Manganese (Mn) is one of the 12 most abundant elements (it comprises approximately 0.1% of the earth’s crust), and can be associated in different ways, finding oxides, sulfides, carbonates, and silicates with greater abundance. Besides, some recent studies mention that the largest manganese reserves in the world (high-grade deposits) are found on the seabed [
1]. At present, 95% of the manganese produced annually is being consumed by the steel industry, and the remaining 5% is being used by other industries, such as the chemical, paint, fertilizer, and battery industries [
2]. Manganese levels in the world range from 20% to 60%, but a significant proportion belongs to the low- and medium-grade (Mn: 20–35%) category [
3]. With the depletion of high-grade manganese resources, low-grade manganese is becoming more important.
Types of low-grade manganese include rhodochrosite, pyrolusite, alabandite, and so forth. When manganese is present in its bivalent soluble form, Mn, manganese salts are generally obtained directly by acid leaching. When manganese in ore is present in the form of manganese dioxide, since MnO
2 is stable in direct acid or alkali dissolution, the possible reduction of the insoluble tetravalent manganese to soluble bivalent manganese is necessary to recover manganese from its ores. In this regard, two processes are followed viz. reduction roasting followed by leaching and reductive leaching [
4]. Zhang et al. [
5] used chemical pure sulfur as a reducing agent to treat low-grade manganese oxide ore through reduction roasting and sulfuric acid leaching. The leaching efficiency was 95.6% for Mn, with a 550 °C roasting temperature, 10 min roasting time, 0.50 of S/Mn, 1.0 mol/L sulfuric acid concentration, 25 °C leaching temperature, 200 r/min stirring rate, 5 min of leaching time, and a 5:1 liquid-to-solid ratio. Alaoui et al. [
6] used potassium oxalate as a reducing agent in sulfuric acid medium to reduced and leached manganese in pyrolusite. The results show that when there is a sulfuric acid concentration of 0.25 to 1 M, oxalic acid concentration of 0.06, 0.09, 0.12, and 0.25 M, leaching temperature of 25 to 40 °C, and manganese ore particle size of −630, −280, −125, and −63 µm, the leaching efficiency of manganese increases with an increase of H
2SO
4 concentration, potassium oxalate concentration, leaching temperature, and decrease of particle size. Oxalic acid concentration has a great influence on Mn extraction, followed by temperature and sulfuric acid concentration. Prasetyo [
7] studied the leaching of manganese from low-grade manganese ore in sulfuric acid mediums with tannic acid as a reducing agent. The results show that the optimum conditions for Mn leaching are tannic acid 40 g/L, sulfuric acid 0.5 M, liquid-solid ratio 5 mL/g, room temperature stirring 6 h, and the leaching efficiency of Mn increases with the increase of tannic acid and sulfuric acid concentration. Lu et al. [
