Parameter Study on the Recycling of LFP Cathode Material Using Hydrometallurgical Methods
Abstract
:1. Introduction
1.1. Research Activities in the Field of Leaching of LFP Cathode Material
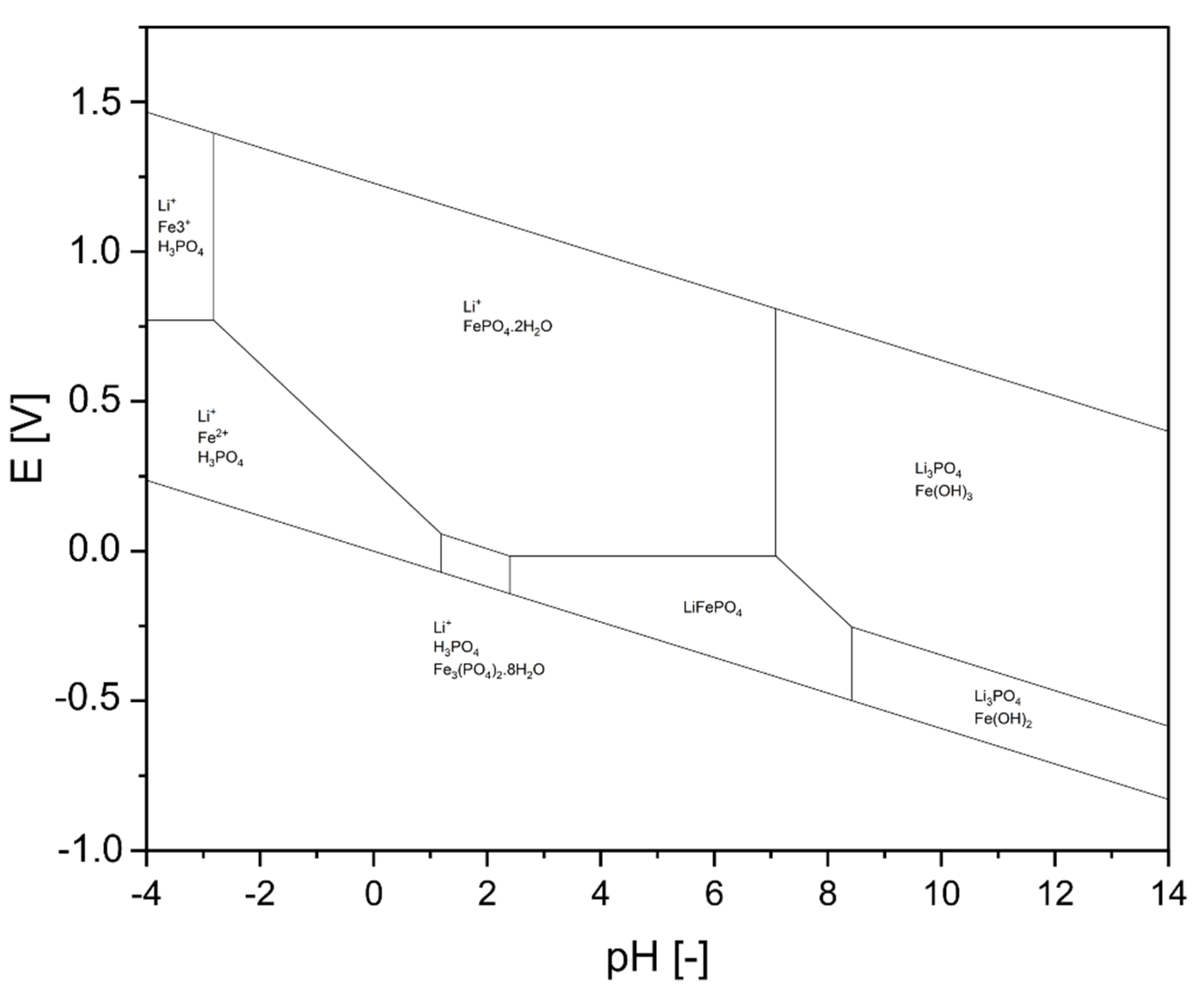
1.2. Physico-Chemical Fundamentals of Hydrometallurgical Decomposition of LFP
2. Materials and Methods
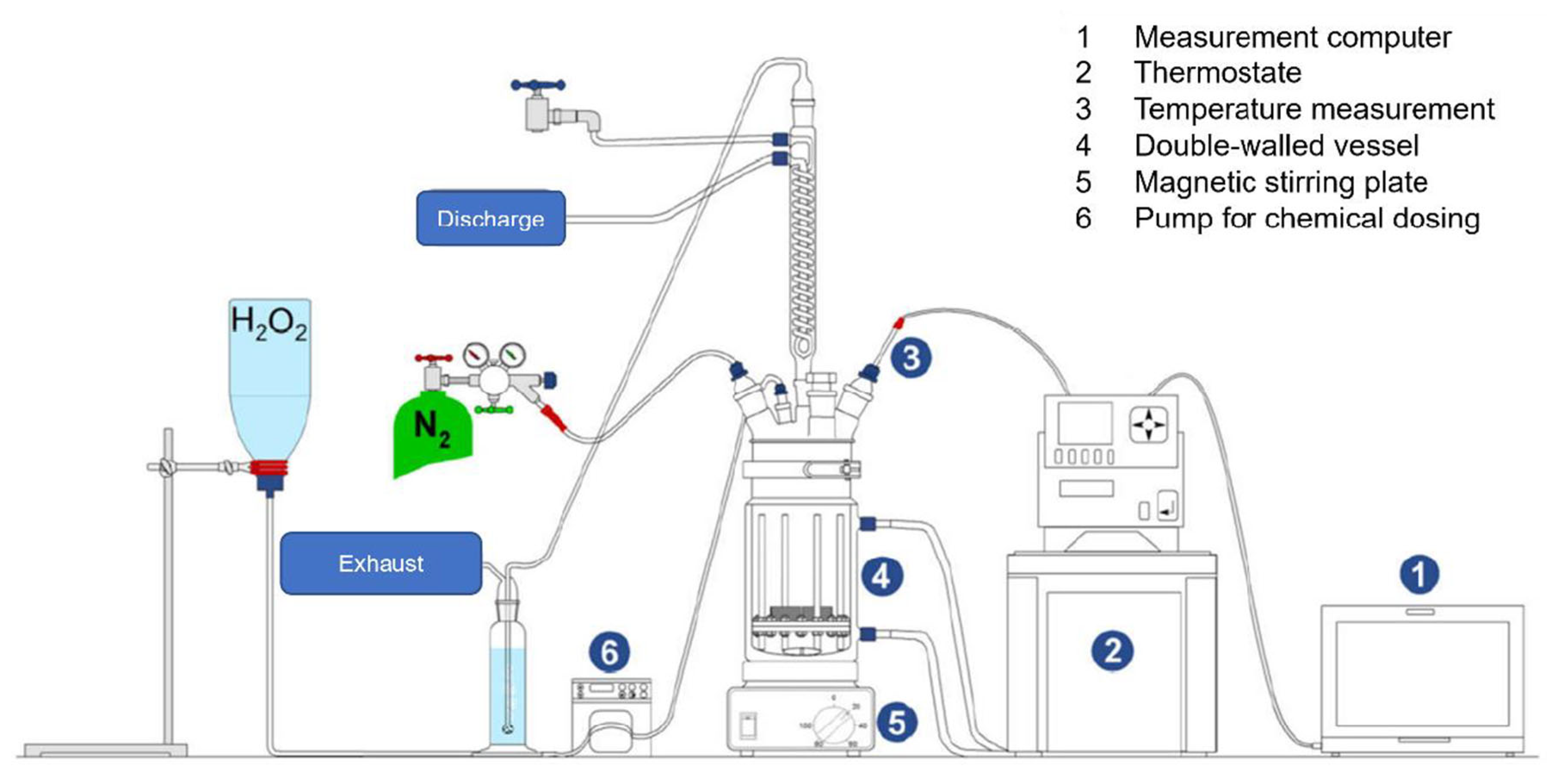
2.1. Experimental Procedure
2.2. Analytical Methods
3. Results
3.1. Evaluation of Leaching Efficiency
3.2. Leaching Behavior of Lithium
3.3. Leaching Behavior of Iron
3.4. Analysis of Solid Residues
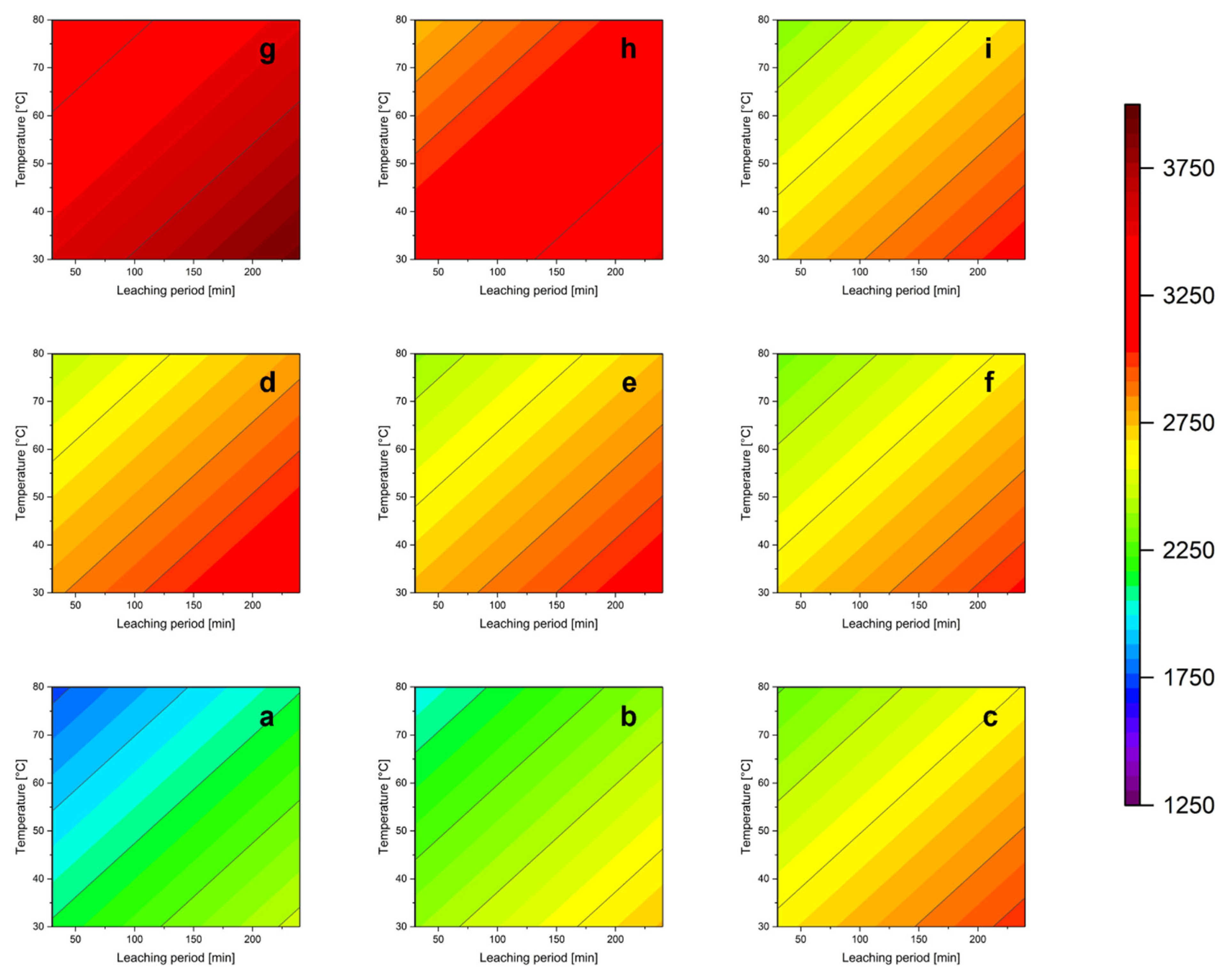
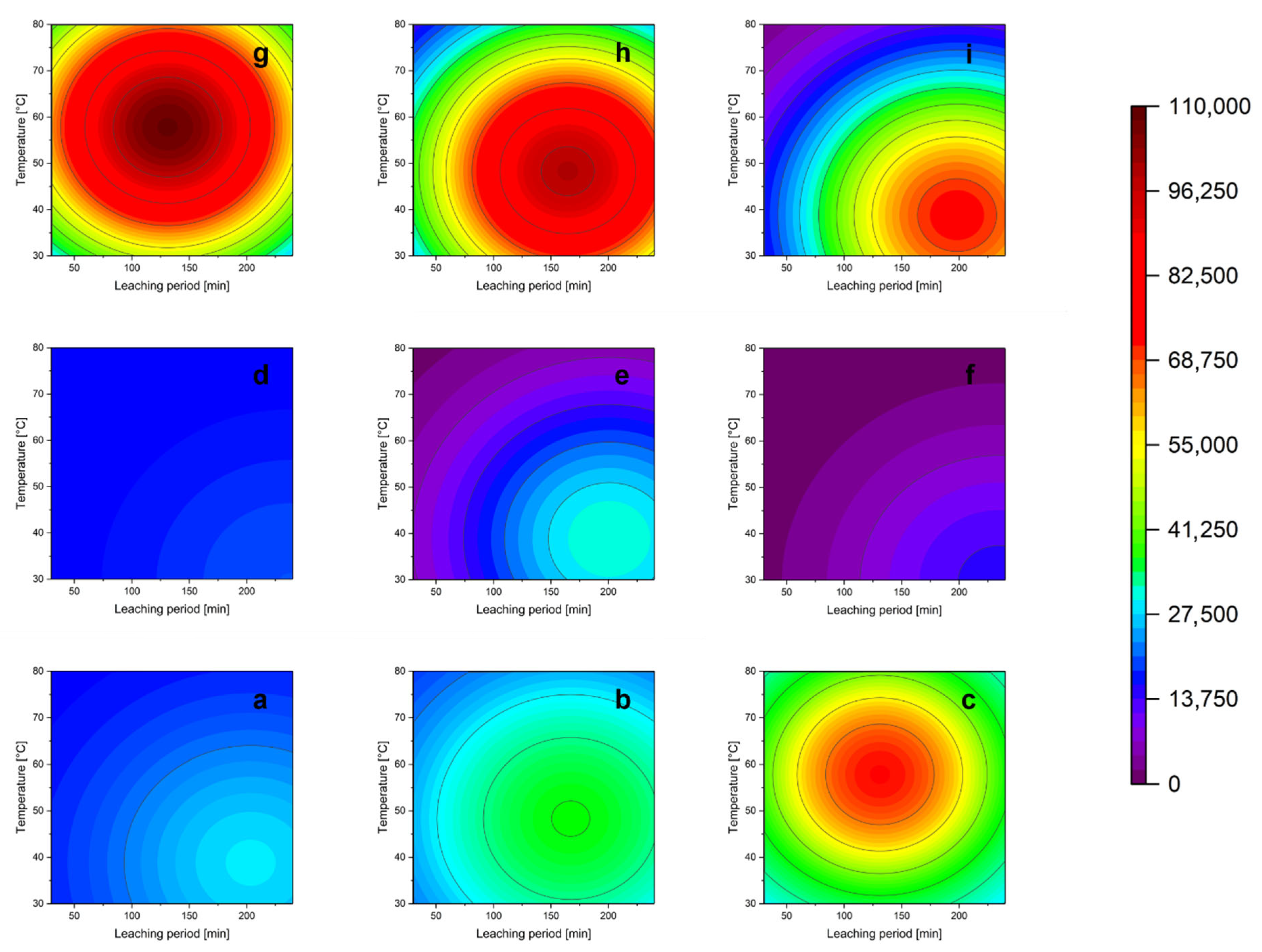
