Features of Cathodic Plasma Electrolytic Nitrocarburizing of Low-Carbon Steel in an Aqueous Electrolyte of Ammonium Nitrate and Glycerin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Processing
2.2. Surface Characterization
3. Results
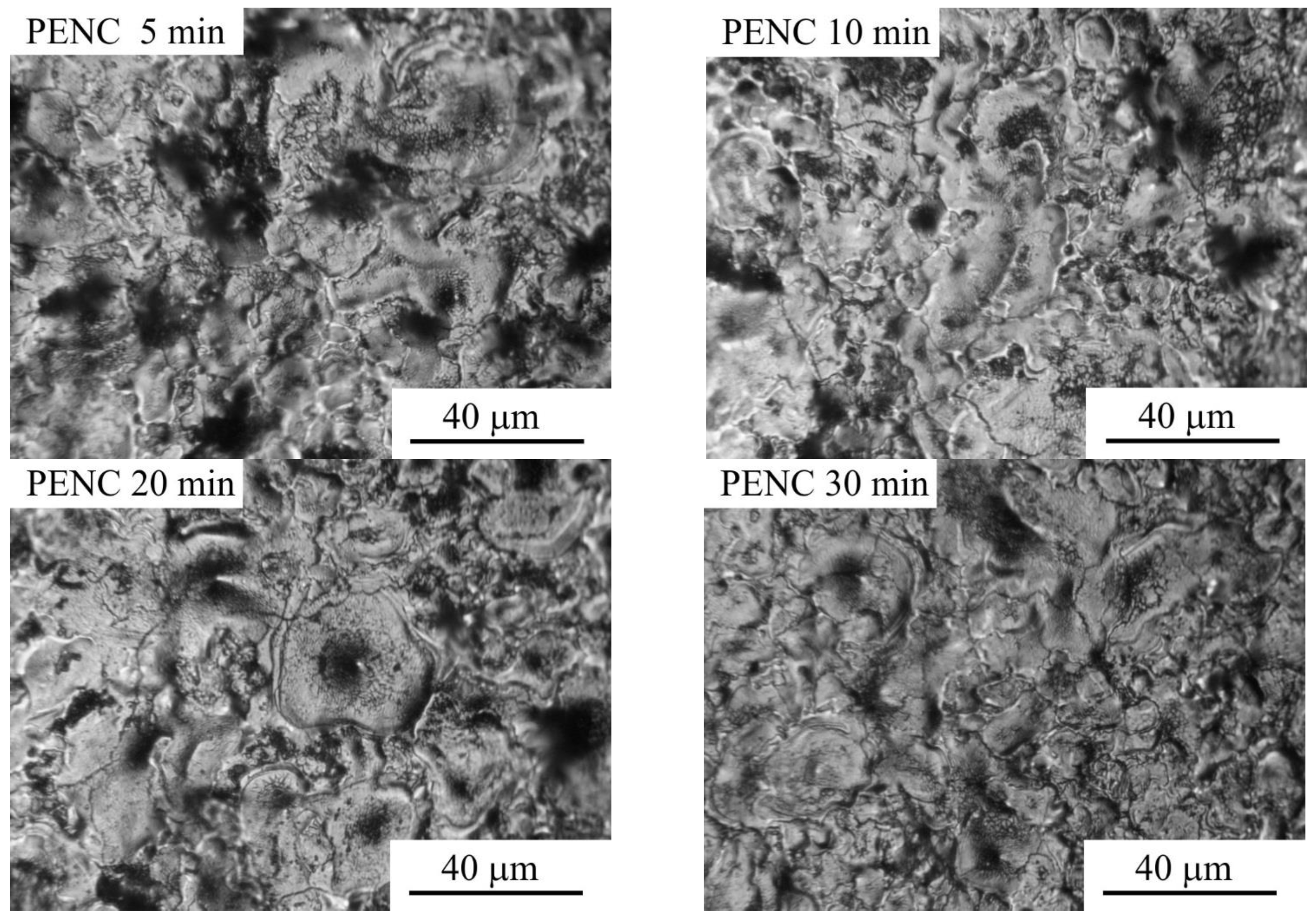
