Development of a Model to Estimate the Thermodynamic Stability of Organic Substances in Leaching Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtention of the Independent Reaction System for the Leaching Process
2.2. Gibbs Free Energy of Formation Calculations
2.3. Obtention of the Pourbaix Diagram
3. Results and Discussions
3.1. Gibbs Free Energy of Formation of Species
3.2. Independent Reaction System Estimation
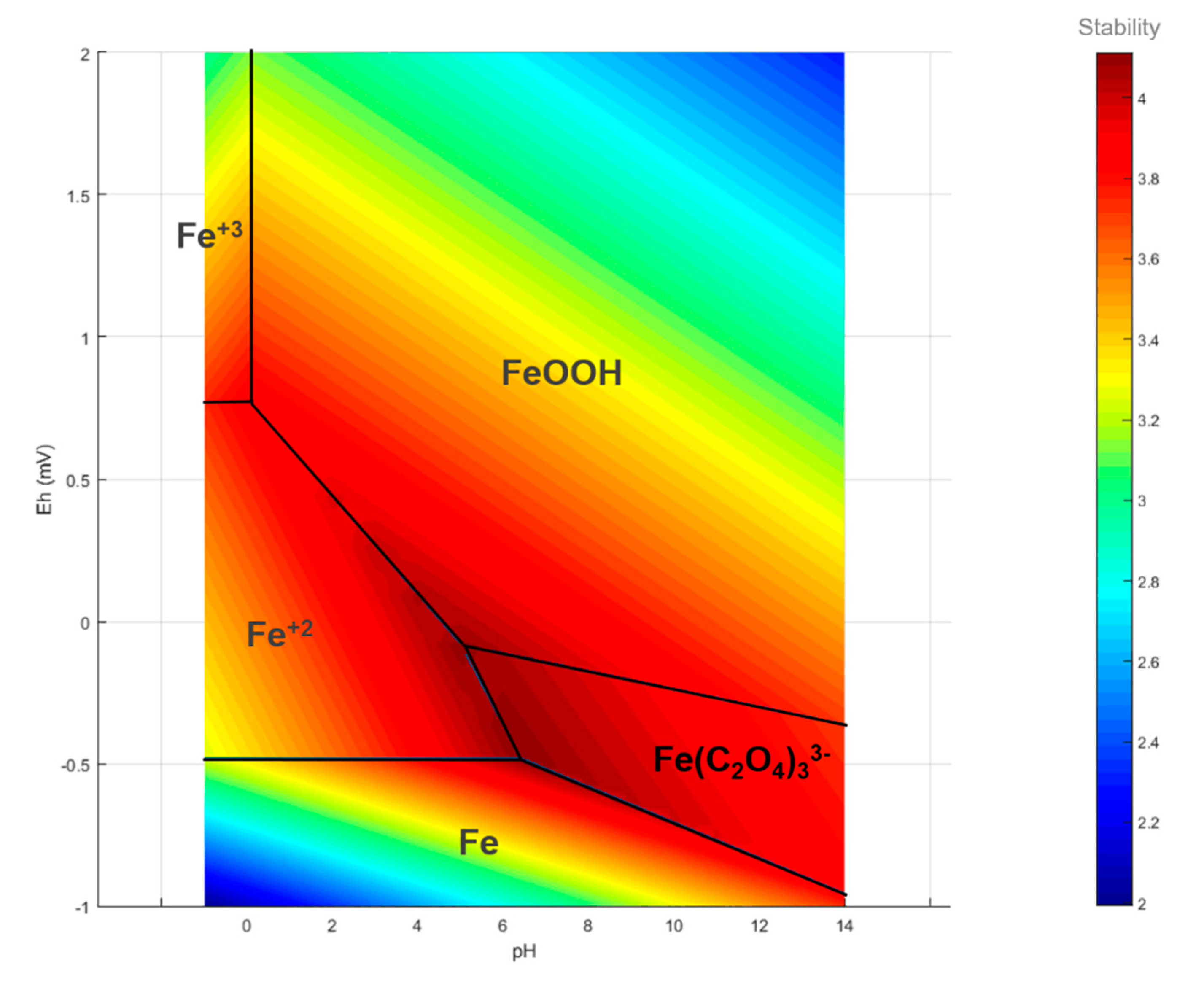
3.3. Stability Functions and Pourbaix Diagrams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saldaña, M.; Gálvez, E.; Robles, P.; Castillo, J.; Toro, N. Copper Mineral Leaching Mathematical Models—A Review. Materials 2022, 15, 1757. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, J.; Wang, H. Research on the Mathematical Model of Local Equilibrium in the Top-Blown Smelting Process of Electronic Waste. Metals 2021, 11, 1500. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, H.; Liang, G.; Zhu, J.; He, C.; Ma, S.; Shuai, Z.; Komarneni, S. Leaching Characteristics and Stabilization of Heavy Metals in Tin-Polymetallic Tailings by Sodium Diethyl Dithiocarbamate Intercalated Montmorillonite (DDTC-Mt). J. Clean. Prod. 2022, 344, 131041. [Google Scholar] [CrossRef]
- Yuksekdag, A.; Kose-Mutlu, B.; Siddiqui, A.F.; Wiesner, M.R.; Koyuncu, I. A Holistic Approach for the Recovery of Rare Earth Elements and Scandium from Secondary Sources under a Circular Economy Framework—A Review. Chemosphere 2022, 293, 133620. [Google Scholar] [CrossRef] [PubMed]
- Moraga, G.A.; Jamett, N.E.; Hernández, P.C.; Graber, T.A.; Taboada, M.E. Chalcopyrite Leaching with Hydrogen Peroxide and Iodine Species in Acidic Chloride Media at Room Temperature: Technical and Economic Evaluation. Metals 2021, 11, 1567. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating Organic Acids as Alternative Leaching Reagents for Metal Recovery from Lithium Ion Batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Pathak, A.; Vinoba, M.; Kothari, R. Emerging Role of Organic Acids in Leaching of Valuable Metals from Refinery-Spent Hydroprocessing Catalysts, and Potential Techno-Economic Challenges: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1–43. [Google Scholar] [CrossRef]
- Panias, D. Mechanisms of Dissolution of Iron Oxides in Aqueous Oxalic Acid Solutions. Hydrometallurgy 1996, 42, 257–265. [Google Scholar] [CrossRef]
- Lee, S.; Tran, T.; Jung, B.; Kim, S.; Kim, M. Dissolution of Iron Oxide Using Oxalic Acid. Hydrometallurgy 2007, 87, 91–99. [Google Scholar] [CrossRef]
- Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; Gómez-Arroyave, L. Mathematical Model for Scaling up Bioprocesses Using Experiment Design Combined with Buckingham Pi Theorem. Appl. Sci. 2021, 11, 11338. [Google Scholar] [CrossRef]
- Saldaña, M.; Neira, P.; Flores, V.; Robles, P.; Moraga, C. A Decision Support System for Changes in Operation Modes of the Copper Heap Leaching Process. Metals 2021, 11, 1025. [Google Scholar] [CrossRef]
- Saldaña, M.; Neira, P.; Flores, V.; Moraga, C.; Robles, P.; Salazar, I. Analysis of the Dynamics of Rougher Cells on the Basis of Phenomenological Models and Discrete Event Simulation Framework. Metals 2021, 11, 1454. [Google Scholar] [CrossRef]
- Huang, H.H. The Eh-PH Diagram and Its Advances. Metals 2016, 6, 23. [Google Scholar] [CrossRef]
- Ocampo-López, C.; Ramírez-Carmona, M.E.; Vélez-Ortiz, E. Thermodynamic Analysis of Stability in Iron Removal from Kaolin by Using Oxalic Acid. Ceramica 2013, 59, 326–330. [Google Scholar] [CrossRef]
- Ramírez-Carmona, M.; Rendon-Castrillon, L.; Ocampo-López, C.; Giraldo-Aristizabal, R. Proceso Para la Separación de Metales en una Matriz Sólida Mediante Lixiviación, Que Emplea una Composición Que Contiene Ácidos Carboxílicos, Monosacáridos, Disacáridos, Aminoácidos, Ácidos Grasos, Alcoholes y Compuestos Fenólicos. Patent Number NC2019/0013648, 2022. 13. [Google Scholar]
- Bhattacharyya, K.G.; Gupta, S. Sen Adsorption of a Few Heavy Metals on Natural and Modified Kaolinite and Montmorillonite: A Review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, Q.; Jin, X.; Chen, Z. Removal of Pb(II) from Aqueous Solution Using Modified and Unmodified Kaolinite Clay. J. Hazard. Mater. 2009, 170, 332–339. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Medici, F.; Pietrelli, L.; Piga, L. Effect of Acid Leaching Pre-Treatment on Gold Extraction from Printed Circuit Boards of Spent Mobile Phones. Materials 2021, 14, 362. [Google Scholar] [CrossRef]
- Fishtik, I. Thermodynamic Stability Relations in Redox Systems. Environ. Sci. Technol. 2006, 40, 1902–1910. [Google Scholar] [CrossRef]
- Fishtik, I. Thermodynamic Stability of Chemical Species in Multiple Reaction Systems. J. Phys. Chem. B 2005, 109, 3851–3859. [Google Scholar] [CrossRef]
- Fishtik, I.; Datta, R. A General Thermodynamic and Stoichiometric Theory of Stability of Chemical Species. Society 2004, 108, 5727–5739. [Google Scholar] [CrossRef]
- Schmidt, J.; Shi, J.; Borlido, P.; Chen, L.; Botti, S.; Marques, M.A.L. Predicting the Thermodynamic Stability of Solids Combining Density Functional Theory and Machine Learning. Chem. Mater. 2017, 29, 5090–5103. [Google Scholar] [CrossRef]
