Studies on the Formation and Processing of Aluminium Dross with Particular Focus on Special Metals
Abstract
:1. Introduction
1.1. Formation and Composition of Aluminium Dross
- Formation of the oxide layer on the melt surface.
- Breaking of the oxide skin by bath movement.
- Sinking and floating of some oxide particles.
- Adhesion of the oxide particles.
- Filling of the cavities with metallic aluminium (capillary action).
- Oxidation of the metallic aluminium finely dispersed in the dross.
- Skimming.
- Processing of the dross outside the furnace.
1.2. Processing of Aluminium Dross
1.3. Special Metals in the Aluminium Recycling Process
2. Materials and Methods
2.1. Origin of the Feedstock
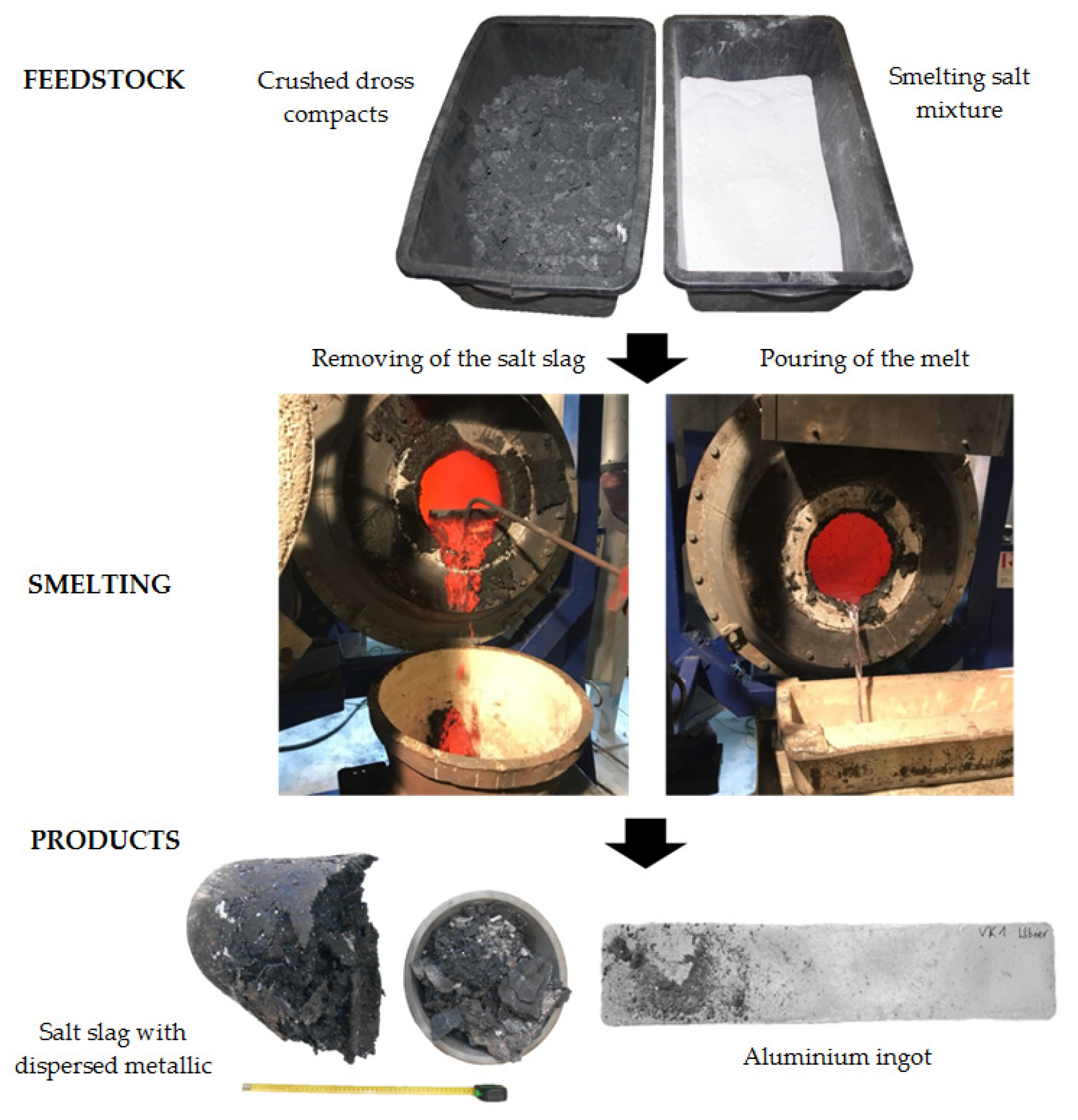
2.2. Pyrometallurgical Treatment of the Dross in the TBRC
- Flow rate of combustion air or fuel gas during melting operation: 3–8 m3/h (for the heating process: 11 m3/h).
