Production of a Novel Biomedical β-Type Titanium Alloy Ti-23.6Nb-5.1Mo-6.7Zr with Low Young’s Modulus
Abstract
:1. Introduction
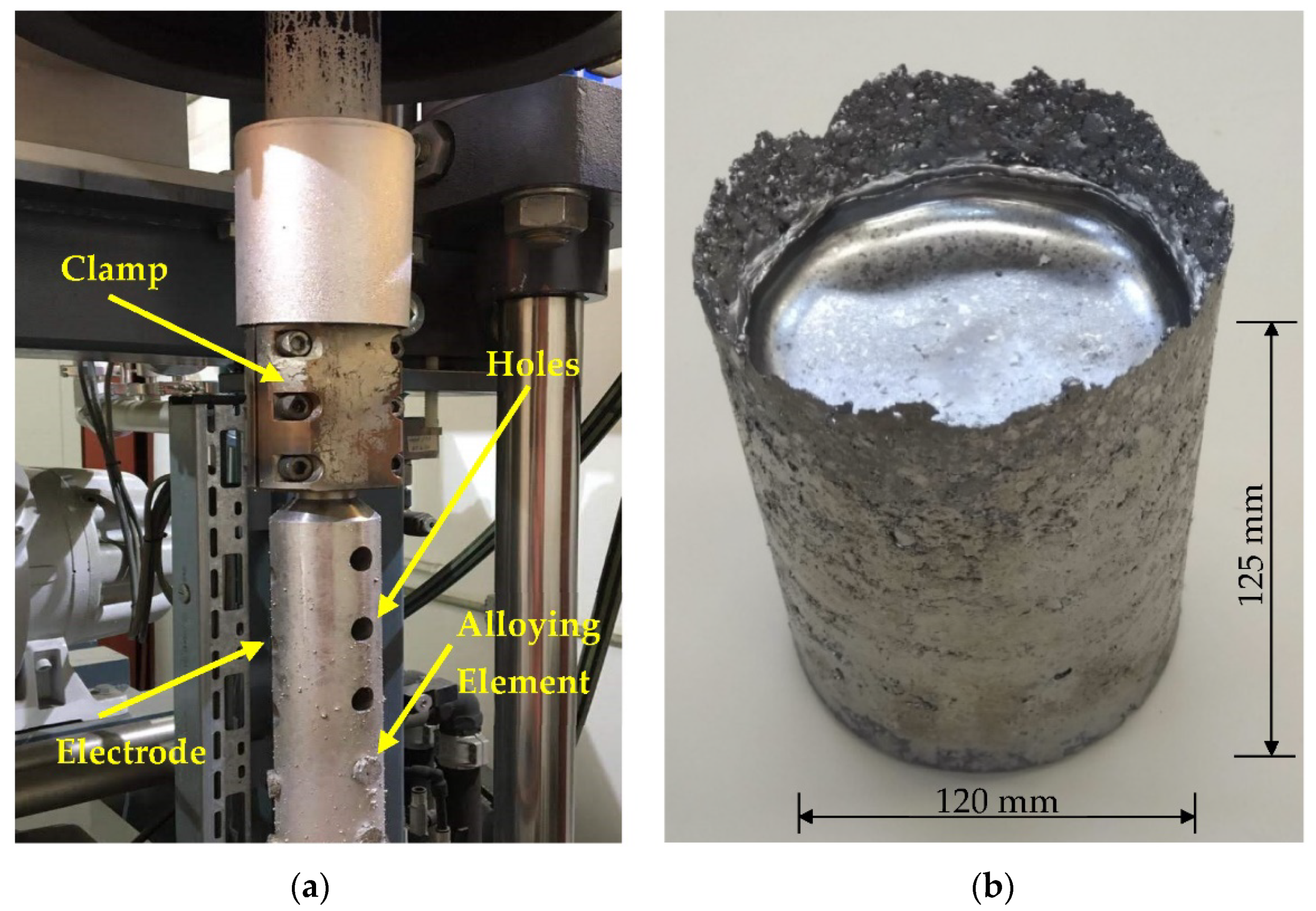
2. Materials and Methods
3. Results and Discussion
3.1. Microstructural Characterization
3.2. Mechanical Properties of the Processed Alloy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-Alloys for Long-Term Implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; da Silva, L.M.; dos Santos Jorge Sousa, K.; Donato, T.A.G.; Grandini, C.R. Preparation, Structural, Microstructural, Mechanical, and Cytotoxic Characterization of Ti-15Nb Alloy for Biomedical Applications. Artif. Organs 2020, 44, 811–817. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Quadros, F.d.F.; Afonso, C.R.M.; Grandini, C.R. The Effect of Solution Heat Treatment Time on the Phase Formation and Selected Mechanical Properties of Ti-25Ta-XZr Alloys for Application as Biomaterials. J. Mater. Eng. Perform. 2021, 30, 5905–5913. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, Q.; Guo, S.; Zhao, X. α′ Type Ti–Nb–Zr Alloys with Ultra-Low Young’s Modulus and High Strength. Prog. Nat. Sci. Mater. Int. 2013, 23, 562–565. [Google Scholar] [CrossRef]
- Kim, K.M.; Al-Zain, Y.; Yamamoto, A.; Daher, A.H.; Mansour, A.T.; AlAjlouni, J.M.; Aloweidi, A.S.; Al-Abbadi, M.A.; Kim, H.Y.; Miyazaki, S. Synthesis and Characterization of a Ti–Zr-Based Alloy with Ultralow Young’s Modulus and Excellent Biocompatibility. Adv. Eng. Mater. 2022, 24, 2100776. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Cheng, X.; Zhao, X. A Metastable β-Type Ti-Nb Binary Alloy with Low Modulus and High Strength. J. Alloys Compd. 2015, 644, 411–415. [Google Scholar] [CrossRef]
- Kuczyńska-Zemła, D.; Kijeńska-Gawrońska, E.; Chlanda, A.; Sotniczuk, A.; Pisarek, M.; Topolski, K.; Swieszkowski, W.; Garbacz, H. Biological Properties of a Novel β-Ti Alloy with a Low Young’s Modulus Subjected to Cold Rolling. Appl. Surf. Sci. 2020, 511, 145523. [Google Scholar] [CrossRef]
- Raganya, L.; Moshokoa, N.; Obadele, B.; Makhatha, E.; Machaka, R. Microstructure and Mechanical Properties of Ti-Mo-Nb Alloys Designed Using the Cluster-plus-Glue-Atom Model for Orthopedic Applications. Int. J. Adv. Manuf. Technol. 2021, 115, 3053–3064. [Google Scholar] [CrossRef]
- Hao, Y.L.; Niinomi, M.; Kuroda, D.; Fukunaga, K.; Zhou, Y.L.; Yang, R.; Suzuki, A. Aging Response of the Young’s Modulus and Mechanical Properties of Ti-29Nb-13Ta-4.6Zr for Biomedical Applications. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2003, 34, 1007–1012. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Hinoshita, K.; Morinaga, M. General Approach to Phase Stability and Elastic Properties of β-Type Ti-Alloys Using Electronic Parameters. Scr. Mater. 2006, 55, 477–480. [Google Scholar] [CrossRef]