8] studied the process of manganese extraction from low-grade manganese oxide ore in sulfuric acid medium with formic acid as a reductant. The process with 15% H
2SO
4, 0.4 mL/g formic acid, a liquid-to-solid ratio of 6, and reaction at 90 °C for 2 h can result in a manganese leaching efficiency of up to 90.05%. It is known that rhodochrosite (MnCO
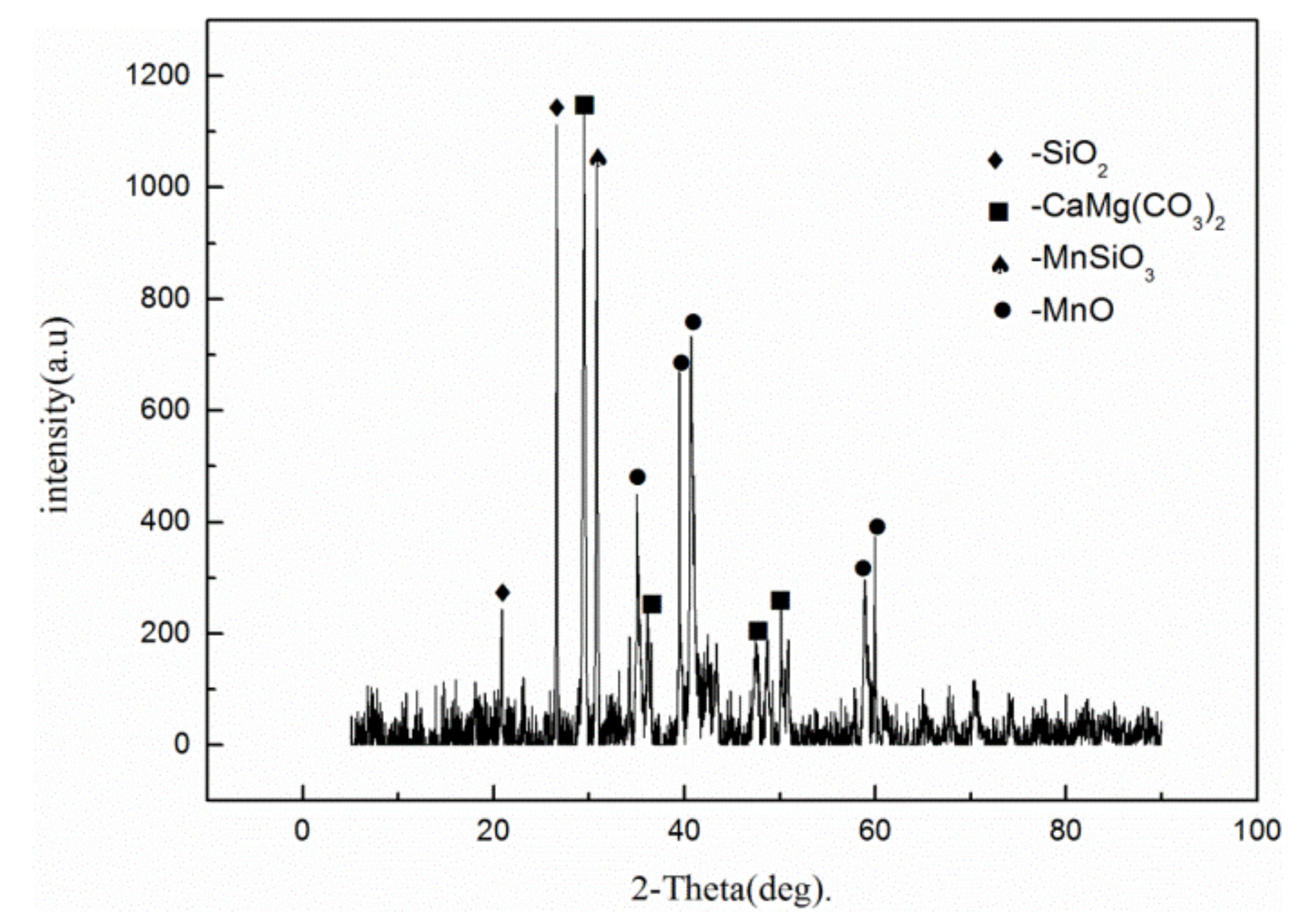
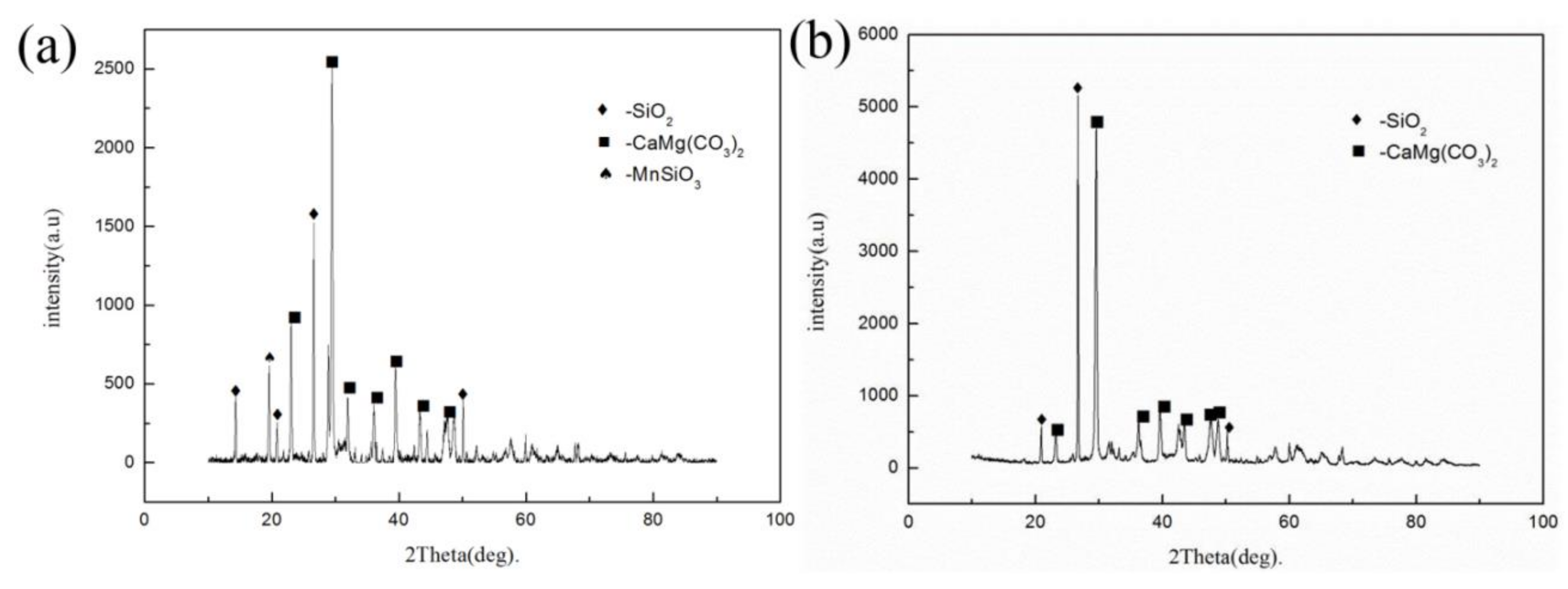
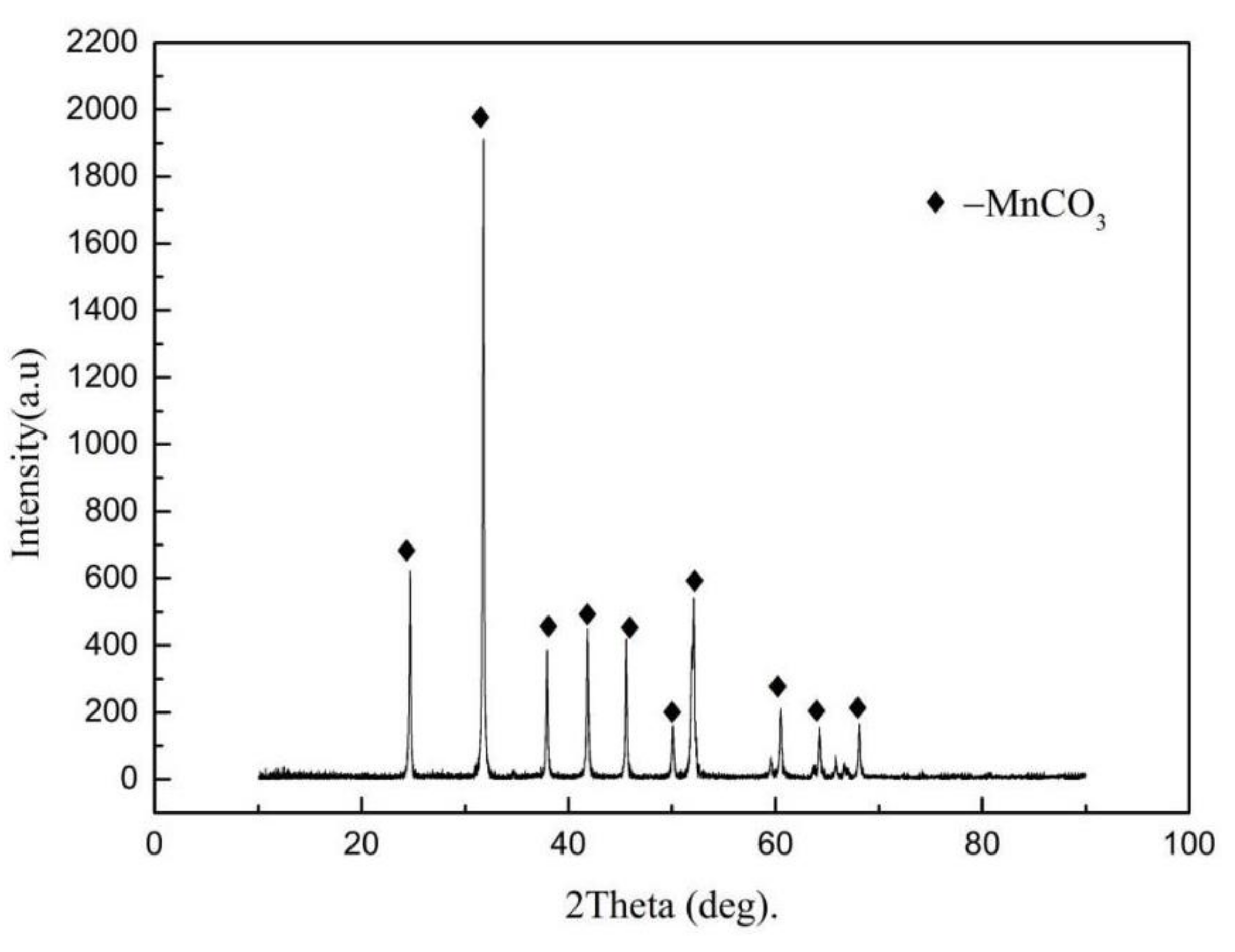
3) exhibits diamagnetic properties and has a magnetic susceptibility that is comparable with that of quartz or illite at room temperature. In addition, rhodochrosite can be converted to MnO by roasting. Thus, the treatment methods of low-grade rhodochrosite mainly include magnetic separation and acid leaching [
9,
10]. Although higher manganese leaching efficiency can be obtained by the above methods, there are some problems, such as high acid consumption, serious environmental pollution and equipment corrosion, and high impurity content. Compared with acid leaching for treating low-grade manganese ore, ammonia leaching makes manganese in manganese ore dissolve into manganese ammonia complexes during the ammonia leaching process, while impurity elements cannot dissolve in ammonia solution, which has good selectivity. Therefore, it is suitable for low-grade minerals with high impurity content, and the ammonia solution can be recycled without waste liquid.
