Effect of Dislocation Slip Mechanism under the Control of Oxygen Concentration in Alpha-Case on Strength and Ductility of TC4 Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
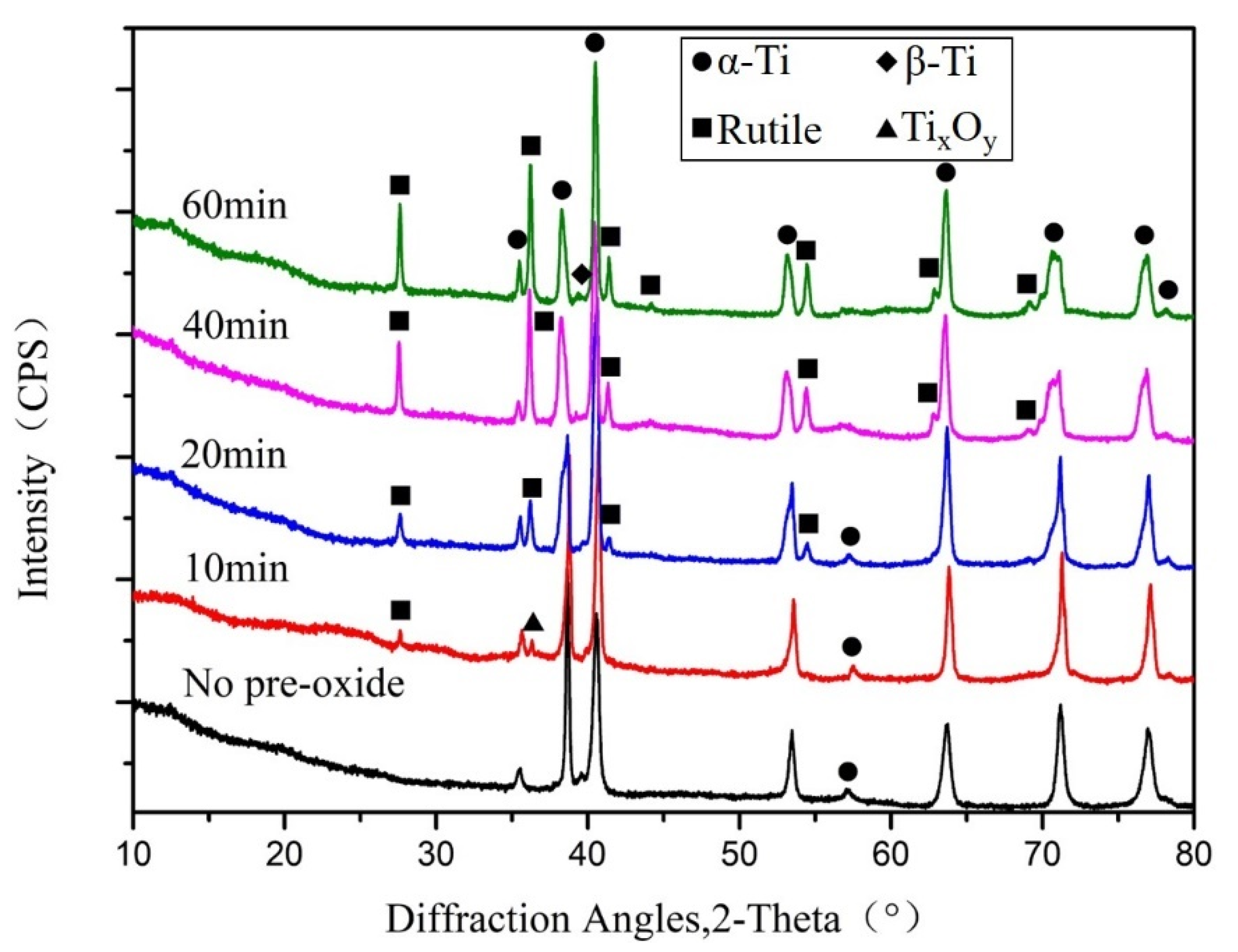
3.1. Phase Composition of TC4 Alloy Surface After Pre-Oxidation
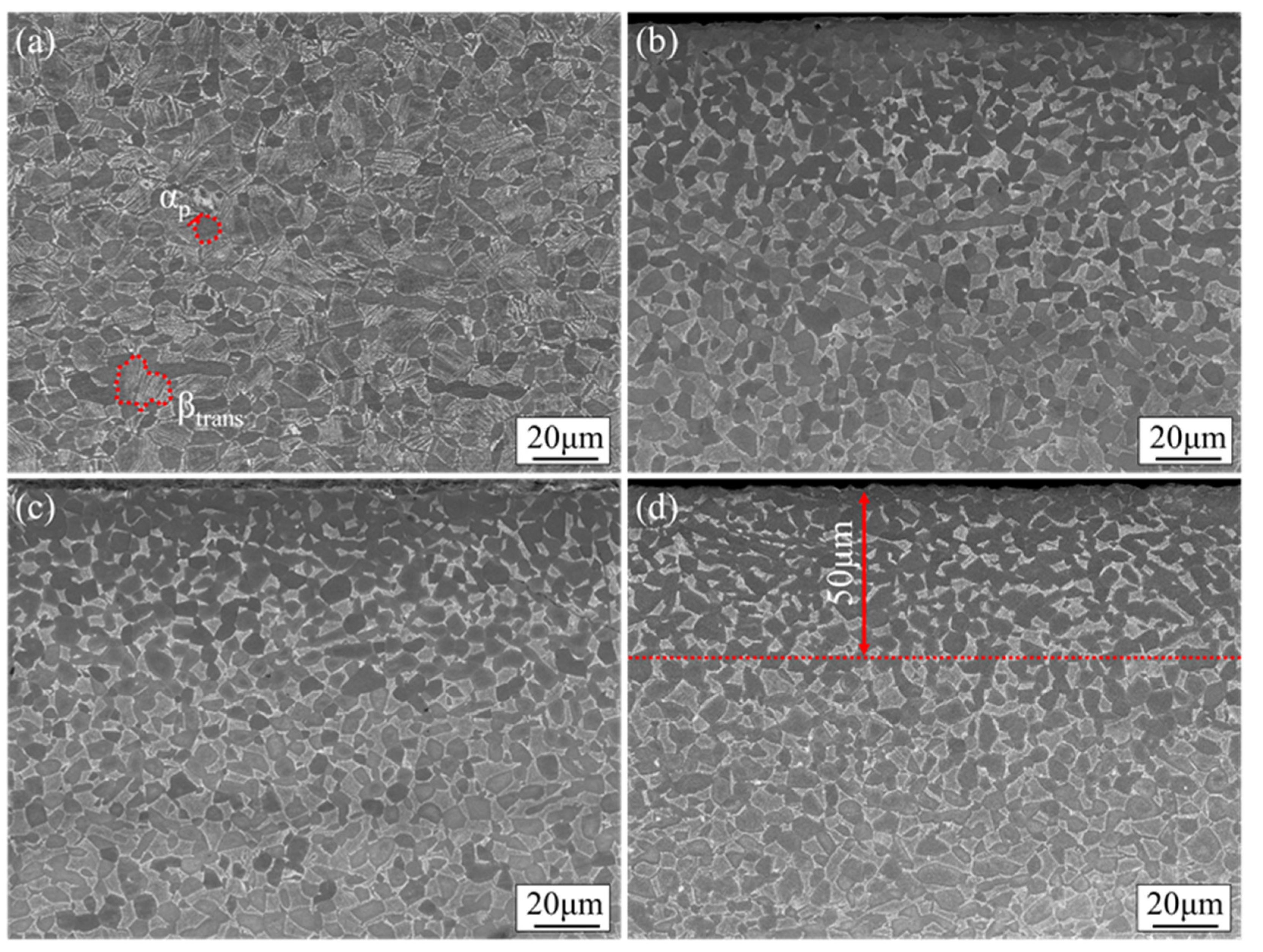