- Stoichiometric combustion air ratio: λ = 1
- Setpoint temperature inside the furnace: approximately 750 °C (adjustable via flow rate).
- Kiln tilt angle: approximately 95°.
- Number of revolutions of the furnace vessel: 1 min−1.
3. Results
4. Discussion
5. Conclusions
- Within the framework of this research, a comprehensive study was conducted on the formation and further processing of aluminium dross.
- In the experimental part, dross formed at an Austrian aluminium smelter was processed in the TBRC with the addition of smelting salt, and the proportion of metallic aluminium contained within was determined. The TBRC corresponded to a tilting drum furnace, which is usually used in secondary aluminium metallurgy for the remelting of dross. The result showed that a 75.7% metal yield could be obtained without taking the proportion of metallic Al in the salt slag into account. Compared to the current processing of the regarded dross, which is carried out externally and results in metal yields of 61–62%, a significant increase and therefore an enormous potential for economic savings are possible.
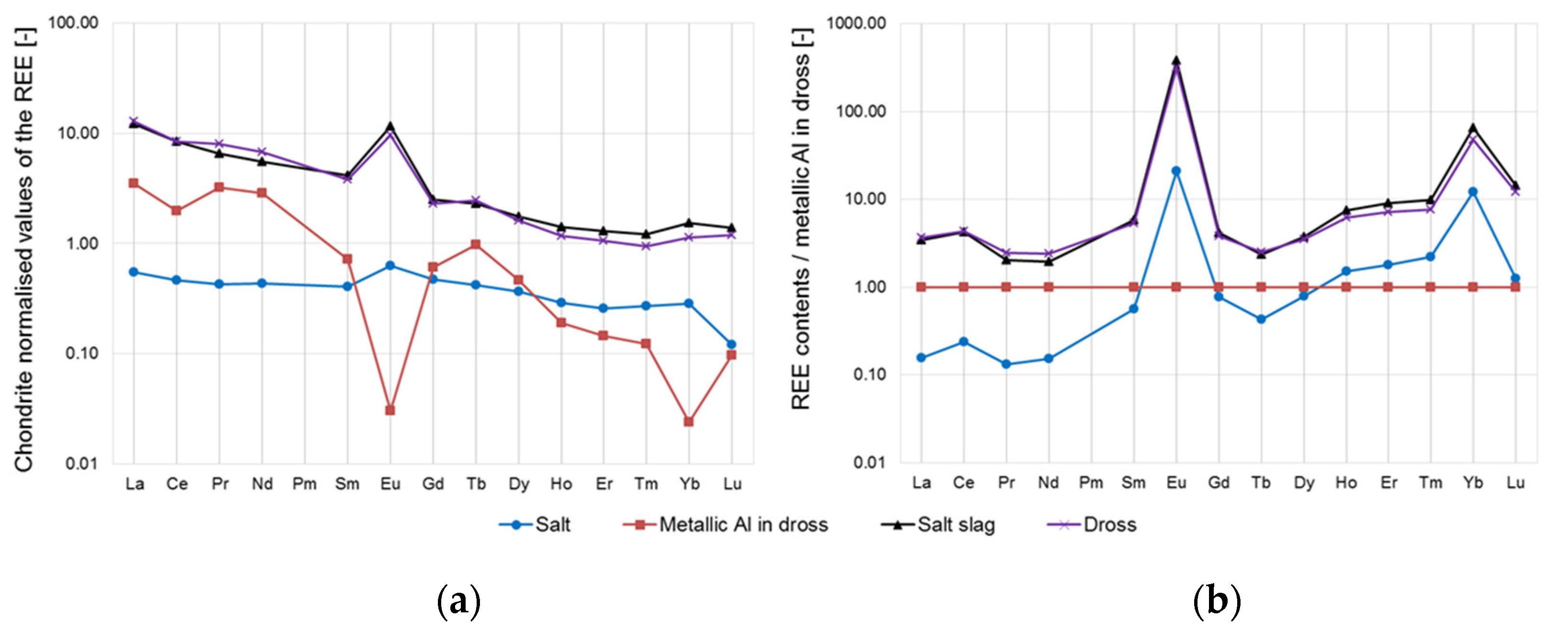
- Another important part of this research was the investigation of the content of rare earths in the phases resulting from the smelting process, the salt slag, and the metallic aluminium. The REE mass fractions in the input materials, the dross, and the smelting salt mixture used were also determined. It was found that the majority of the REEs directly passed from the dross into the salt slag. Already during the remelting of aluminium scrap in the two-chamber furnace, the rare earths preferentially accumulated in the dross, whereby they mainly entered the melting process via the scrap in the form of their oxides. The oxidation state did not change during the transition from the dross to the salt slag, which could be demonstrated, in particular, by the constant europium anomaly. The behaviour of special metals, such as rare earths, in aluminium recycling still requires comprehensive research and will become more important in the future, since these elements will also increasingly enter the aluminium recycling cycle due to their growing usage.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Bureau of Metal Statistics. Metal Statistics 2009–2019, 107th ed.; World Bureau of Metal Statistics: Ware, UK, 2019. [Google Scholar]