3.5. Parameter Study
3.5.1. Influence of the Acid Concentration
3.5.2. Influence of Oxidizing Agent
3.5.3. Influence of the Leaching Temperature
3.5.4. Influence of the Solid/Liquid Ratio
3.5.5. Influence of the Leaching Time
4. Discussion
- Acid concentration
- Amount of oxidant
- Temperature
- Solid/liquid ratio
- Leaching time
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blengini, G.A.; Cynthia, E.L.; Umberto, E.; Cristina, T.D.M.; Dominic, W.; Konstantinos, G.; Claudiu, P.; Samuel, C.; Lucia, M.; Manuela, U.; et al. Study on the EU’s List of Critical Raw Materials (2020); Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Statista. Anzahl der Neuzulassungen von Elektroautos Weltweit von 2012 und 2021. Available online: https://de.statista.com/statistik/daten/studie/406683/umfrage/anzahl-der-verkaeufe-von-elektroautos-weltweit-prognose/ (accessed on 23 August 2022).
- IEA. Global EV Outlook 2022. Available online: https://www.iea.org/reports/global-ev-outlook-2022 (accessed on 23 August 2022).
- Korthauer, R. Handbuch Lithium-Ionen-Batterien; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Windisch-Kern, S.; Gerold, E.; Nigl, T.; Jandric, A.; Altendorfer, M.; Rutreche, B.; Silvia, S.; Raupenstrauch, H.; Pomberger, R.; Antrekowitsch, H.; et al. Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Manag. 