High-Temperature Corrosion Behavior of Bi3.75La0.25Ti3O12 and Bi3La1Ti3O12 Coating Prepared by rf Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. High-Temperature Corrosion
3.2. Electrochemical Impedance Spectroscopy
3.3. X-ray Diffraction
3.4. Transmission Electron Microscopy
3.5. Scanning Electron Microscopy
3.6. Energy Activation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grao, M.; Redfern, J.; Kelly, P.J.; Ratova, M. Magnetron co-sputtered Bi12TiO20/Bi4Ti3O12 composite—An efficient photocatalytic material with photoinduced oxygen vacancies for water treatment application. Appl. Surf. Sci. 2021, 552, 149486. [Google Scholar] [CrossRef]
- Das, K.; Bariki, R.; Majhi, D.; Mishra, A.; Das, K.K.; Dhiman, R.; Mishra, B.G. Facile synthesis and application of CdS/Bi20TiO32/Bi4Ti3O12 ternary heterostructure: A synergistic multi-heterojunction photocatalyst for enhanced endosulfan degradation and hydrogen evolution reaction. Appl. Catal. B 2022, 303, 120902. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Fang, Y.; Zhang, Y.; Jia, M.; Li, R.; Huang, Y. Bi4Ti3O12 Synthesized by High Temperature Solid Phase Method and its Visible Catalytic Activity. Procedia Environ. Sci. Eng. Manag. 2013, 18, 547–558. [Google Scholar] [CrossRef]
- Lin, C.; Ma, J.; Yi, F.; Zhang, H.; Qian, Y.; Zhang, K. Ag NPs modified plasmonic Z-scheme photocatalyst Bi4Ti3O12/Ag/Ag3PO4 with improved performance for pollutants removal under visible light irradiation. Ceram. Int. 2020, 46, 14650–14661. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, K.; Sun, S.; Qin, L.; Tang, L.; Li, Z. One-step synthesis of Bi4Ti3O12/Bi2O3/Bi12TiO20 spherical ternary heterojunctions with enhanced photocatalytic properties via sol-gel method. Solid State Sci. 2020, 100, 106098. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, K.; Sun, S.; Qin, L.; Tang, L.; Li, Z. Characterization of the full matrix constants of Bi4Ti3O12 ceramics. Ceram. Int. 2021, 47, 23518–23527. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Zambrano, G.; Aperador, W.; Escobar-Alarcon, L.; Camps, E. Mechanical and electrochemical characterization of vanadium nitride (VN) thin film. Appl. Surf. Sci. 2011, 258, 312–320. [Google Scholar] [CrossRef]
- Piedrahita, W.F.; Aperador, W.; Caicedo, J.C.; Prieto, P. Evolution of physical properties in hafnium carbonitride thin films. J. Alloys Compd. 2017, 690, 485–496. [Google Scholar] [CrossRef]
- Macías, H.A.; Yate, L.; Coy, L.E.; Aperador, W.; Olaya, J.J. Influence of Si-addition on wear and oxidation resistance of TiWSixN thin films. Ceram. Int. 2019, 45, 17363. [Google Scholar] [CrossRef]