- Krone, K. Aluminiumrecycling; Aluminium-Verlag: Düsseldorf, Germany, 2000. [Google Scholar]
- Becker, E. Reststoffe des Aluminium-Recyclings. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 1993. [Google Scholar]
- Rao, S.R. Resource Recovery and Recycling from Metallurgical Wastes; Elsevier: Amsterdam, The Netherlands; London, UK, 2006; Volume 7. [Google Scholar]
- Antrekowitsch, H.; Paulitsch, H.; Pirker, A. Reststoffe aus der Aluminium-Sekundärindustrie. In Proceedings of the der Berliner Schlackenkonferenz, Berlin, Germany, 24 September 2013; pp. 615–638. [Google Scholar]
- Zauner, J. Konzept zur Verringerung des Aluminiumgehaltes der Krätze und zur Verwertung bzw. Entsorgung des verbleibenden Rückstandes. Master’s Thesis, University of Leoben, Leoben, Austria, 2000. [Google Scholar]
- Manfredi, O.; Wuth, W.; Bohlinger, I. Characterizing the Physical and Chemical Properties of Aluminum Dross. JOM 1997, 49, 48–51. [Google Scholar] [CrossRef]
- Schmitz, C. Handbook of Aluminium Recycling; Vulkan-Verlag: Essen, Germany, 2006. [Google Scholar]
- Alfaro, I. Technische und wirtschaftliche Gesichtspunkte bei der Entstehung und der Verarbeitung von Aluminiumkrätze. Aluminium 1986, 62, 259–266. [Google Scholar]
- Manfredi, O. Beitrag zur Charakterisierung von Aluminiumkrätzen. Ph.D. Thesis, Technical University of Berlin, Berlin, Germany, 1997. [Google Scholar]
- Kvithyld, A.; Meskers, C.E.M.; Gaal, S.; Reuter, M.; Engh, T.A. Recycling Light Metals: Optimal Thermal De-coating. JOM 2008, 60, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Feige, R.; Merker, G. SEROX—Ein synthetischer Al-Glasrohstoff. Presentation, Sitzung des Fachausschusses III “Glasrohstoffe und Glasschmelze”; Würzburg, Germany, 2006. Available online: https://docplayer.org/16234564-Serox-ein-synthetischer-al-glasrohstoff.html (accessed on 10 July 2021).