- Niinomi, M.; Akahori, T.; Takeuchi, T.; Katsura, S.; Fukui, H.; Toda, H. Mechanical Properties and Cyto-Toxicity of New Beta Type Titanium Alloy with Low Melting Points for Dental Applications. Mater. Sci. Eng. C 2005, 25, 417–425. [Google Scholar] [CrossRef]
- Zhou, Z.; Lai, M.; Tang, B.; Kou, H.; Chang, H.; Zhu, Z.; Li, J.; Zhou, L. Non-Isothermal Phase Transformation Kinetics of ω Phase in TB-13 Titanium Alloys. Mater. Sci. Eng. A 2010, 527, 5100–5104. [Google Scholar] [CrossRef]
- Kuroda, P.; Lourenço, M.; Correa, D.; Grandini, C. Thermomechanical Treatments Influence on the Phase Composition, Microstructure, and Selected Mechanical Properties of Ti–20Zr–Mo Alloys System for Biomedical Applications. J. Alloys Compd. 2019, 812, 152108. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M. Microstructures and Mechanical Properties of Ti-50 Mass% Ta Alloy for Biomedical Applications. J. Alloys Compd. 2008, 466, 535–542. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Effects of Ta Content on Young’s Modulus and Tensile Properties of Binary Ti-Ta Alloys for Biomedical Applications. Mater. Sci. Eng. A 2004, 371, 283–290. [Google Scholar] [CrossRef]
- Hanada, S.; Masahashi, N.; Jung, T.K.; Yamada, N.; Yamako, G.; Itoi, E. Fabrication of a High-Performance Hip Prosthetic Stem Using β Ti-33.6Nb-4Sn. J. Mech. Behav. Biomed. Mater. 2014, 30, 140–149. [Google Scholar] [CrossRef]
- Hanada, S.; Masahashi, N.; Jung, T.K. Effect of Stress-Induced α″ Martensite on Young’s Modulus of β Ti-33.6Nb-4Sn Alloy. Mater. Sci. Eng. A 2013, 588, 403–410. [Google Scholar] [CrossRef]
- Laheurte, P.; Eberhardt, A.; Philippe, M.J. Influence of the Microstructure on the Pseudoelasticity of a Metastable Beta Titanium Alloy. Mater. Sci. Eng. A 2005, 396, 223–230. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Fuwa, H.; Hinoshita, K.; Kimura, H.; Shinzato, Y.; Morinaga, M. Phase Stability Change with Zr Content in β-Type Ti-Nb Alloys. Scr. Mater. 2007, 57, 1000–1003. [Google Scholar] [CrossRef]
- Lopes, E.S.N.; Cremasco, A.; Contieri, R.; Caram, R. Effects of Aging Heat Treatment on the Microstructure of Ti-Nb and Ti-Nb-Sn Alloys Employed as Biomaterials. Adv. Mater. Res. 2011, 324, 61–64. [Google Scholar] [CrossRef]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical Titanium Alloys with Young’s Moduli Close to That of Cortical Bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef]
- Mythili, R.; Paul, V.T.; Saroja, S.; Vijayalakshmi, M.; Raghunathan, V.S. Study of Transformation Behavior in a Ti-4.4 Ta-1.9 Nb Alloy. Mater. Sci. Eng. A 2005, 390, 299–312. [Google Scholar] [CrossRef]
- Nunes, A.R.V.; Borborema, S.; Araújo, L.S.; Malet, L.; Dille, J.; Henrique de Almeida, L. Influence of Thermo-Mechanical Processing on Structure and Mechanical Properties of a New Metastable β Ti–29Nb–2Mo–6Zr Alloy with Low Young’s Modulus. J. Alloys Compd. 2020, 820, 153078. [Google Scholar] [CrossRef]
