Cs4PbBr6 Combined with Graphite as Anode for High-Performance Lithium Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of CQDs and CQDs/G
2.2. Electrochemical Evaluation
3. Result and Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Palacín, M.R.; de Guibert, A. Why do batteries fail? Science 2016, 351, 1253292. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. Energy storage materials: A perspective. Energy Storage Mater. 2015, 1, 158–161. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Qi, Z.; Wang, H.; Shalaginov, M.Y.; Goncalves, C.; Kang, M.; Richardson, K.A.; Guerrero-Sanchez, J.; Moreno-Armenta, M.G.; Pol, V.G. Ge2Sb2Se5 Glass as High-Capacity Promising Lithium-Ion Battery Anode. Nano Energy 2020, 68, 104326. [Google Scholar] [CrossRef]
- He, W.; Zhang, X.; Jin, C.; Wang, Y.; Mossin, S.; Yue, Y. Nano-glass ceramic cathodes for Li+/Na+ mixed-ion batteries. J. Power Sources 2017, 342, 717–725. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Yan, Q.; Yu, S.; He, X.; Chen, Y.; Zhang, R.; Ma, L.; Liu, T.; Li, M. A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 2020, 585, 63–67. [Google Scholar] [CrossRef]
- Poli, I.; Hintermair, U.; Regue, M.; Kumar, S.; Sackville, E.V.; Baker, J.; Watson, T.M.; Eslava, S.; Cameron, P.J. Graphite-protected CsPbBr3 perovskite photoanodes functionalised with water oxidation catalyst for oxygen evolution in water. Nat. Commun. 2019, 10, 2097. [Google Scholar] [CrossRef]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef]
- Lv, P.; Zhao, H.; Gao, C.; Du, Z.; Wang, J.; Liu, X. SiOx–C dual-phase glass for lithium ion battery anode with high capacity and stable cycling performance. J. Power Sources 2015, 274, 542–550. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Z.; Liu, J.; Yin, G.; Gu, M. PbO@C core–shell nanocomposites as an anode material of lithium-ion batteries. Electrochem. Commun. 2009, 11, 917–920. [Google Scholar] [CrossRef]