- King, R.C.; Armstrong, G.T. Heat of Combustion and Heat of Formation of Aluminum Carbide. J. Res. Natl. Bur. Standards. Sect. A Phys. Chem. 1964, 68A, 661–668. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Kopac, J. Aluminum recovery as a product with high added value using aluminum hazardous waste. J. Hazard. Mater. 2013, 261, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. State of the art in aluminum recycling from aluminum dross. Light Metals 2006, 2006, 931–936. [Google Scholar]
- Prillhofer, R.; Prillhofer, B.; Antrekowitsch, H. Verwertung von Reststoffen beim Aluminium-Recycling. BHM Berg Hüttenmännische Mon. 2008, 153, 103–108. [Google Scholar] [CrossRef]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross. Resour. Conserv. Recycl. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Ünlü, N.; Drouet, M.G. Comparison of salt-free aluminum dross treatment processes. Resour. Conserv. Recycl. 2002, 36, 61–72. [Google Scholar] [CrossRef]
- Geochemie der Seltenen Erden. Available online: https://www.agw.kit.edu/downloads/Studiengang/Geochemie%20der%20Seltenen%20Erden%20(Stosch,%208%20MB).pdf (accessed on 13 February 2021).
- Lohmann, D. Kampf um Seltene Erden—Hightech-Rohstoffe als Mangelware. In Im Fokus: Bodenschätze; Lohmann, D., Podbregar, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 7–15. [Google Scholar]
- Untersuchung zu Seltenen Erden: Permanentmagnete im industriellen Einsatz in Baden-Württemberg. Available online: http://ressourcenfieber.net/publications/reports/SelteneErden_PM_BaWue_Report_2014_de.pdf (accessed on 4 October 2020).
- Study on Rare Earths and Their Recycling. Available online: http://www.ressourcenfieber.eu/publications/reports/Rare%20earths%20study_Oeko-Institut_Jan%202011.pdf (accessed on 4 October 2020).
- Luidold, S.; Antrekowitsch, H. Gewinnung von Technologiemetallen aus alternativen Rohstoffquellen. BHM Berg Hüttenmännische Mon. 2012, 157, 32–37. [Google Scholar] [CrossRef]
- Wasson, J.T.; Kallemeyn, G.W. Compositions of chondrites. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1988, 325, 535–544. [Google Scholar]
- Bendel, V. Volatilitätskontrollierte Fraktionierung refraktär-lithophiler Elemente in Meteoriten und der Erde. Ph.D. Thesis, Georg-August-University Göttingen, Göttingen, Germany, 2013. [Google Scholar]
- Rare-Earth-Element (REE) Behaviour in the Strange Lake Intrusion, Labrador: Resource Estimation Using Predictive Methods. Available online: https://www.gov.nl.ca/iet/files/mines-geoscience-publications-currentresearch-2013-kerr-2013.pdf (accessed on 20 January 2021).
- Adler, B.; Müller, R. Seltene Erdmetalle; Univ.-Verlag Ilmenau: Ilmenau, Germany, 2014; Volume 10. [Google Scholar]
- Recherche zur Rohstoffgruppe der Seltenen Erden. Available online: http://www.reuse-computer.org/fileadmin/user_upload/documents/Artikel/UM_Recherche_Seltenerdmetalle.pdf (accessed on 4 October 2020).