- Amaya, C.; Aperador, W.; Caicedo, J.C.; Espinoza-Beltrán, F.J.; Muñoz-Saldaña, J.; Zambrano, G.; Prieto, P. Corrosion study of Alumina/Yttria-Stabilized Zirconia (Al2O3/YSZ) nanostructured Thermal Barrier Coatings (TBC) exposed to high temperature treatment. Corros. Sci. 2009, 51, 2994–2999. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, C.; Xie, C.; Li, W. Flexible organic solar cells: Materials, large-area fabrication techniques and potential applications. Nano Energy 2021, 89, 106399. [Google Scholar] [CrossRef]
- Zhang, M.; Jeerh, G.; Zou, P.; Lan, R.; Wang, M.; Wang, H.; Tao, S. Recent development of perovskite oxide-based electrocatalysts and their applications in low to intermediate temperature electrochemical devices. Mater. Today 2021, 49, 351–359. [Google Scholar] [CrossRef]
- Song, D.; Song, T.; Paik, U.; Lyu, G.; Kim, J.; Yang, S.; Jung, Y. Hot-corrosion resistance and phase stability of Yb2O3–Gd2O3–Y2O3 stabilized zirconia-based thermal barrier coatings against Na2SO4 + V2O5 molten salts. Surf. Coat. Technol. 2020, 400, 126197. [Google Scholar] [CrossRef]
- Yate, L.; Coy, L.E.; Aperador, W. Robust tribo-mechanical and hot corrosion resistance of ultra-refractory Ta-Hf-C ternary alloy films. Sci. Rep. 2017, 7, 3080. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yoon, D.J. Site Preference of La in Bi3.75La0.25Ti3O12 Using Neutron Powder Diffraction and Raman Scattering. J. Electrocera 2005, 14, 265–271. [Google Scholar] [CrossRef]
- Perez, F.J.; Hierro, M.P.; Dudley, D.; Gomez, C.; Romero, M.; Daza, L. Hot-corrosion studies of separator plates of Aisi-310 stainless steels in molten-carbonate fuel cells. Oxid. Met. 2000, 53, 375–398. [Google Scholar] [CrossRef]
- Zeng, C.L.; Guo, P.Y.; Wu, W.T. Electrochemical impedance spectra for the corrosion of two-phase Cu–15Al alloy in eutectic (Li, K)2CO3 at 650 °C in air. Electrochim. Acta 2004, 49, 1445–1450. [Google Scholar] [CrossRef]
- Ni, C.S.; Lu, L.Y.; Zeng, C.L.; Niu, Y. Electrochemical impedance studies of the initial-stage corrosion of 310S stainless steel beneath thin film of molten (0.62Li,0.38K)2CO3 at 650 °C. Corros. Sci. 2011, 53, 1018–1024. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Li, J.; Shi, H.; Liu, F.; Han, E. Self-healing epoxy coating based on tung oil-containing microcapsules for corrosion protection. Prog. Org. Coat. 2021, 156, 106236. [Google Scholar] [CrossRef]