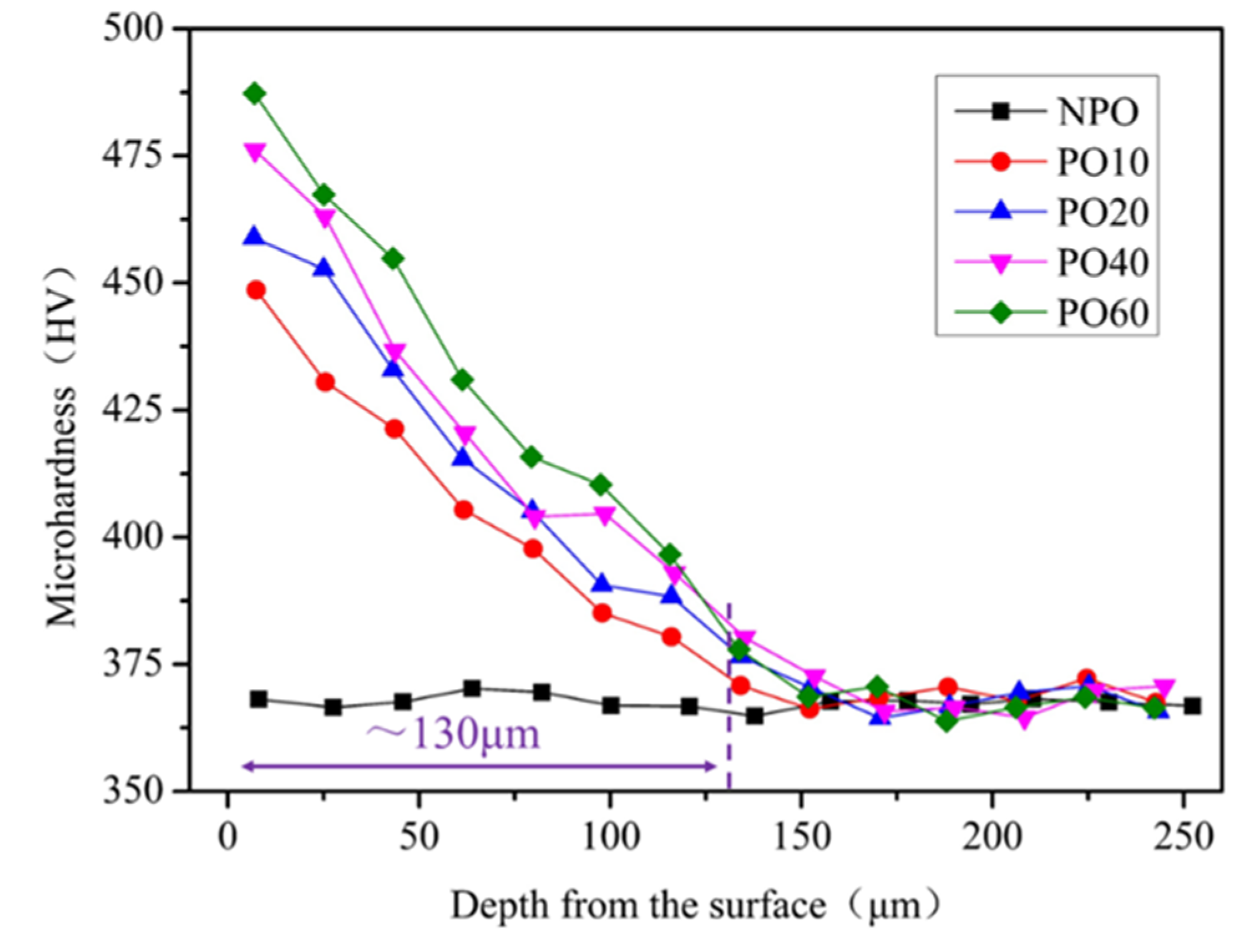
3.2. Microstructure, Microhardness, and Distribution of Surface Layer Oxygen and Main Alloying Elements after Diffusion and Aging Heat Treatment
3.3. Tensile Properties
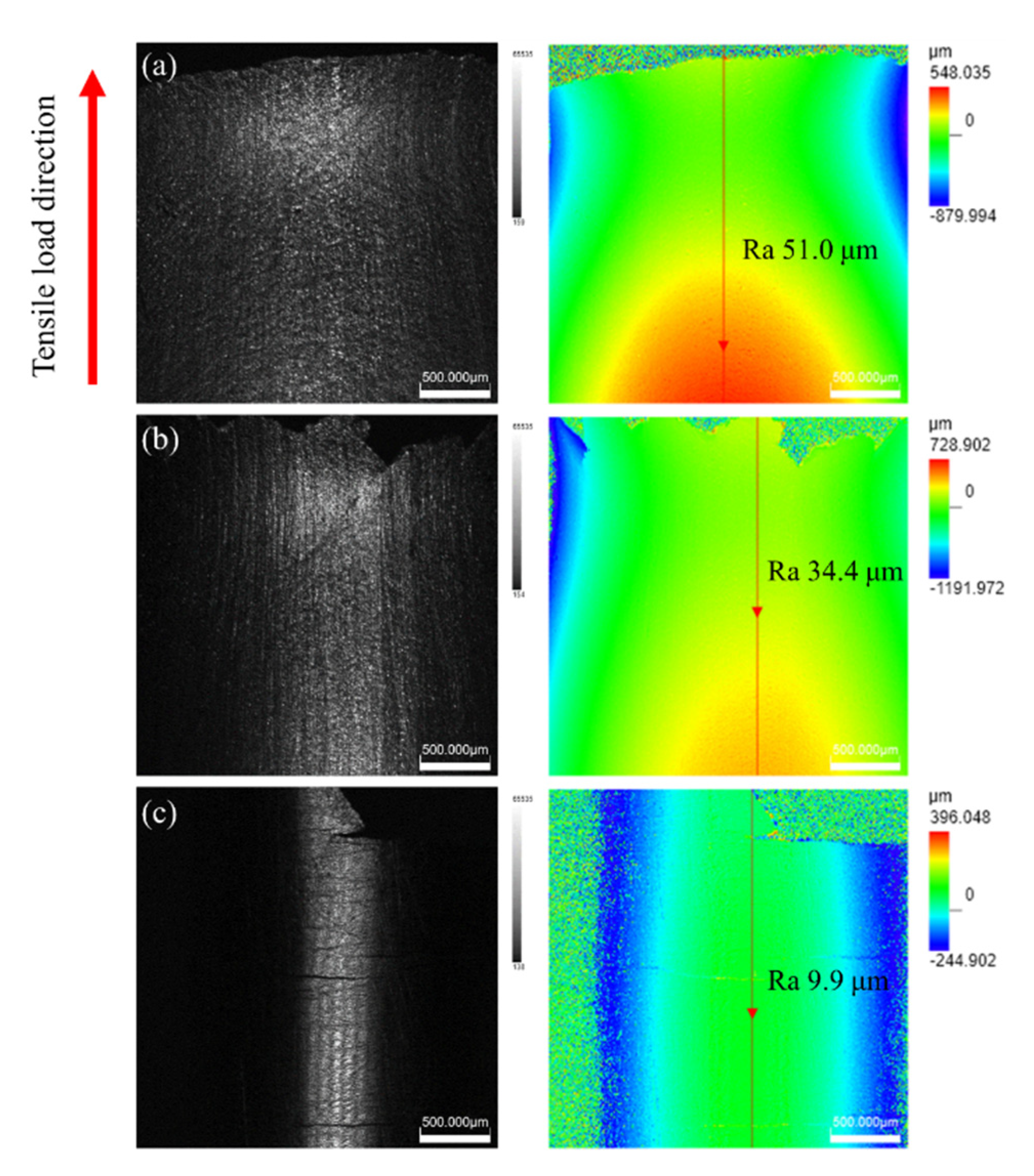
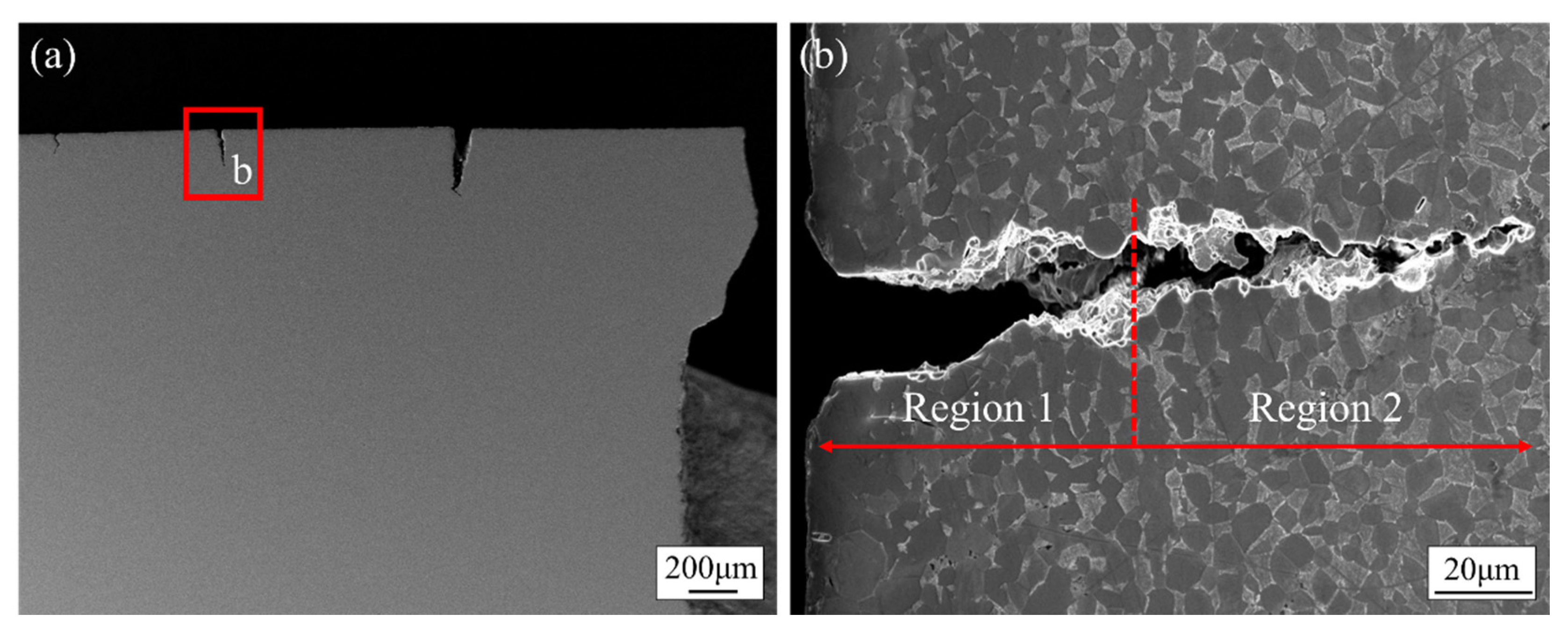
3.4. Fracture Behavior of TC4 Alloy
4. Discussion
4.1. Formation of Alpha-Case under Different Oxygen Diffusion Processes
4.2. Effect of Alpha-Case on Strength and Ductility of TC4 Alloy
4.3. Evolution of the Slip Mechanism in the Alpha-Case
5. Conclusions
- After the rolled TC4 alloy is treated by oxygen diffusion process, the surface layer forms a typical alpha-case gradient structure in which the volume fraction of αp phase gradually decreases and the volume fraction of βtrans gradually increases. The oxygen concentration and microhardness of the alpha-case increases with an increase in pre-oxidation time.