- Nunes, A.R.V.; Borborema, S.; Araújo, L.S.; Dille, J.; Malet, L.; de Almeida, L.H. Production, Microstructure and Mechanical Properties of Cold-Rolled Ti-Nb-Mo-Zr Alloys for Orthopedic Applications. J. Alloys Compd. 2018, 743, 141–145. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Dille, J.; Rezende, M.C.; Mei, P.; Almeida, L.H.D.; Baldan, R.; Nunes, C.A. Mechanical Characterization of Ti-12Mo-13Nb Alloy for Biomedical Application Hot Swaged and Aged. Mater. Res. 2015, 18, 8–12. [Google Scholar] [CrossRef]
- Jawed, S.F.; Rabadia, C.D.; Liu, Y.J.; Wang, L.Q.; Li, Y.H.; Zhang, X.H.; Zhang, L.C. Mechanical Characterization and Deformation Behavior of β-Stabilized Ti-Nb-Sn-Cr Alloys. J. Alloys Compd. 2019, 792, 684–693. [Google Scholar] [CrossRef]
- Ho, W.F.; Ju, C.P.; Lin, J.H.C. Structure and Properties of Cast Binary Ti - Mo Alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef]
- Lan, C.; Wu, Y.; Guo, L.; Chen, F. Effects of Cold Rolling on Microstructure, Texture Evolution and Mechanical Properties of Ti-32.5Nb-6.8Zr-2.7Sn-0.3O Alloy for Biomedical Applications. Mater. Sci. Eng. A 2017, 690, 170–176. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.; Qin, J.; Zhang, F.; Zhang, D. Microstructure and Mechanical Properties of Cold-Rolled TiNbTaZr Biomedical β Titanium Alloy. Mater. Sci. Eng. A 2008, 490, 421–426. [Google Scholar] [CrossRef]
- Gao, J.; Huang, Y.; Guan, D.; Knowles, A.J.; Ma, L.; Dye, D.; Rainforth, W.M. Deformation Mechanisms in a Metastable Beta Titanium Twinning Induced Plasticity Alloy with High Yield Strength and High Strain Hardening Rate. Acta Mater. 2018, 152, 301–314. [Google Scholar] [CrossRef]
- Jonas, J.J.; Aranas, C.; Fall, A.; Jahazi, M. Transformation Softening in Three Titanium Alloys. Mater. Des. 2017, 113, 305–310. [Google Scholar] [CrossRef]
- Zhao, G.H.; Xu, X.; Dye, D.; Rivera-Díaz-del-Castillo, P.E.J. Microstructural Evolution and Strain-Hardening in TWIP Ti Alloys. Acta Mater. 2020, 183, 155–164. [Google Scholar] [CrossRef]
- Nunes, A.R.V.; Borborema, S.; Malet, L.; Dille, J.; De Almeida, L.H. Microstructural Evolution of Cold-Rolled β Metastable Ti -29Nb-2Mo-6Zr Alloy. Microsc. Microanal. 2019, 25, 2634–2635. [Google Scholar] [CrossRef]
- Xu, T.W.; Li, J.S.; Zhang, S.S.; Zhang, F.S.; Liu, X.H. Cold Deformation Behavior of the Ti-15Mo-3Al-2.7Nb-0.2Si Alloy and Its Effect on α Precipitation and Tensile Properties in Aging Treatment. J. Alloys Compd. 2016, 682, 404–411. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Dasgupta, A.; Khatirkar, R.; Saroja, S.; Samajdar, I.; Vijayalakshmi, M. Effect of Cooling Rate on Transformation Texture and Variant Selection during Β→α Transformation in Ti-5Ta-1.8Nb Alloy. Mater. Sci. Eng. A 2010. [Google Scholar] [CrossRef]