- Feng, J.; Ming-Wang, Z.; Zheng, D.; Song, G.-L. Influence of dissolved oxygen on the corrosion of mild steel in a simulated cement pore solution under supercritical carbon dioxide. Constr. Build. Mater. 2021, 311, 125270. [Google Scholar] [CrossRef]
- David, C.; Ruel, F.; Boissy, C.; Roche, V.; Véron, M.; Nogueira, R.P. On the effect of plastic pre-straining on the corrosion behaviour of a duplex stainless steel and how the emergence of slip steps affects the hydrogen evolution reaction kinetics. Corros. Sci. 2020, 179, 109167. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, W.; Liu, Y.; Niu, X.; Wang, H.; Cui, L.W. Effect of alloyed Ca on the microstructure and corrosion behavior of extruded Mg-Bi-Al-based alloys. Mater. Charact. 2020, 163, 110292. [Google Scholar] [CrossRef]
- Yuan, G.; Zhang, G.; Li, K.; Li, F.; Cao, Y.; He, J.; Huang, Z.; Jia, Q.; Zhang, S.; Zhang, H. Preparation and Photocatalytic Performance for Degradation of Rhodamine B of AgPt/Bi4Ti3O12 Composites. Nanomaterials 2020, 10, 2206. [Google Scholar] [CrossRef]
- Thilagavathi, R.; Prithiba, A.; Rajalakshmi, R. Assessment of Passiflora vitifolia Leaves Extract as a Potential Inhibitor for Mild Steel Acid Corrosion. Rasayan J. Chem. 2019, 12, 431–449. [Google Scholar] [CrossRef]
- Bautista, A.; González-Centeno, A.; Blanco, G.; Guzmán, S. Application of EIS to the study of corrosion behaviour of sintered ferritic stainless steels before and after high-temperature exposure. Mater. Charact. 2008, 59, 32–39. [Google Scholar] [CrossRef]
- Encinas-Sánchez, V.; de Miguel, M.T.; Lasanta, M.I.; García-Martín, G.; Pérez, F.J. Electrochemical impedance spectroscopy (EIS): An efficient technique for monitoring corrosion processes in molten salt environments in CSP application. Sol. Energy Mater. Sol. Cells 2019, 191, 157–163. [Google Scholar] [CrossRef]
- Lario, J.; Escuder, A.; Segovia, F.; Amigó, V. Electrochemical corrosion behavior of Ti–35Nb–7Zr–5Ta powder metallurgic alloys after Hot Isostatic Process in fluorinated artificial saliva. J. Mater. Res. Technol. 2022, 16, 1435–1444. [Google Scholar] [CrossRef]
- Sun, L.; Fu, Q.; Sun, J.; Zhang, G. Comparison investigation of hot corrosion exposed to Na2SO4 salt and oxidation of MoSi2-based coating on Nb alloy at 100 °C. Surf. Coat. Technol. 2020, 385, 125388. [Google Scholar] [CrossRef]
- Operator, W.; Bautista-Ruiz, J.; Caicedo, J. Synergistic corrosion-wear effect in austenitic powder metallurgical steel with different titanium additions. Rasayan J. Chem. 2022, 15, 57–64. [Google Scholar]