- Xie, W.; Cao, J.; Li, P.; Fan, M.; Xu, S.; Zhang, J. Heterogeneity-control-engineered glass anode containing perovskite quantum dots for high rate lithium-ion batteries. ACS Appl. Energy Mater. 2022, 5, 42–45. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. NPJ Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Vassiliev, S.Y.; Levin, E.E.; Nikitina, V.A. Kinetic analysis of lithium intercalating systems: Cyclic voltammetry. Electrochim. Acta 2016, 190, 1087–1099. [Google Scholar] [CrossRef]
- Tan, L.P.; Lu, Z.; Tan, H.T.; Zhu, J.; Rui, X.; Yan, Q.; Hng, H.H. Germanium nanowires-based carbon composite as anodes for lithium-ion batteries. J. Power Sources 2012, 206, 253–258. [Google Scholar] [CrossRef]
- Li, C.; Lu, X.; Ding, W.; Feng, L.; Gao, Y.; Guo, Z. Formability of ABX3 (X = F, Cl, Br, I) Halide perovskites. Acta Crystallogr. Sect. B Struct. Sci. 2008, 64, 702–707. [Google Scholar] [CrossRef]
- Cao, Y.; Hatchard, T.D.; Dunlap, R.A.; Obrovac, M.N. Mechanofusion-derived Si-alloy/graphite composite electrode materials for Li-ion batteries. J. Mater. Chem. A 2019, 7, 8335–8343. [Google Scholar] [CrossRef]
- Chen, J.; Fan, X.; Ji, X.; Gao, T.; Hou, S.; Zhou, X.; Wang, L.; Wang, F.; Yang, C.; Chena, L.; et al. Intercalation of Bi nanoparticles into graphite results in an ultra-fast and ultra-stable anode material for sodium-ion batteries. Energy Environ. Sci. 2018, 11, 1218–1225. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L., III. The state of understanding of the lithium ion battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, C.; Su, X.; Abudoureheman, M.; Pan, S.; Yang, Z. LiGeBO4: A nonlinear optical material with a balance between deep-ultraviolet cut-off edge and large SHG response induced by hand-in-hand tetrahedra. Inorg. Chem. Front. 2019, 6, 914–919. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [Green Version]