- Elmay, W.; Berveiller, S.; Patoor, E.; Gloriant, T.; Prima, F.; Laheurte, P. Texture Evolution of Orthorhombic A″ Titanium Alloy Investigated by in Situ X-Ray Diffraction. Mater. Sci. Eng. A 2017, 679, 504–510. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yi, D.Q.; Liu, H.Q.; Wu, X.Y.; Wang, B.; Yang, F.L. Effects of Cold Deformation on Microstructure, Texture Evolution and Mechanical Properties of Ti-Nb-Ta-Zr-Fe Alloy for Biomedical Applications. Mater. Sci. Eng. A 2012, 547, 64–71. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.H. A Review on Alloy Design, Biological Response, and Strengthening of β-Titanium Alloys as Biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef]
- Lee, C.M.; Ju, C.P.; Chern Lin, J.H. Structure-Property Relationship of Cast Ti-Nb Alloys. J. Oral Rehabil. 2002, 29, 314–322. [Google Scholar] [CrossRef]





| Composition (% Mass) | |||||
|---|---|---|---|---|---|
| Ti | Nb | Mo | Zr | O | N |
| Balanced | 23.57 ± 0.13 | 5.07 ± 0.05 | 6.72 ± 0.09 | 0.105 | 0.0098 |
| Alloy | Thermomechanical Processing | Temp. (°C) | Time | Hardness (HV-0,2) | YM (GPa) | HV/E | Phases (DRX) |
|---|---|---|---|---|---|---|---|
| Ti-23.6Nb-5.1Mo-6.7Zr | ST | 1000 | 24 h | 228.90 ± 2.08 | NM | NM | β |
| 50% CR | - | - | 271.80 ± 6.70 | 57.62 ± 0.32 | 4.72 | β + ⍺″ | |
| Annealed (After 50% CR) | 950 | 1 h | 246.90 ± 2.92 | 58.59 ± 0.37 | 4.21 | β | |
| 50% CR and Aged | 300 | 2 h | 343.70 ± 4.22 | 63.40 ± 0.63 | 5.42 | β + ⍺″ | |
| 50% CR and Aged | 400 | 2 h | 395.00 ± 4.11 | 78.57 ± 2.00 | 5.03 | β + ⍺ + ω | |
| 50% CR and Aged | 500 | 2 h | 351.60 ± 3.60 | 69.47 ± 1.14 | 5.06 | β + ⍺ | |
| Ti-6Al-4V | As Cast | - | - | 337 ± 2.0 | 120 ± 3.0 | 2.81 | ⍺ + β |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.R.V.; Borborema, S.; Araújo, L.S.; de Almeida, L.H.; Kaufman, M.J. Production of a Novel Biomedical β-Type Titanium Alloy Ti-23.6Nb-5.1Mo-6.7Zr with Low Young’s Modulus. Metals 2022, 12, 1588. https://doi.org/10.3390/met12101588
Nunes ARV, Borborema S, Araújo LS, de Almeida LH, Kaufman MJ. Production of a Novel Biomedical β-Type Titanium Alloy Ti-23.6Nb-5.1Mo-6.7Zr with Low Young’s Modulus. Metals. 2022; 12(10):1588. https://doi.org/10.3390/met12101588
Chicago/Turabian StyleNunes, Aline Raquel Vieira, Sinara Borborema, Leonardo Sales Araújo, Luiz Henrique de Almeida, and Michael J. Kaufman. 2022. "Production of a Novel Biomedical β-Type Titanium Alloy Ti-23.6Nb-5.1Mo-6.7Zr with Low Young’s Modulus" Metals 12, no. 10: 1588. https://doi.org/10.3390/met12101588
APA StyleNunes, A. R. V., Borborema, S., Araújo, L. S., de Almeida, L. H., & Kaufman, M. J. (2022). Production of a Novel Biomedical β-Type Titanium Alloy Ti-23.6Nb-5.1Mo-6.7Zr with Low Young’s Modulus. Metals, 12(10), 1588. https://doi.org/10.3390/met12101588