2022, 138, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Edison. Mit dem LFP-Akku Beginnt Die Chinesische Eisenzeit. Available online: https://edison.media/mit-lfp-akkus-beginnt-die-chinesischeeisenzeit/25225920/#:~:text=Das%20K%C3%BCrzel%20steht%20f%C3%BCr%20%E2%80%9EContemporary,des%20Tesla%20Model%203%20liefert (accessed on 23 August 2022).
- Habib, A.K.M.R.R.; Butler, K. Alternatives to lithium-ion batteries in electric vehicles. Future Technol. 2022, 1, 33–34. [Google Scholar] [CrossRef]
- Elwert, T.; Hua, Q.S.; Schneider, K. Recycling of Lithium Iron Phosphate Batteries: Future Prospects and Research Needs. Mater. Sci. Forum 2019, 959, 49–68. [Google Scholar] [CrossRef]
- London Metal Exchange. Available online: https://www.lme.com/en (accessed on 12 September 2022).
- Buchert, M.; Sutter, J. Ökobilanzen zum Recyclingverfahren; LithoRec II für Lithium-Ionen-Batterien: Berlin/Darmstadt, Germany, 2015. [Google Scholar]
- Metalary.com: Manganese Price. Available online: https://www.metalary.com/manganese-price/ (accessed on 12 September 2022).
- Statista. Durchschnittspreise Ausgewählter Mineralischer Rohstoffe in den Jahren 2014 bis 2020. Available online: https://de.statista.com/statistik/daten/studie/260427/umfrage/durchschnittspreise-ausgewaehlter-mineralischer-rohstoffe/ (accessed on 29 August 2022).