- Akkerman, Q.A.; Manna, L. What defines a halide perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef]
- Chiara, R.; Ciftci, Y.O.; Queloz, V.I.; Nazeeruddin, M.K.; Grancini, G.; Malavasi, L. Green-Emitting lead-free Cs4SnBr6 zero-dimensional perovskite nanocrystals with improved air stability. J. Phys. Chem. Lett. 2020, 11, 618–623. [Google Scholar] [CrossRef]
- Xiao, Z.; Yan, Y. Progress in theoretical study of metal halide perovskite solar cell materials. Adv. Energy Mater. 2017, 7, 1701–1736. [Google Scholar] [CrossRef]
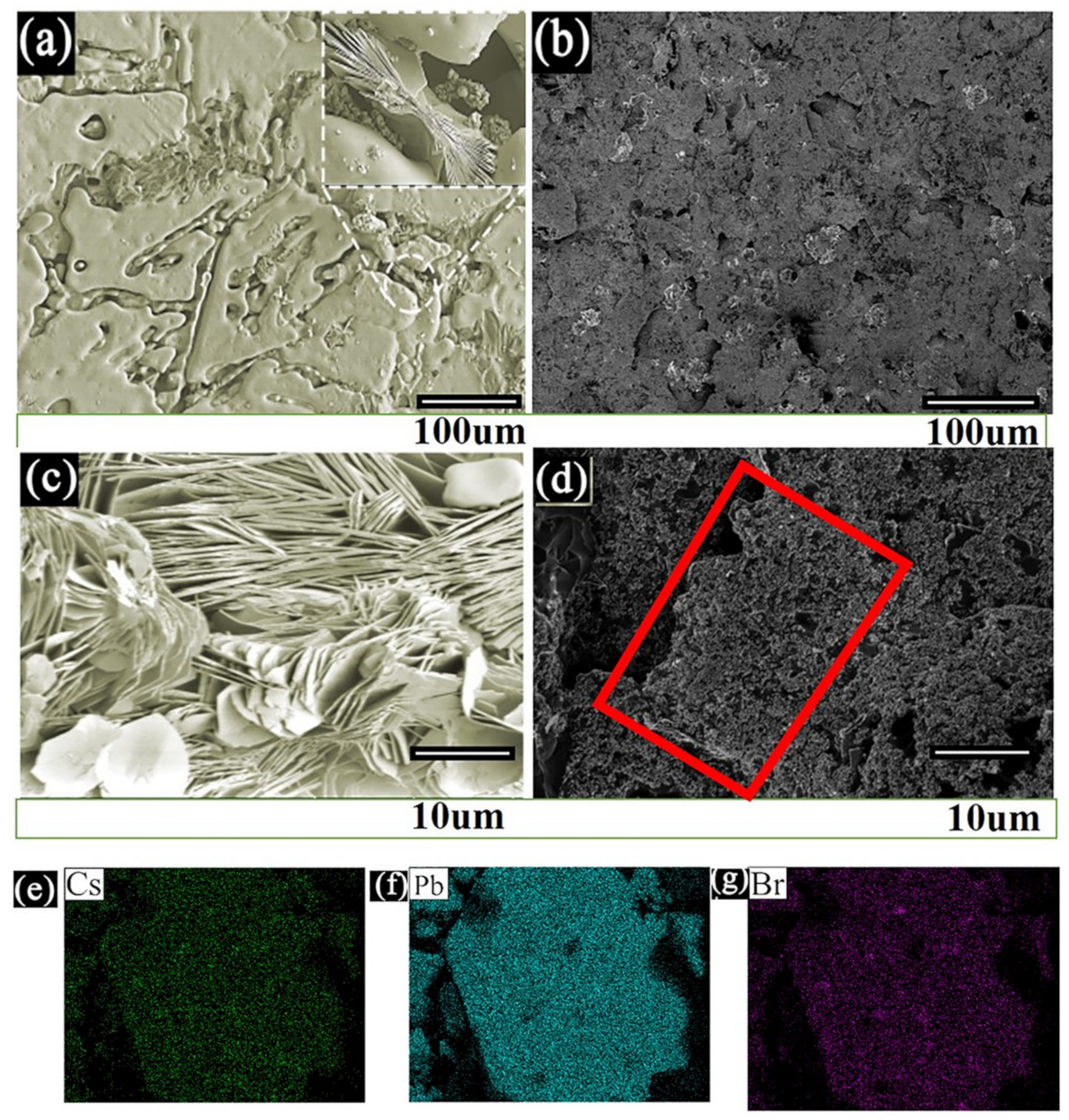

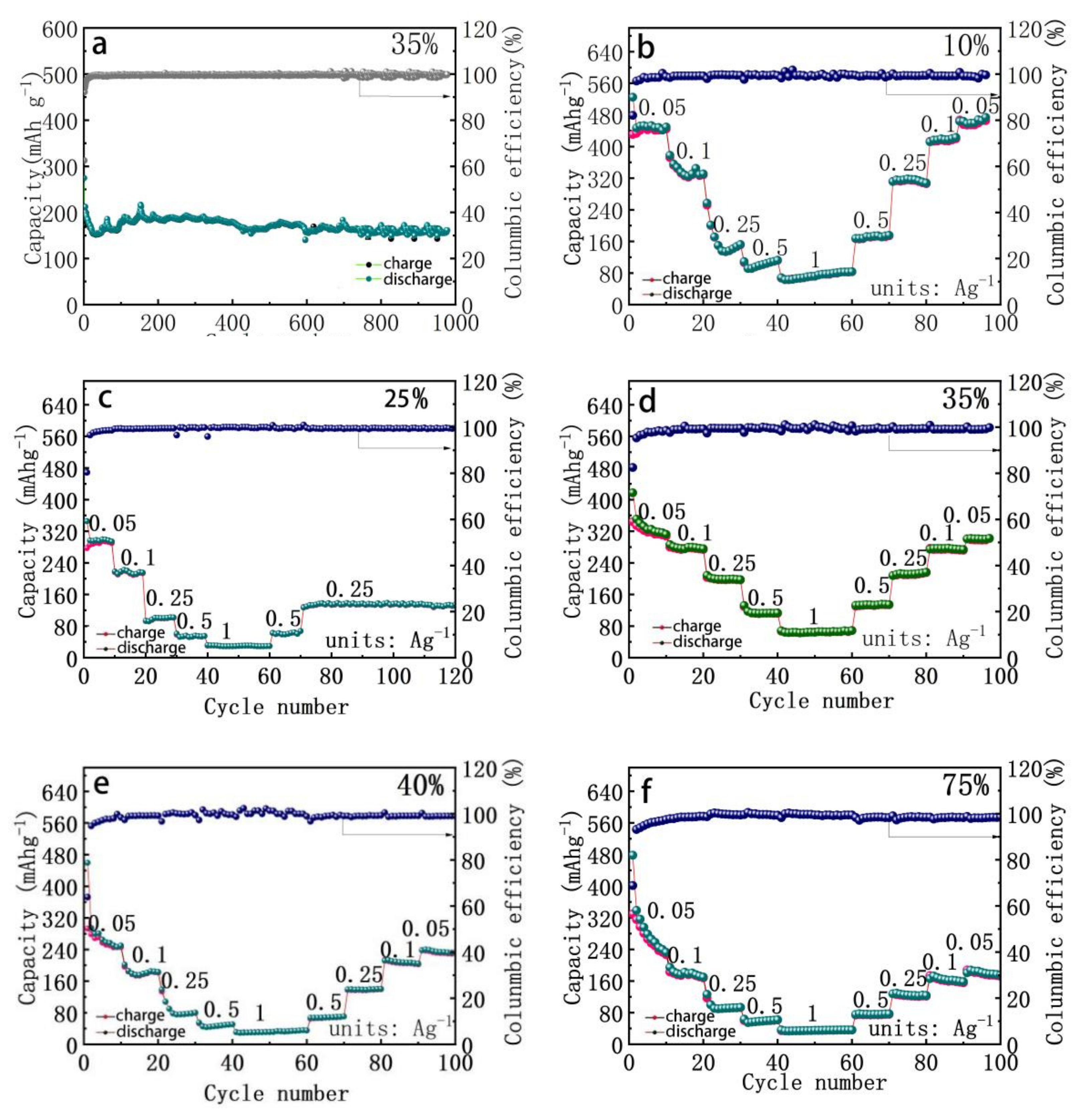


| 10% | 25% | 35% | 40% | 75% | |
|---|---|---|---|---|---|
| Before ρ (mΩ/cm2) | 101.3 | 125.8 | 93.1 | 138.8 | 158.8 |
| After ρ (mΩ/cm2) | 120.7 | 138.2 | 113.5 | 151.5 | 167.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Liu, C.; Yin, X. Cs4PbBr6 Combined with Graphite as Anode for High-Performance Lithium Batteries. Metals 2022, 12, 1584. https://doi.org/10.3390/met12101584
Zhao W, Liu C, Yin X. Cs4PbBr6 Combined with Graphite as Anode for High-Performance Lithium Batteries. Metals. 2022; 12(10):1584. https://doi.org/10.3390/met12101584
Chicago/Turabian StyleZhao, Weigang, Cuirong Liu, and Xu Yin. 2022. "Cs4PbBr6 Combined with Graphite as Anode for High-Performance Lithium Batteries" Metals 12, no. 10: 1584. https://doi.org/10.3390/met12101584
APA StyleZhao, W., Liu, C., & Yin, X. (2022). Cs4PbBr6 Combined with Graphite as Anode for High-Performance Lithium Batteries. Metals, 12(10), 1584. https://doi.org/10.3390/met12101584