- Panias, D. Dissolution of Hematite in Acidic Oxalate Solutions: The Effect of Ferrous Ions Addition. Hydrometallurgy 1996, 43, 219–230. [Google Scholar] [CrossRef]
- Reklaitis, G. Introduction to Material and Energy Balances, 1st ed.; John Wiley: New York, NY, USA, 1991; ISBN 978-0-471-04131-3. [Google Scholar]
- Curtiss, L.A.; Raghavachari, K.; Redfern, P.C.; Rassolov, V.; Pople, J.A. Gaussian-3 (G3) Theory for Molecules Containing First and Second-Row Atoms. J. Chem. Phys. 1998, 109, 7764–7776. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-4 Theory. J. Chem. Phys. 2007, 126, 084108. [Google Scholar] [CrossRef]
- Zuluaga, J. Instalación de Gaussian en un Cluster Rocks; Universidad de Antioquia: Medellín, Colombia, 2006. [Google Scholar]
- Patel, A.M.; Nørskov, J.K.; Persson, K.A.; Montoya, J.H. Efficient Pourbaix Diagrams of Many-Element Compounds. Phys. Chem. Chem. Phys. 2019, 21, 25323–25327. [Google Scholar] [CrossRef]
- Lema, J.M.; Cameselle, C.; Ricart, M.T.; Nu, M.J. Iron Removal from Kaolin. Comparison between “in Situ” and “Two-Stage” Bioleaching Processes. Hydrometallurgy 2003, 68, 97–105. [Google Scholar]
- Lee, S.; Tran, T.; Park, Y.; Kim, S.; Kim, M. Study on the Kinetics of Iron Oxide Leaching by Oxalic Acid. Int. J. Miner. Process. 2006, 80, 144–152. [Google Scholar] [CrossRef]
- Deng, B.; Wang, B.; Su, S.; Ding, S.; Sun, W. Recovery of Iron from Pyrolusite Leaching Slag by a Lab-Scale Circulation Process of Oxalic Acid Leaching and Ultraviolet Irradiation. Metals 2017, 8, 8. [Google Scholar] [CrossRef]
- Ocampo-López, C. Estudio Termodinámico de Remoción Biotecnológica de Hierro Presente en Caolín; Universidad Pontificia Bolivariana: Medellín, Colombia, 2011. [Google Scholar]
- Vincze, L.; Papp, S. Individual Quantum Yields of Fe3+OXn2−Hm+ Complexes in Aqueous Acidic Solutions (OX2− ≡ C2O42−, n = 1–3, m = 0, 1). J. Photochem. 1987, 36, 289–296. [Google Scholar] [CrossRef]
- Bourdoiseau, J.A.; Sabot, R.; Jeannin, M.; Termemil, F.; Refait, P. Determination of Standard Gibbs Free Energy of Formation of Green Rusts and Its Application to the Fe(II–III) Hydroxy-Oxalate. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 72–80. [Google Scholar] [CrossRef]
- Uchimiya, M.; Stone, A.T. Redox Reactions between Iron and Quinones: Thermodynamic Constraints. Geochim. Cosmochim. Acta 2006, 70, 1388–1401. [Google Scholar] [CrossRef]
- Refait, P.; Bon, C.; Simon, L.; Bourrié, G.; Trolard, F.; Bessière, J.; Gènin, J.-M.R. Chemical Composition and Gibbs Standard Free Energy of Formation of Fe(II)-Fe(III) Hydroxysulphate Green Rust and Fe(II) Hydroxide. Clay Miner. 1999, 34, 499–510. [Google Scholar] [CrossRef]