- Holzer, A.; Windisch-Kern, S.; Ponak, C.; Raupenstrauch, H. A Novel Pyrometallurgical Recycling Process for Lithium-Ion Batteries and Its Application to the Recycling of LCO and LFP. Metals 2021, 11, 149. [Google Scholar] [CrossRef]
- Gerold, E.; Antrekowitsch, H. Potenzialabschätzung von Synergieeffekten zur simultanen Rückgewinnung von Wertmetallen aus komplexen, metallhaltigen Reststoffen. Osterr. Wasser-Und Abfallwirtsch. 2022, 74, 22–31. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- uropean Parliament and the Council of the European Union–Directive 2006/66/EC of the European Parliament and of the Council of 6. September 2006 on Batteries and Accumulators and Waste Batteries and Accumulators and Repealing Directive 91/157/EEC. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006L0066-20131230&rid=1 (accessed on 10 September 2022).
- Li, H.; Xing, S.; Liu, Y.; Li, F.; Guo, H.; Kuang, G. Recovery of Lithium, Iron, and Phosphorus from Spent LiFePO 4 Batteries Using Stoichiometric Sulfuric Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 8017–8024. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Zanoni, R.; Branchi, M.; Marcucci, C.; Zamparelli, C.; Altimari, P.; Navarra, M.A.; Pagnanelli, F. Upcycling Real Waste Mixed Lithium-Ion Batteries by Simultaneous Production of rGO and Lithium-Manganese-Rich Cathode Material. ACS Sustain. Chem. Eng. 2021, 9, 13303–13311. [Google Scholar] [CrossRef]
- Qin, X.; Yang, G.; Cai, F.; Wang, B.; Jiang, B.; Chen, H.; Tan, C. Recovery and Reuse of Spent LiFePO4 Batteries. J. New Mater. Electrochem. Syst. 2019, 22, 119–124. [Google Scholar] [CrossRef]
- Lou, W.; Zhang, Y.; Zheng, S.; Sun, P.; Wang, X.; Li, J.; Qiao, S.; Zhang, Y.; Wenzel, M.; Weigand, J.J. Leaching performance of Al-bearing spent LiFePO4 cathode powder in H2SO4 aqueous solution. Trans. Nonferrous Met. Soc. China 2021, 31, 817–831. [Google Scholar] [CrossRef]
- Tang, H.; Dai, X.; Qiang, L.; Qiao, Y.; Tan, F. Selective Leaching of LiFePO4 by H2SO4 in the Presence of NaClO3. Rev. De Chim. 2020, 71, 248–254. [Google Scholar] [CrossRef]
- Dyana, Z.N.F.; Perdana, I.; Praseta, A. Kintics Study on Lithium Leaching of Spent Lithium Iron Phosphate Batteries in Low Concentration of Sulfuric Acid; Materials Science: Yogyakarta, Indonesia, 2020. [Google Scholar]
- Wang, M.; Liu, K.; Dutta, S.; Alessi, D.S.; Rinklebe, J.; Sik Ok, Y.; Tsang, D.C.W. Recycling of lithium iron phosphate batteries: Status, technologies, challenges, and prospects. Renew. Sustain. Energy Rev. 2022, 163, 112515. [Google Scholar] [CrossRef]