- Bokhari, S.N.H.; Meisel, T.C. Method Development and Optimisation of Sodium Peroxide Sintering for Geological Samples. Geostand. Geoanalytical Res. 2016, 41, 181–195. [Google Scholar] [CrossRef]
- Houk, R.S.; Fassel, V.A.; Flesch, G.D.; Svec, H.J.; Gray, A.L.; Taylor, C.E. Inductively coupled argon plasma as an ion source for mass spectrometric determination of trace elements. Anal. Chem. 1980, 52, 2283–2289. [Google Scholar] [CrossRef] [Green Version]
- Wibner, S. Verhalten Aluminiumhaltiger Reststoffe und Minderwertiger Aluminiumschrotte beim Recycling Unter Berücksichtigung von Sondermetallen. Ph.D. Thesis, University of Leoben, Leoben, Austria, 2021. [Google Scholar]
- Deady, E.; Mouchos, E.; Goodenough, K.; Williamson, B.; Wall, F. Rare Earth Elements in Karst-Bauxites: A Novel Untapped European Resource? In Proceedings of the ERES2014: 1st European Rare Earth Resources Conference, Milos, Greece, 4–7 September 2014; pp. 1–12. [Google Scholar]



| Element | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 1.57 | 0.236 | 0.616 | 0.0929 | 0.457 | 0.149 | 0.056 | 0.197 | 0.0355 | 0.245 | 0.0546 | 0.166 | 0.0242 | 0.159 | 0.0245 |
| Designation | Fractions | Fractions of the Salt Slag | Mass [kg] |
|---|---|---|---|
| Feedstock | Dross | 35.00 | |
| Smelting salt mixture | 25.00 | ||
| Sum input | 60.00 | ||
| Products | Aluminium ingot | 26.48 | |
| Salt slag | Salt slag (including metallic Al and Fe) | 32.21 | |
| Metallic Al > 1 mm | 1.59 | ||
| Metallic Al 0.5–1 mm | 0.48 | ||
| Fe in salt slag | 0.16 | ||
| Salt slag (metallic Al and Fe deducted) | 29.98 | ||
| Sum Al output | 28.55 | ||
| Sum output | 58.69 | ||
| Difference (sum input − sum output) | 1.31 | ||
| Metal yield I [%] (sum Al output/dross) | 81.6 | ||
| Metal yield II [%] (aluminium ingot/dross) | 75.7 | ||
| REE | Dross | Metallic Al (Ingot) | Salt | Salt Slag |
|---|---|---|---|---|
| Sc | <0.5 | 0.18 | <1 | <1 |
| Y | 3.31 | 0.28 | 1.10 | 4.51 |
| La | 3.02 | 0.83 | 0.13 | 2.85 |
| Ce | 5.18 | 1.21 | 0.29 | 5.13 |
| Pr | 0.73 | 0.30 | 0.040 | 0.61 |
| Nd | 3.08 | 1.29 | 0.20 | 2.53 |
| Pm | - | - | - | - |
| Sm | 0.57 | 0.11 | 0.060 | 0.61 |
| Eu | 0.53 | 0.002 | 0.035 | 0.65 |
| Gd | 0.45 | 0.12 | 0.092 | 0.49 |
| Tb | 0.087 | 0.035 | 0.015 | 0.081 |
| Dy | 0.39 | 0.11 | 0.089 | 0.43 |
| Ho | 0.063 | 0.010 | 0.016 | 0.077 |
| Er | 0.17 | 0.024 | 0.043 | 0.22 |
| Tm | 0.023 | 0.003 | 0.007 | 0.029 |
| Yb | 0.18 | 0.004 | 0.045 | 0.24 |
| Lu | 0.029 | 0.002 | 0.003 | 0.034 |
| Σ SEE | 17.8 | 4.5 | 2.2 | 18.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wibner, S.; Antrekowitsch, H.; Meisel, T.C. Studies on the Formation and Processing of Aluminium Dross with Particular Focus on Special Metals. Metals 2021, 11, 1108. https://doi.org/10.3390/met11071108
Wibner S, Antrekowitsch H, Meisel TC. Studies on the Formation and Processing of Aluminium Dross with Particular Focus on Special Metals. Metals. 2021; 11(7):1108. https://doi.org/10.3390/met11071108
Chicago/Turabian StyleWibner, Stefan, Helmut Antrekowitsch, and Thomas C. Meisel. 2021. "Studies on the Formation and Processing of Aluminium Dross with Particular Focus on Special Metals" Metals 11, no. 7: 1108. https://doi.org/10.3390/met11071108
APA StyleWibner, S., Antrekowitsch, H., & Meisel, T. C. (2021). Studies on the Formation and Processing of Aluminium Dross with Particular Focus on Special Metals. Metals, 11(7), 1108. https://doi.org/10.3390/met11071108