The ammonia leaching method has been widely used in nickel, cobalt, zinc, copper minerals, or slag [
11,
12,
13]. Chen et al. [
14] used the reductive roasting-ammonia leaching method to extract nickel and cobalt from low-grade nickel-containing laterite ore. The leaching efficiency of nickel and cobalt reached 86.25% and 60.84%, respectively. At the same time, the leaching agent could also be recovered at normal temperature and pressure. Li et al. [
15] treated low-grade zinc oxide minerals by irradiation roasting and ammonia leaching processes, and the leaching efficiency of zinc was able to reach 88.3%. Bidari [
16] used the ammonia leaching method with ammonia and ammonium carbonate as leachables to extract 78% copper from copper smelter residue. It is thus concluded that ammonia leaching is well-suited for the treatment of low-grade minerals. At the same time, ammonia leaching has been regarded as one of the possible methods for manganese extraction by researchers because of its advantages, such as recycling of leaching agents, good selectivity to manganese, and less waste liquid production [
17].
There are also some reports about leaching of manganese by the ammonia leaching method, such as Chen’s [
18] study of manganese extraction from permanganite by the ammonia leaching method, which reached up to 59.9–64.8%. Chen et al. [
19] applied the ammonia leaching method to extract manganese from low-grade manganese, and the results showed that the ammonia to ammonium carbonate concentration ratio, leaching temperature, liquid-solid ratio and leaching time had significant effects on the leaching of manganese. The U.S. mineral administration [
20] studied the ammonia leaching method for recovery of manganese from flat furnace residue, showing that manganese is dissolved in the form of its amine complexes. Mcintosh [
21] studied the recovery of manganese from several BOF slags by the ammonia leaching method, showing that approximately 80% of manganese can be leached. From the current research situation, the research on ammonia leaching treatment of low-grade manganese ore is not deep enough. Our group [
22] employed the roasting ammonia leaching method to leach manganese from low-grade rhodochrosite, and the leaching efficiency of manganese could reach 85.6% under optimal conditions. Through the above studies, it was found that the ammonia leaching method has the deficiency of low manganese leaching efficiency. In order to solve this problem, this study aims to select an appropriate additive on the basis of previous studies to improve the leaching efficiency of manganese in the ammonia leaching process.
The addition of additives in the leaching process is an effective way to enhance the leaching efficiency of minerals, which has been confirmed by many studies. For example, Sun [
23] used Al
2(SO
4)
3 and Al
2O
3 as additives in the leaching of coal system goethite, and Zhou [
24] used lauryl alcohol as an additive in leaching potassium from phosphorus potassium-associated minerals, and found that the addition of additives enhanced the leaching of minerals equally well. Fluoride as an effective co-immersion agent can effectively improve the leaching efficiency of minerals [
25]. Zhang et al. [
26] used NH
4F as an additive for leaching vanadium from sulfuric acid, and it was concluded that fluoride ions can promote the dissolution of poorly soluble aluminosilicate minerals, such as mica. Govindaiah [
27] found that polytetrafluoroethylene is a promising additive for warm pressurised oxidative leaching in copper high sulfide, and the leaching efficiency and final extraction yield of copper can be significantly improved in the presence of polytetrafluoroethylene beads. Obviously, the addition of suitable additives in different mineral leaching processes can enhance the leaching efficiency of minerals. However, there is no report about adding additives in the leaching process of manganese by ammonia leaching.