- Hernández-Cuevas, G.; Leyva Mendoza, J.R.; García-Casillas, P.E.; Olivas-Armendariz, I.; Mani-González, P.G.; Díaz de la Torre, S. Synthesis and characterization of niobium doped bismuth titanate. Boletín de la Sociedad Española de Cerámica y Vidrio 2021, in press. [Google Scholar] [CrossRef]
- Nogueira, A.E.; Lima, A.R.F.; Longo, E.; Leite, E.R.; Camargo, E. Effect of lanthanum and lead doping on the microstructure and visible light photocatalysis of bismuth titanate prepared by the oxidant peroxide method (OPM). J. Photochem. Photobiol. A 2015, 312, 55–63. [Google Scholar] [CrossRef]
- Simões, A.Z.; Piano, R.F.; Riccardi, C.S.; Cavalcante, L.S.; Longo, E.; Varela, J.A. Dielectric properties of pure and lanthanum modified bismuth titanate thin films. J. Alloys Compd. 2008, 454, 66–71. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Zhao, X.; Deng, X.; Zhang, W. High temperature electrical insulation and adhesion of nanocrystalline YSZ/Al2O3 composite film for thin-film thermocouples on Ni-based superalloy substrates. Appl. Surf. Sci. 2022, 579, 152169. [Google Scholar] [CrossRef]
- Obitte, B.C.N.; Ikhioya, I.L.; Whyte, G.M.; Chime, U.K.; Ezekoye, B.A.; Ekwealor, A.B.C.; Maaza, M. The effects of doping and temperature on properties of electrochemically deposited Er3+ doped ZnSe thin films. Opt. Mater. 2022, 124, 111979. [Google Scholar] [CrossRef]
- Ganesha Krishna, V.S.; Mahesha, M.G. Influence of the substrate temperature on the structural, optical, and morphological properties of (101) oriented ZnSe thin films for window applications in solar cells. Mater. Today 2022, 58, 623–626. [Google Scholar]


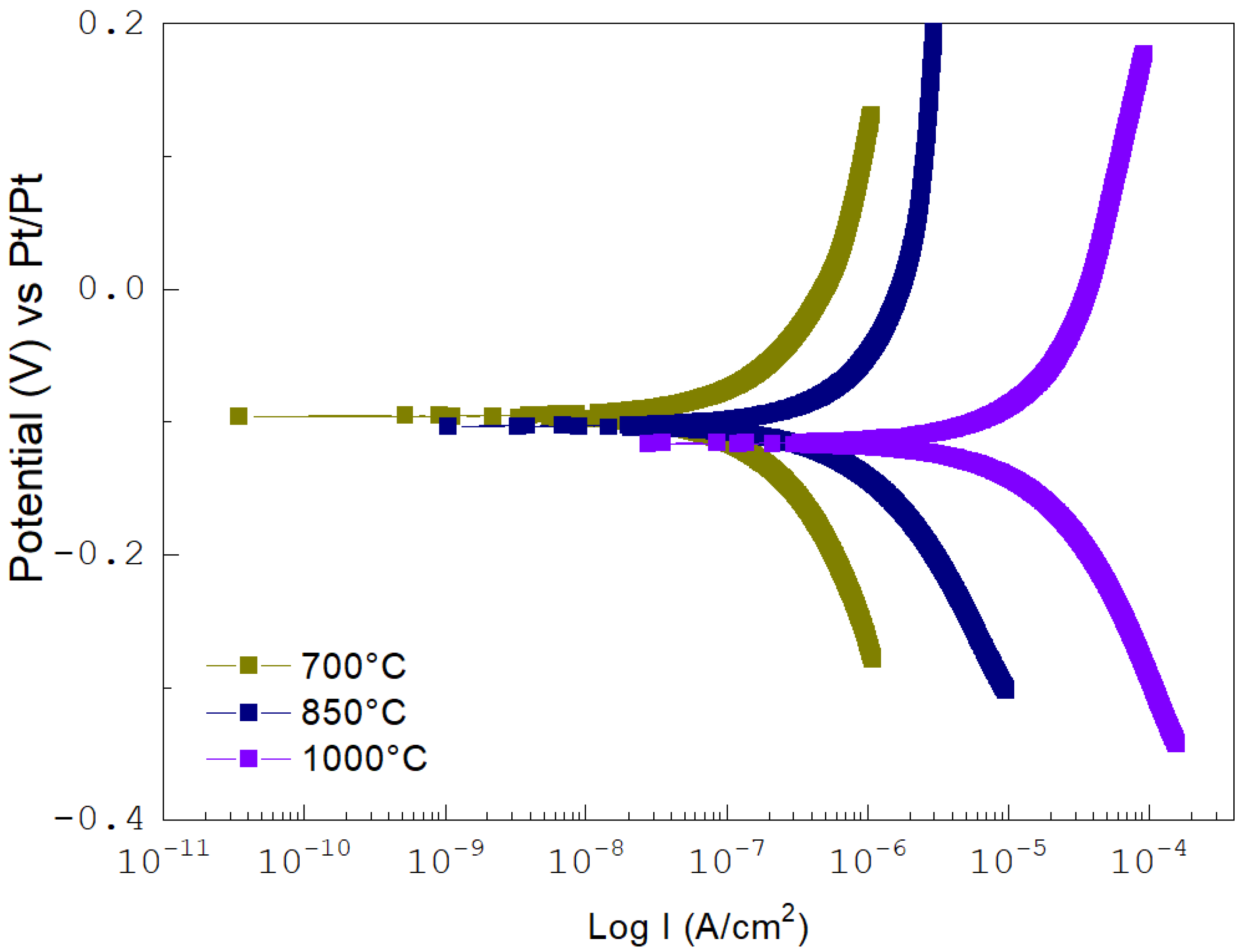








| Temperature (°C) | Error (V vs. Pt) | Icorr (μA/cm2) | Corrosion Rate (μm/Year) |
|---|---|---|---|
| 700 | −1.47 | 8.18 × 10−6 | 94.28 |
| 850 | −1.44 | 16.95 × 10−6 | 195.36 |
| 1000 | −1.49 | 77.44 × 10−6 | 892.55 |
| Temperature (°C) | Error (V vs. Pt) | Icorr (μA/cm2) | Corrosion Rate (μm/Year) |
|---|---|---|---|
| 700 | −0.09 | 1.01 × 10−6 | 1.12 |
| 850 | −0.10 | 0.68 × 10−6 | 7.82 |
| 1000 | −0.11 | 16.10 × 10−6 | 185.55 |
| Temperature (°C) | Error (V vs. Pt) | Icorr (μA/cm2) | Corrosion Rate (μm/Year) |
|---|---|---|---|
| 700 | 0.21 | 0.10 × 10−6 | 1.18 |
| 850 | 0.18 | 0.59 × 10−6 | 4.47 |
| 1000 | 0.16 | 8.14 × 10−6 | 93.83 |
| Temperature (°C) | Rs (Ω cm2) | CPE1 (μF cm−2 s−(1−α1) | α1 | Rp1 (Ω cm2) | CPE2 μF cm−2 s−(1−α2) | α2 | Rp2 103(Ω cm2) |
|---|---|---|---|---|---|---|---|
