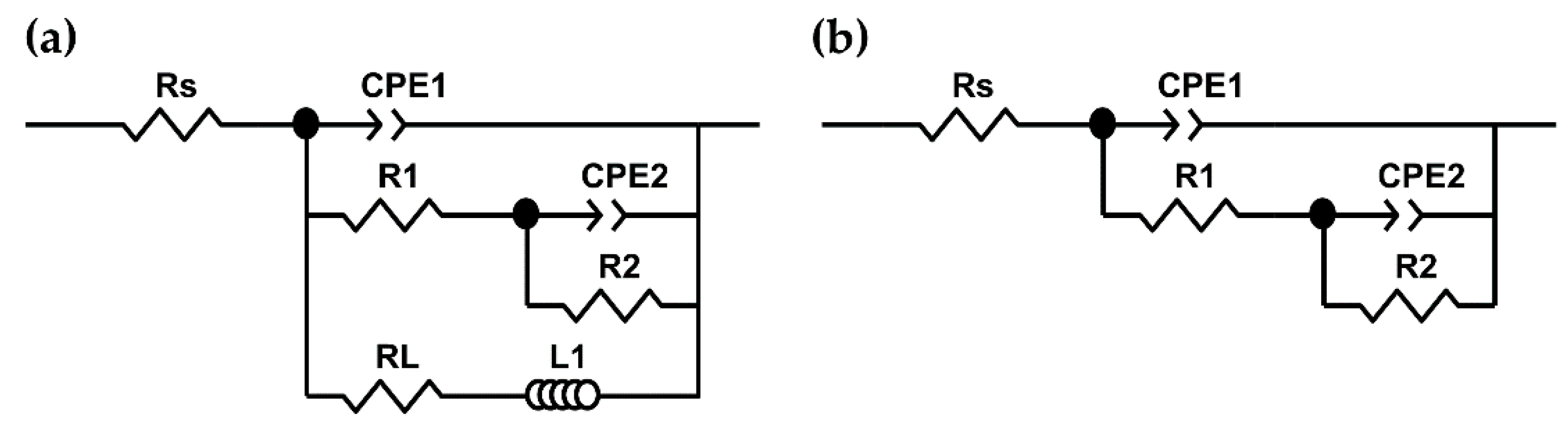
| Material | AM60-AlN | AM60 |
|---|---|---|
| Vickers hardness (HVs-longitudinal section) | 71.0 ± 1.5 | 66.1 ± 3.9 |
| Vickers hardness (HVs-cross section) | 64.0 ± 1.8 | 68.0 ± 3.6 |
| Grain size-longitudinal section (µm) | 2.9 ± 0.1 | 3.3 ± 1.1 |
| Grain size-cross-section (µm) | 2.9 ± 0.4 | 3.3 ± 0.2 |
| Zone | C | N | O | Mg | Al | Mn |
|---|---|---|---|---|---|---|
| 1 | - | - | 3.6 | 90.1 | 6.3 | - |
| 2 | 4.6 | - | 5.3 | 7.6 | 32.4 | 50.1 |
| 3 | - | 2.5 | 5.4 | 38.5 | 26.9 | 26.7 |
| 4 | - | 1.2 | 64.4 | 32.9 | - |
| Zone | C | O | Mg | Al | Si | Mn |
|---|---|---|---|---|---|---|
| 1 | 6.1 | 35.9 | 18.6 | 13.3 | - | 26.1 |
| 2 | 4.6 | 33.0 | 56.3 | 5.8 | 0.2 | - |
| 3 | 6.3 | 63.2 | 25.8 | 4.7 | - | - |
| OCP (V vs. SCE) | ||||||
|---|---|---|---|---|---|---|
| Time (Days) | Initial | 1 | 7 | 10 | 15 | 30 |
| AM60-AlN | −1.59 ± 0.07 | −1.55 ± 0.04 | −1.49 ± 0.02 | −1.50 ± 0.03 | −1.50 ± 0.02 | −1.49 ± 0.01 |
| AM60 | −1.59 ± 0.09 | −1.56 ± 0.02 | −1.52 ± 0.02 | −1.52 ± 0.01 | −1.52 ± 0.01 | −1.53 ± 0.01 |
| Rn (kΩ cm−2) | ||||||
|---|---|---|---|---|---|---|
| Time (Days) | Initial | 1 | 7 | 10 | 15 | 30 |
| AM60-AlN | 1.81 | 2.23 | 2.58 | 3.70 | 2.40 | 0.80 |
| AM60 | 2.74 | 1.02 | 1.41 | 0.90 | 0.95 | 0.37 |
| Circuit Elements | 1 h | 24 h | 15 Days |
|---|---|---|---|
| Rs (kΩ) | 0.56 ± 0.01 | 0.62 ± 0.01 | 0.66 ± 0.01 |
| CPE1 (µsn/Ω cm2) | 7.95 ± 0.66 | 20.20 ± 0.12 | 49.5 ± 3.53 |
| n1 | 0.91 ± 0.02 | 0.90 ± 0.01 | 0.87 ± 0.01 |
| R1 (kΩ cm2) | 2.44 ± 0.08 | 3.78 ± 0.09 | 11.34 ± 0.38 |
| CPE2 (msn/Ω cm2) | 0.89 ± 0.11 | 1.58 ± 0.28 | 0.58± 0.10 |