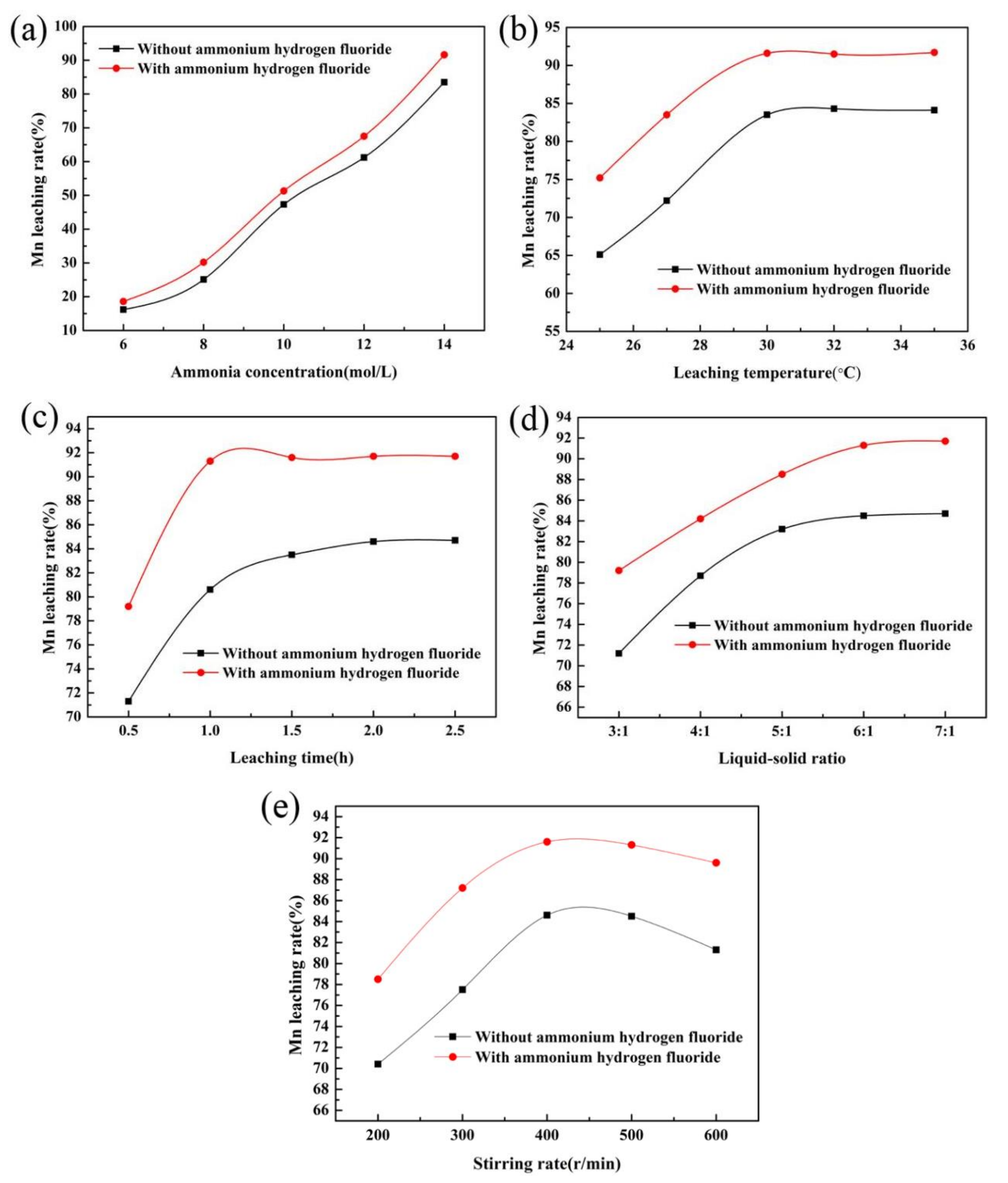
In order to improve the manganese leaching efficiency of low-grade rhodochrosite treated by ammonia leaching, in this paper, ammonium hydrogen fluoride was selected as an additive during ammonia leaching treatment, and the effects of ammonium hydrogen fluoride with or without an additive on the leaching efficiency of manganese were comparatively studied using a single-factor method. The ammonia leaching process with the addition of ammonium hydrogen fluoride was also optimized by response surface methodology.