| 700 | 4.10 (0.3%) | 92.56 (4%) | 0.75 | 19.20 (3%) | 342.83 (3%) | 0.79 | 2.43 (0.2%) |
| 850 | 5.24 (0.2%) | 87.12 (4%) | 0.69 | 5.31 (2%) | 546.23 (3%) | 0.75 | 1.02 (0.3%) |
| 1000 | 5.91 (0.3%) | 92.53 (5%) | 0.74 | 2.84 (2%) | 653.76 (4%) | 0.67 | 0.05 (0.2%) |
| Temperature (°C) | Rs (Ω cm2) | CPE1 (μF cm−2 s−(1−α1) | α1 | Rp1 (Ω cm2) | CPE2 μF cm−2 s−(1−α2) | α2 | Rp2 103(Ω cm2) |
|---|---|---|---|---|---|---|---|
| 700 | 1.31 (0.4%) | 121.05 (2%) | 0.54 | 5.3 (0.1%) | 564.21 (3%) | 0.56 | 0.08 (0.01%) |
| 850 | 3.22 (0.6%) | 243.13 (3%) | 0.59 | 2.3 (0.2%) | 853.28 (2%) | 0.67 | 0.04 (0.02%) |
| 1000 | 2.54 (0.6%) | 322.25 (3%) | 0.64 | 1.3 (0.3%) | 1035.25 (2%) | 0.64 | 0.01 (0.02%) |
| System | C | O | Na | Si | S | La | Ti | Cr | Mn | Fe | Ni | V | Bi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | |||||||||||||
| Substrate | 1 | 16 | 4 | 3 | 6 | 0 | 0 | 14 | 3 | 39 | 4 | 10 | 0 |
| Bi3.75La0.25Ti3O12 | 1 | 15 | 1 | 2 | 1 | 6 | 18 | 7 | 1 | 10 | 5 | 8 | 25 |
| Bi3La1Ti3O12 | 2 | 13 | 1 | 2 | 1 | 9 | 19 | 8 | 1 | 9 | 5 | 7 | 23 |
| System | C | O | Na | Si | S | La | Ti | Cr | Mn | Fe | Ni | V | Bi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | |||||||||||||
| Substrate | 1 | 22 | 4 | 1 | 2 | 0 | 0 | 4 | 1 | 49 | 5 | 16 | 0 |
| Bi3.75La0.25Ti3O12 | 3 | 19 | 14 | 2 | 4 | 3 | 14 | 2 | 0 | 4 | 5 | 21 | 12 |
| Bi3La1Ti3O12 | 4 | 18 | 12 | 1 | 5 | 4 | 16 | 1 | 0 | 7 | 1 | 19 | 12 |
| System | C | O | Na | Si | S | La | Ti | Cr | Mn | Fe | Ni | V | Bi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | |||||||||||||
| Substrate | 1 | 18 | 2 | 1 | 2 | 0 | 0 | 2 | 1 | 46 | 2 | 25 | 0 |
| Bi3.75La0.25Ti3O12 | 1 | 22 | 16 | 1 | 2 | 2 | 12 | 0 | 0 | 2 | 1 | 29 | 12 |
| Bi3La1Ti3O12 | 1 | 25 | 19 | 2 | 1 | 1 | 11 | 0 | 0 | 2 | 1 | 22 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Ruiz, J.; Sánchez-Molina, J.; Aperador, W. High-Temperature Corrosion Behavior of Bi3.75La0.25Ti3O12 and Bi3La1Ti3O12 Coating Prepared by rf Magnetron Sputtering. Metals 2022, 12, 1585. https://doi.org/10.3390/met12101585
Bautista-Ruiz J, Sánchez-Molina J, Aperador W. High-Temperature Corrosion Behavior of Bi3.75La0.25Ti3O12 and Bi3La1Ti3O12 Coating Prepared by rf Magnetron Sputtering. Metals. 2022; 12(10):1585. https://doi.org/10.3390/met12101585
Chicago/Turabian StyleBautista-Ruiz, Jorge, Jorge Sánchez-Molina, and Willian Aperador. 2022. "High-Temperature Corrosion Behavior of Bi3.75La0.25Ti3O12 and Bi3La1Ti3O12 Coating Prepared by rf Magnetron Sputtering" Metals 12, no. 10: 1585. https://doi.org/10.3390/met12101585