- Relative to the tensile properties of TC4 alloy without alpha-case, the ductility of TC4 alloy with the alpha-case is decreased. Moreover, the ductility decreases with an increase in oxygen concentration. Ductility value is the lowest on PO60 specimen, with EI and RA values being 3.6% and 5.5%, respectively. The strength first increases and then decreases, while the highest strength is observed on PO20 specimen with lower oxygen concentration. In this state, UTS and YS increase from 1085 MPa and 970 MPa without the alpha-case specimens to 1155 MPa and 1003 MPa, respectively.
- When the oxygen concentration is low, the alpha-case still exhibits a certain degree of plasticity, and the crack sensitivity is low. Moreover, the alpha-case strengthened by the oxygen in solid solution contributed to a certain extent to the macroscopic strength. More specifically, it improves the strength of the TC4 alloy. When the oxygen concentration is high, the alpha-case turns into a brittle layer, and the crack sensitivity is increased. As a result, cracks start to initiate and expand rapidly for a relatively small amount of deformation. Furthermore, the bearing area of the specimen is reduced, which results in a decrease in strength and plasticity of TC4 alloy.
- An increase in the amount of solid solution of oxygen in the alpha-case, which makes the slip highly localized, resulting in the slip shift from the wavy form to the planar form. This, in turn, causes cracks in the alpha-case to initiate at a small amount of strain, and leads to embrittlement of the alpha-case. At the same time, narrow and strong planar slip is restricted in front of the crack tip, which provides a strong driving force for the crack propagation. This leads to rapid crack growth, which does not favorably affect the strength and ductility of TC4 alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, M.; Kumpfert, J.; Ward, C.H.; Leyens, C. Titanium Alloys for Aerospace Applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Application; Wiley-VCH: Köln, Germany, 2003. [Google Scholar]
- Lütjering, G.G.; Williams, J.C. Titanium, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Conrad, H. Effect of interstitial solutes on the strength and ductility of titanium. Prog. Mater. Sci. 1981, 26, 123–403. [Google Scholar] [CrossRef]
- Kant, A.; Strauss, B. Dissociation Energies of Diatomic Molecules of the Transition Elements. II. Titanium, Chromium, Manganese, and Cobalt. J. Chem. Phys. 1964, 41, 3806–3808. [Google Scholar] [CrossRef]
- Andreeva, V.V. Behavior and Nature of Thin Oxide Films on Some Metals in Gaseous Media and in Electrolyte Solutions. Corrosion 1964, 20, 35t–46t. [Google Scholar] [CrossRef]
- Gaddam, R.; Sefer, B.; Pederson, R.; Antti, M. Oxidation and alpha-case formation in Ti–6Al–2Sn–4Zr–2Mo alloy. Mater. Charact. 2015, 99, 166–174. [Google Scholar] [CrossRef]
- Moiseyev, V.N. Titanium Alloys: Russian Aircraft and Aerospace Applications. Metallovedenie i termicheskaya obrabotka metallov. 2006, 8, 48. [Google Scholar]