- Xu, N.; Gao, Y. Characterization of Hematite Dissolution Affected by Oxalate Coating, Kinetics and PH. Appl. Geochem. 2008, 23, 783–793. [Google Scholar] [CrossRef]


| Species | Source | |
|---|---|---|
| 0.00 | [19,34] | |
| −21.88 | [35] | |
| −4.11 | [19] | |
| −1407.51 | [14,32] | |
| −2308.38 | This study | |
| −3068.89 | This study | |
| −3753.88 | This study | |
| −117.07 | [19] | |
| −116.39 | [36] | |
| −242.65 | [36] | |
| +7281.06 | [36] | |
| +1541.17 | [35] | |
| −166.52 | [35] | |
| 0.00 | [19] | |
| 0.00 | [19] | |
| −56.58 | [35] |
| Species | Bi | e− | H | O | Fe | C2O42− |
|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 0 | 0 | |
| 2 | −1 | 1 | 0 | 0 | 0 | |
| 3 | 0 | 2 | 1 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 1 | 0 | |
| 5 | 0 | 2 | 0 | 0 | 1 | |
| 6 | −2 | 0 | 0 | 1 | 0 | |
| 7 | −3 | 0 | 0 | 1 | 0 | |
| 8 | 0 | 0 | 0 | 1 | 1 | |
| 9 | 2 | 0 | 0 | 1 | 2 | |
| 10 | 1 | 0 | 0 | 1 | 2 | |
| 11 | 3 | 0 | 0 | 1 | 3 | |
| 12 | 0 | 1 | 2 | 1 | 0 | |
| 13 | 0 | 2 | 2 | 1 | 0 | |
| 14 | 0 | 0 | 4 | 3 | 0 | |
| 15 | 2 | 0 | 0 | 0 | 1 | |
| 16 | 1 | 1 | 0 | 0 | 1 |
| ν | B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 | B10 | B11 | B12 | B13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| −1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| −1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −1 | |
| −1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −2 | |
| −1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | −2 | |
| −1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | −3 | |
| −1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| −3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | −1 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | −1 |
| Independent Reaction | Stoichiometric Reaction |
|---|---|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocampo-López, C.; Ospina-Sanjuan, Á.; Ramírez-Carmona, M.; Rendón-Castrillón, L. Development of a Model to Estimate the Thermodynamic Stability of Organic Substances in Leaching Processes. Metals 2022, 12, 1424. https://doi.org/10.3390/met12091424
Ocampo-López C, Ospina-Sanjuan Á, Ramírez-Carmona M, Rendón-Castrillón L. Development of a Model to Estimate the Thermodynamic Stability of Organic Substances in Leaching Processes. Metals. 2022; 12(9):1424. https://doi.org/10.3390/met12091424
Chicago/Turabian StyleOcampo-López, Carlos, Álvaro Ospina-Sanjuan, Margarita Ramírez-Carmona, and Leidy Rendón-Castrillón. 2022. "Development of a Model to Estimate the Thermodynamic Stability of Organic Substances in Leaching Processes" Metals 12, no. 9: 1424. https://doi.org/10.3390/met12091424