| n2 | 0.67 ± 0.10 | 0.95 ± 0.09 | 0.72 ± 0.01 |
| R2 (kΩ cm2) | 2.15 ± 0.41 | 1.31 ± 0.16 | 6.39 ± 0.27 |
| L1 (kH cm2) | 408.0 ± 1.5 | - | - |
| RL (kΩ cm2) | 0.17 ± 0.01 | - | - |
| Rp (kΩ cm2) | 0.16 | 5.09 | 17.73 |
| Circuit Elements | 1 h | 24 h | 15 Days |
|---|---|---|---|
| Rs (kΩ) | 0.53 ± 0.01 | 0.53 ± 0.01 | 0.67 ± 0.01 |
| CPE1 (µsn/Ω cm2) | 9.24 ± 0.61 | 27.5 ± 1.17 | 63.87 ± 1.31 |
| n1 | 0.91 ± 0.01 | 0.89 ± 0.01 | 0.84 ± 0.01 |
| R1 (kΩ cm2) | 2.10 ± 0.03 | 3.75 ± 0.08 | 12.71 ± 0.28 |
| CPE2 (msn/Ω cm2) | 0.98 ± 0.19 | 1.77 ± 0.39 | 1.71 ± 0.61 |
| n2 | 0.71 ± 0.07 | 1.00 ± 0.10 | 1.00 ± 0.14 |
| R2 (kΩ cm2) | 1.57 ± 0.07 | 1.15 ± 0.14 | 3.82 ± 0.73 |
| L1 (kH cm2) | 266 ± 6.2 | - | - |
| RL (kΩ cm2) | 0.42 ± 0.06 | - | - |
| Rp (kΩ cm2) | 0.37 | 4.90 | 16.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez, L.; Veleva, L.; Feliu, S., Jr.; Giannopoulou, D.; Dieringa, H. Corrosion Behavior of Extruded AM60-AlN Metal Matrix Nanocomposite and AM60 Alloy Exposed to Simulated Acid Rain Environment. Metals 2021, 11, 990. https://doi.org/10.3390/met11060990
Chávez L, Veleva L, Feliu S Jr., Giannopoulou D, Dieringa H. Corrosion Behavior of Extruded AM60-AlN Metal Matrix Nanocomposite and AM60 Alloy Exposed to Simulated Acid Rain Environment. Metals. 2021; 11(6):990. https://doi.org/10.3390/met11060990
Chicago/Turabian StyleChávez, Luis, Lucien Veleva, Sebastián Feliu, Jr., Danai Giannopoulou, and Hajo Dieringa. 2021. "Corrosion Behavior of Extruded AM60-AlN Metal Matrix Nanocomposite and AM60 Alloy Exposed to Simulated Acid Rain Environment" Metals 11, no. 6: 990. https://doi.org/10.3390/met11060990