- Forte, F.; Pietrantonio, M.; Pucciarmatia, S.; Puzone, M.; Fontana, D. Lithium iron phosphate batteries recycling: An assessment of current status. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2232–2259. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, N.; Solangi, N.; Safdar, F.; Kumar, J. A short overview of recycling and treatment of spent LiFePO4 battery. North Am. Acad. Res. 2022, 5, 76–87. [Google Scholar]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Wu, D.; Wang, D.; Liu, Z.; Rao, S.; Zhang, K. Selective recovery of lithium from spent lithium iron phosphate batteries using oxidation pressure sulfuric acid leaching system. Trans. Nonferrous Met. Soc. China 2022, 32, 2071–2079. [Google Scholar] [CrossRef]
- Tao, S.; Li, J.; Zhou, H.; Wang, L.; Hu, H. A method for recovering Li3PO4 from spent lithium iron phosphate cathode material through high-temperature activation. Ionics 2019, 25, 5643–5653. [Google Scholar] [CrossRef]
- Dokko, K.; Koizumi, S.; Nakano, H.; Kanamura, K. Particle morphology, crystal orientation, and electrochemical reactivity of LiFePO4 synthesized by the hydrothermal method at 443 K. J. Mater. Chem. 2007, 17, 4803. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, J.; Liu, Y.; Yang, C.; Ma, B.; Chen, Y.; Wang, C. E-pH Diagrams for the Li-Fe-P-H2O System from 298 to 473 K: Thermodynamic Analysis and Application to the Wet Chemical Processes of the LiFePO4 Cathode Material. J. Phys. Chem. C 2019, 123, 14207–14215. [Google Scholar] [CrossRef]




| Component | Price [US-$/t] | LFP | NMC |
|---|---|---|---|
| Li | 76.886 | 2.78% | 4.76% |
| Ni | 2.200 | - | 7.94% |
| Co | 51.520 | - | 3.97% |
| Mn | 2.060 | - | 29.37% |
| Fe | 835 | 21.53% | - |
| Al | 2.280 | 42.36% | 24.60% |
| Other | - | - | 7.54% |
| P | 320 | 11.81% | - |
| O2 | - | 24.31% | 21.83% |
| Reference | Acid | Conc. | Oxidising Agent | Conc. | Temperature | Leaching Time | Stirring Speed | Efficiency |
|---|---|---|---|---|---|---|---|---|
| Li et al. (2017) [17] | H2SO4 | 0.3 M | H2O2 | 2.07 mol% H2O2/Li | 60 °C | 120 min | - | 95.56% Li |
| Jung et al. (2021) [27] | H2SO4 | 0.28 M | - | - | 85 °C | 240 min | - | 98.46% Li |
| Jung et al. (2021) [27] | H2SO4 | 2.5 M | - | - | 60 °C | 240 min | - | 97% Li |
| Jung et al. (2021) [27] | H2SO4 | - | O2/H2O2- | - | 80–120 °C | 120 min | - | 92% Li |
| Lou et al. (2021) [20] | H2SO4 | 0.35 mol% (H2SO4/LFP) | - | - | 20 °C | 90 min | 800 rpm | 91.53% LFP |
| Tang et al. (2020) [21] | H2SO4 | 1 M | NaClO3 | 25 mass% NaClO3/LFP | 90 °C | 60 min | 150 rpm | 97.23% Li |
| Wu et al. (2022) [28] | H2SO4 | 0.6 M | O2 | 1.3 MPa | 120 °C | 90 min | - | 95.74%Li |
| Tao et al. (2019) [29] | H2SO4 | 0.28 M | H2O2 | - | 85 °C | 240 min | - | 98.48% Li |
| Dyana et al. (2020) [22] | H2SO4 | 0.1 M | H2O2 | 2 vol% | 60 °C | 60 min | - | 74.74% Li |