- Chamorro, X.; Herrero-Dorca, N.; Rodríguez, P.P.; Andrés, U.; Azpilgain, Z. α-Case formation in Ti-6Al-4V investment casting using ZrSiO4 and Al2O3 moulds. J. Mater. Process. Technol. 2017, 243, 75–81. [Google Scholar] [CrossRef]
- Gurrappa, I. An oxidation model for predicting the life of titanium alloy components in gas turbine engines. J. Alloys Compd. 2005, 389, 190–197. [Google Scholar] [CrossRef]
- Jia, Y.; Pan, R.; Zhang, P.; Sun, Z.; Chen, X.; Zhang, X.; Wu, X. Enhanced surface strengthening of titanium treated by combined surface deep-rolling and oxygen boost diffusion technique. Corros. Sci. 2019, 157, 256–267. [Google Scholar] [CrossRef]
- Satko, D.P.; Shaffer, J.B.; Tiley, J.S.; Semiatin, S.L.; Pilchak, A.L.; Kalidindi, S.R.; Kosaka, Y.; Glavicic, M.G.; Salem, A.A. Effect of microstructure on oxygen rich layer evolution and its impact on fatigue life during high-temperature application of α/β titanium. Acta Mater. 2016, 107, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Davies, P.; Pederson, R.; Coleman, M.; Birosca, S. The hierarchy of microstructure parameters affecting the tensile ductility in centrifugally cast and forged Ti-834 alloy during high temperature exposure in air. Acta Mater. 2016, 117, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Rosa, C.J. Oxygen Diffusion in Alpha and Beta Titanium in the Temperature Range of 932 to 1142 C. Metall. Mater. Trans. B 1970, 1, 2517–2522. [Google Scholar]
- Bache, M.R.; Evans, W.J.; Davies, H.M. Electron back scattered diffraction (EBSD) analysis of quasi-cleavage and hydrogen induced fractures under cyclic and dwell loading in titanium alloys. J. Mater. Sci. 1997, 32, 3435–3442. [Google Scholar] [CrossRef]
- Mahoney, M.W.; Paton, N.E. Fatigue and fracture characteristics of silicon-bearing titanium alloys. Metall. Trans. A 1978, 9, 1497–1501. [Google Scholar] [CrossRef]
- Gaddam, R.; Antti, M.; Pederson, R. Influence of alpha-case layer on the low cycle fatigue properties of Ti-6Al-2Sn-4Zr-2Mo alloy. Mater. Sci. Eng. A 2014, 599, 51–56. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Porter, W.J.; Boone, S.; John, R.; Martin, P. Life prediction under tension of titanium alloys that develop an oxygenated brittle case during use. Scripta Mater. 2011, 65, 420–423. [Google Scholar] [CrossRef]
- Chan, K.S.; Chan, K.S.; Koike, M.; Koike, M.; Johnson, B.W.; Johnson, B.W.; Okabe, T.; Okabe, T. Modeling of Alpha-Case Formation and Its Effects on the Mechanical Properties of Titanium Alloy Castings. Metall. Mater. Trans. A 2008, 39, 171–180. [Google Scholar] [CrossRef]
- Jia, W.; Zeng, W.; Liu, J.; Zhou, Y.; Wang, Q. Influence of thermal exposure on the tensile properties and microstructures of Ti60 titanium alloy. Mater. Sci. Eng. A 2011, 530, 511–518. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M.; Weinem, D.; Kaysser, W.A. Influence of Long-Term Annealing on Tensile Properties and Fracture of Near-a Titanium Alloy Ti-6AI-2.75Sn-4Zr-0.4Mo-0.45Si. Metall. Mater. Trans. A 1996, 27, 1709–1717. [Google Scholar] [CrossRef]
- Naydenkin, E.V.; Mishin, I.P.; Ratochka, I.V.; Lykova, O.N.; Zabudchenko, O.V. The effect of alpha-case formation on plastic deformation and fracture of near β titanium alloy. Mater. Sci. Eng. A 2020, 769, 138495. [Google Scholar] [CrossRef]
- Madsen, A.; Ghonem, H. Effects of aging on the tensile and fatigue behavior of the near-α Ti-1100 at room temperature and 593 °C. Mater. Sci. Eng. A 1994, 177, 63–73. [Google Scholar] [CrossRef]
- Dobeson, R.; Petrazoller, N.; Dargusch, M.; McDonald, S. Effect of thermal exposure on the room temperature tensile properties of Grade 2 titanium. Mater. Sci. Eng. A 2011, 528, 3925–3929. [Google Scholar] [CrossRef]
- Simbi, D.J.; Scully, J.C. The effect of residual interstitial elements and iron on mechanical properties of commercially pure titanium. Mater. Lett. 1996, 26, 35–39. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Dong, H.; Bell, T.; Xu, B. The effect of treatment condition on boost diffusion of thermally oxidised titanium alloy. J. Alloy. Compd. 2007, 431, 93–99. [Google Scholar] [CrossRef]
- Dong, H.; Li, X.Y. Oxygen boost diffusion for the deep-case hardening of titanium alloys. Mater. Sci. Eng. A 2000, 280, 303–310. [Google Scholar] [CrossRef]
- Yan, M.; Dargusch, M.S.; Ebel, T.; Qian, M. A transmission electron microscopy and three-dimensional atom probe study of the oxygen-induced fine microstructural features in as-sintered Ti–6Al–4V and their impacts on ductility. Acta Mater. 2014, 68, 196–206. [Google Scholar] [CrossRef]
- Zabler, S. Interstitial Oxygen diffusion hardening—A practical route for the surface protection of titanium. Mater. Charact. 2011, 62, 1205–1213. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Z.; Dong, H.; Bell, T. The computer simulation of the boost diffusion oxidation process for the deep-case hardening of titanium alloys. J. Phys. IV 2004, 120, 259–268. [Google Scholar]
- Pilchak, A.L.; Porter, W.J.; John, R. Room temperature fracture processes of a near-α titanium alloy following elevated temperature exposure. J. Mater. Sci. 2012, 47, 7235–7253. [Google Scholar] [CrossRef]
- Chong, Y.; Bhattacharjee, T.; Park, M.; Shibata, A.; Tsuji, N. Factors determining room temperature mechanical properties of bimodal microstructures in Ti-6Al-4V alloy. Mater. Sci. Eng. A 2018, 730, 217–222. [Google Scholar] [CrossRef]
- Williams, J.C.; Sommer, A.W.; Tung, P.P. The influence of oxygen concentration on the internal stress and dislocation arrangements in α titanium. Metall. Trans. 1972, 3, 2979–2984. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, Z.; Qiu, C.; Qiu, C.; Venkatesh, V.; Venkatesh, V.; Fraser, H.L.; Fraser, H.L.; Williams, R.E.A.; Williams, R.E.A.; et al. The Influence of Precipitation of Alpha2 on Properties and Microstructure in TIMETAL 6-4. Metall. Mater. Trans. A 2013, 44, 1706–1713. [Google Scholar] [CrossRef]
- Bagot, P.A.J.; Radecka, A.; Magyar, A.P.; Gong, Y.; Bell, D.C.; Smith, G.D.W.; Moody, M.P.; Dye, D.; Rugg, D. The effect of oxidation on the subsurface microstructure of a Ti-6Al-4V alloy. Scripta Mater. 2018, 148, 24–28. [Google Scholar] [CrossRef]