| Parameter | Minimum Value | Maximum Value |
|---|---|---|
| H2SO4 [mol/L] | 0.3 | 2.3 |
| H2O2 [vol%] | 0 | 16.5 |
| Temperature [°C] | 30 | 80 |
| S/L ratio [g/L] | 50 | 110 |
| Leaching time [min] | 30 | 240 |
| Nb. | Acid Concentration | Oxidant Concentration | Leaching Time | Temperature | Solid/Liquid Ratio |
|---|---|---|---|---|---|
| - | [mol/L] | [vol%] | [min] | [°C] | [g/L] |
| N1 | 1.6 | 10.7 | 100 | 46.7 | 71.3 |
| N2 | 1.0 | 5.2 | 170 | 46.7 | 69.6 |
| N3 | 1.0 | 5.2 | 100 | 46.7 | 87.0 |
| N4 | 1.6 | 5.3 | 170 | 46.7 | 89.1 |
| N5 | 1.6 | 5.3 | 100 | 63.3 | 71.3 |
| N6 | 1.0 | 10.4 | 170 | 63.3 | 87.0 |
| N7 | 1.9 | 2.7 | 65 | 38.3 | 63.3 |
| N8 | 0.6 | 12.9 | 65 | 38.3 | 60.0 |
| N9 | 0.6 | 2.6 | 205 | 38.3 | 94.3 |
| N10 | 1.9 | 13.6 | 65 | 71.7 | 63.4 |
| N11 | 0.6 | 2.6 | 205 | 71.7 | 600 |
| N12 | 0.6 | 2.6 | 65 | 71.7 | 94.3 |
| N13 | 1.9 | 13.6 | 205 | 71.7 | 99.6 |
| N14 | 2.3 | 0.0 | 30 | 30.0 | 55.2 |
| N15 | 0.3 | 15.2 | 30 | 30.0 | 50.7 |
| N16 | 0.3 | 0.0 | 240 | 30.0 | 50.7 |
| N17 | 2.3 | 16.5 | 240 | 30.0 | 55.2 |
| N18 | 0.3 | 0.0 | 30 | 30.0 | 101.5 |
| N19 | 2.3 | 16.5 | 30 | 30.0 | 110.3 |
| N20 | 2.3 | 0.0 | 240 | 30.0 | 110.3 |
| N21 | 0.3 | 15.2 | 240 | 30.0 | 101.4 |
| N22 | 0.3 | 0.0 | 30 | 80.0 | 50.7 |
| N23 | 2.3 | 16.2 | 30 | 80.0 | 55.1 |
| N24 | 2.3 | 16.2 | 240 | 80.0 | 55.2 |
| N25 | 0.3 | 15.2 | 240 | 80.0 | 50.7 |
| N26 | 2.3 | 0.0 | 30 | 80.0 | 110.3 |
| N27 | 0.3 | 15.2 | 30 | 80.0 | 101.4 |
| N28 | 0.3 | 0.0 | 240 | 80.0 | 101.5 |
| N29 | 2.3 | 16.5 | 240 | 80.0 | 110.3 |
| N30 | 1.3 | 7.9 | 135 | 55.0 | 79.3 |
| N31 | 1.3 | 7.9 | 135 | 55.0 | 79.2 |
| N32 | 1.3 | 7.9 | 135 | 55.0 | 79.3 |
| N33 | 1.3 | 7.9 | 135 | 55.0 | 79.5 |
| N34 | 1.3 | 7.9 | 135 | 55.0 | 79.3 |
| N35 | 1.3 | 7.9 | 135 | 55.0 | 79.5 |
| Parameter | Value | Unit |
|---|---|---|
| Acid concentration | 2.00 | mol/L |
| Oxidant concentration | 0.02 | vol% |
| Leaching time | 240 | min |
| Solid/liquid ratio | 96.88 | g/L |
| Temperature | 30.02 | °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerold, E.; Lerchbammer, R.; Antrekowitsch, H. Parameter Study on the Recycling of LFP Cathode Material Using Hydrometallurgical Methods. Metals 2022, 12, 1706. https://doi.org/10.3390/met12101706
Gerold E, Lerchbammer R, Antrekowitsch H. Parameter Study on the Recycling of LFP Cathode Material Using Hydrometallurgical Methods. Metals. 2022; 12(10):1706. https://doi.org/10.3390/met12101706
Chicago/Turabian StyleGerold, Eva, Reinhard Lerchbammer, and Helmut Antrekowitsch. 2022. "Parameter Study on the Recycling of LFP Cathode Material Using Hydrometallurgical Methods" Metals 12, no. 10: 1706. https://doi.org/10.3390/met12101706