- Marcinkowski, M.J. The Relationship between Atomic Order and the Mechanical Properties of Alloys. Treatise Mater. Sci. Technol. 1974, 5, 181–287. [Google Scholar]
- Gysler, A.; Weissmann, S. Effect of order in Ti3Al particles and of temperature on the deformation behavior of age-hardened TiAl alloys. Mater. Sci. Eng. 1977, 27, 181–193. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Qin, J.; Chen, Y.; Lu, W.; Zhang, D. The effect of heat treatment on thermal stability of Ti matrix composite. J. Alloy. Compd. 2011, 509, 52–56. [Google Scholar] [CrossRef]
- Boonchuduang, T.; Bootchanont, A.; Klysubun, W.; Amonpattaratkit, P.; Khamkongkaeo, A.; Puncreobutr, C.; Yimnirun, R.; Lohwongwatana, B. Formation of Alpha-Case Layer During Investment Casting of Pure Ti and Ti-6Al-4V Using Comparative XRD and EXAFS Investigation. Metall. Mater. Trans. 2020, 51, 586–596. [Google Scholar] [CrossRef]
- Montanari, R.; Costanza, G.; Tata, M.E.; Testani, C. Lattice expansion of Ti–6Al–4V by nitrogen and oxygen absorption. Mater. Charact. 2008, 59, 334–337. [Google Scholar] [CrossRef] [Green Version]
- Kwasniak, P.; Garbacz, H.; Kurzydlowski, K.J. Solid solution strengthening of hexagonal titanium alloys: Restoring forces and stacking faults calculated from first principles. Acta Mater. 2016, 102, 304–314. [Google Scholar] [CrossRef]





| Al | V | Fe | C | O | N | H | Ti |
|---|---|---|---|---|---|---|---|
| 5.97 | 4.03 | 0.04 | 0.012 | 0.11 | 0.0052 | 0.0023 | Bal. |
| Yield Strength /MPa | Tensile Strength /MPa | Elongation /% | Percentage of Area Reduction /% |
|---|---|---|---|
| 899 | 1039 | 14.0 | 50.3 |
| Specimen Name | Step I (Pre-Oxidation) | Step II (Diffusion) | Step III (Aging) | |||
|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Temperature (°C) | Time (h) | Temperature (°C) | Time (h) | |
| NPO | - | 930 | 2 | 500 | 6 | |
| PO10 | 700 | 10 | ||||
| PO20 | 20 | |||||
| PO40 | 40 | |||||
| PO60 | 60 | |||||
| Specimen | NPO | PO10 | PO20 | PO40 | PO60 |
|---|---|---|---|---|---|
| αp (Vol.%) | 45.7 | 55.6 | 58.8 | 62.5 | 65.4 |
| βtrans (Vol.%) | 54.3 | 44.4 | 41.2 | 37.5 | 34.6 |
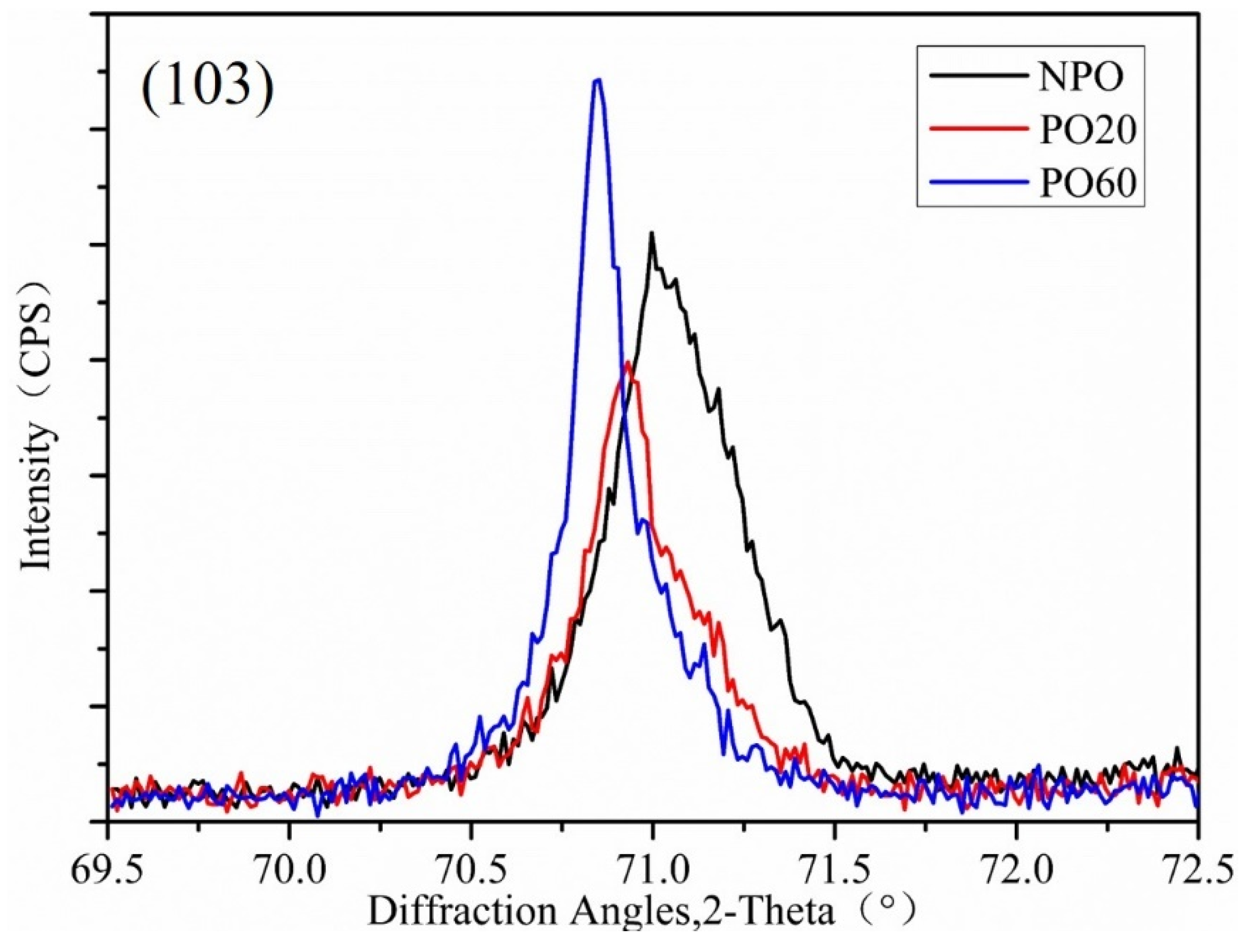
| Specimen | Lattice Parameter (Å) | |
|---|---|---|
| a | c | |
| NPO | 2.94757 | 4.66054 |
| PO20 | 2.94759 | 4.67616 |
| PO60 | 2.94982 | 4.68729 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Liang, Y.; Sun, H.; Wang, S. Effect of Dislocation Slip Mechanism under the Control of Oxygen Concentration in Alpha-Case on Strength and Ductility of TC4 Alloy. Metals 2021, 11, 1057. https://doi.org/10.3390/met11071057
Feng X, Liang Y, Sun H, Wang S. Effect of Dislocation Slip Mechanism under the Control of Oxygen Concentration in Alpha-Case on Strength and Ductility of TC4 Alloy. Metals. 2021; 11(7):1057. https://doi.org/10.3390/met11071057
Chicago/Turabian StyleFeng, Xin, Yilong Liang, Hao Sun, and Shu Wang. 2021. "Effect of Dislocation Slip Mechanism under the Control of Oxygen Concentration in Alpha-Case on Strength and Ductility of TC4 Alloy" Metals 11, no. 7: 1057. https://doi.org/10.3390/met11071057