3.1. Morphology, Phase Composition, and Roughness of the Surface
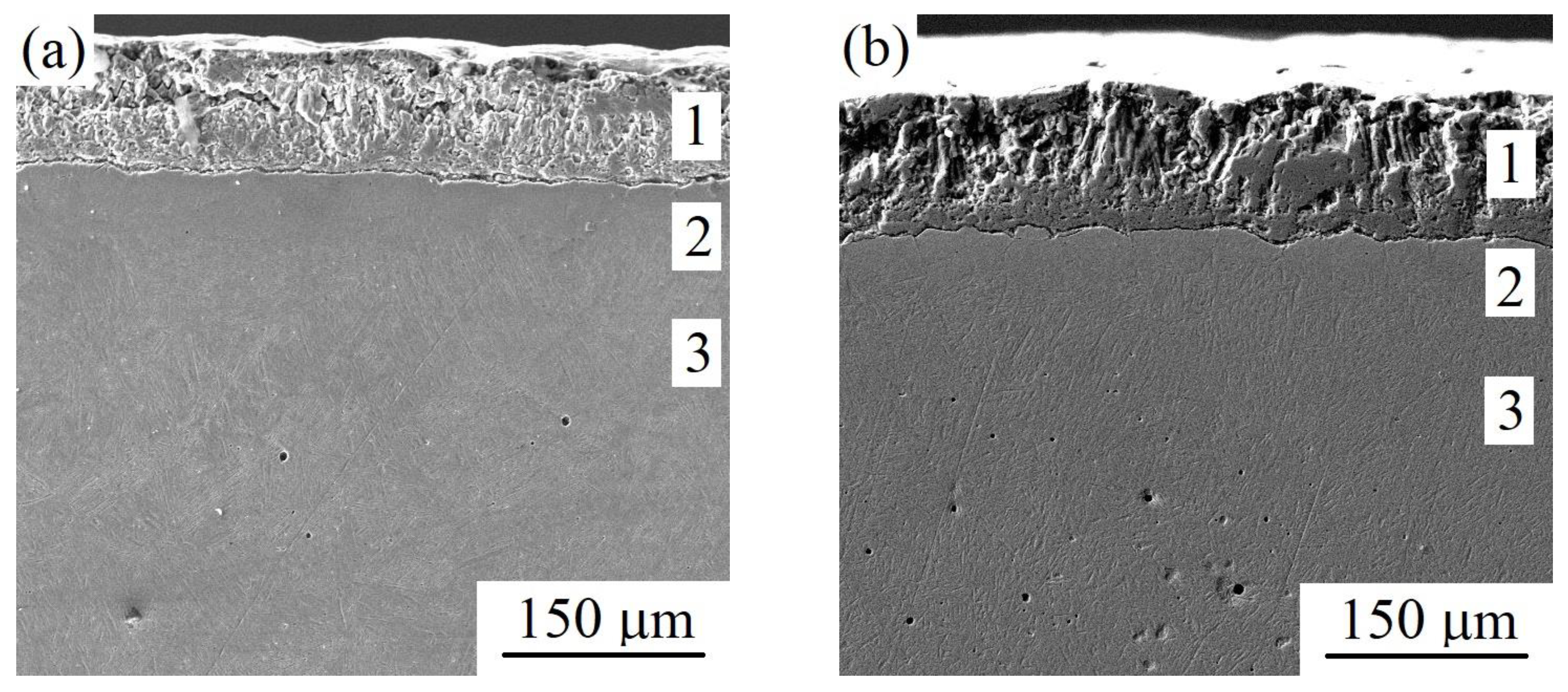
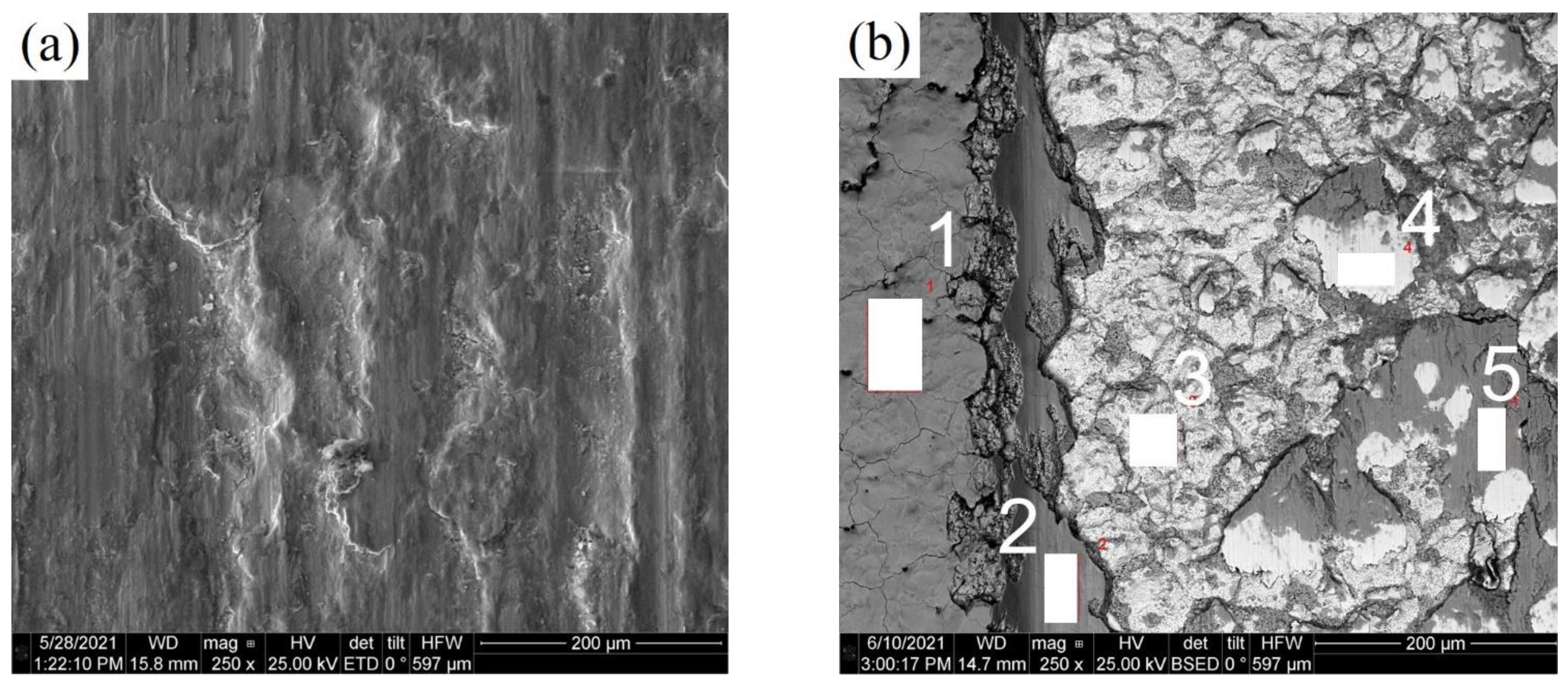
3.2. Cross-Sectional SEM and EDX Analysis of PENC Samples
3.3. Calculation of the Diffusion Coefficients of Nitrogen and Carbon
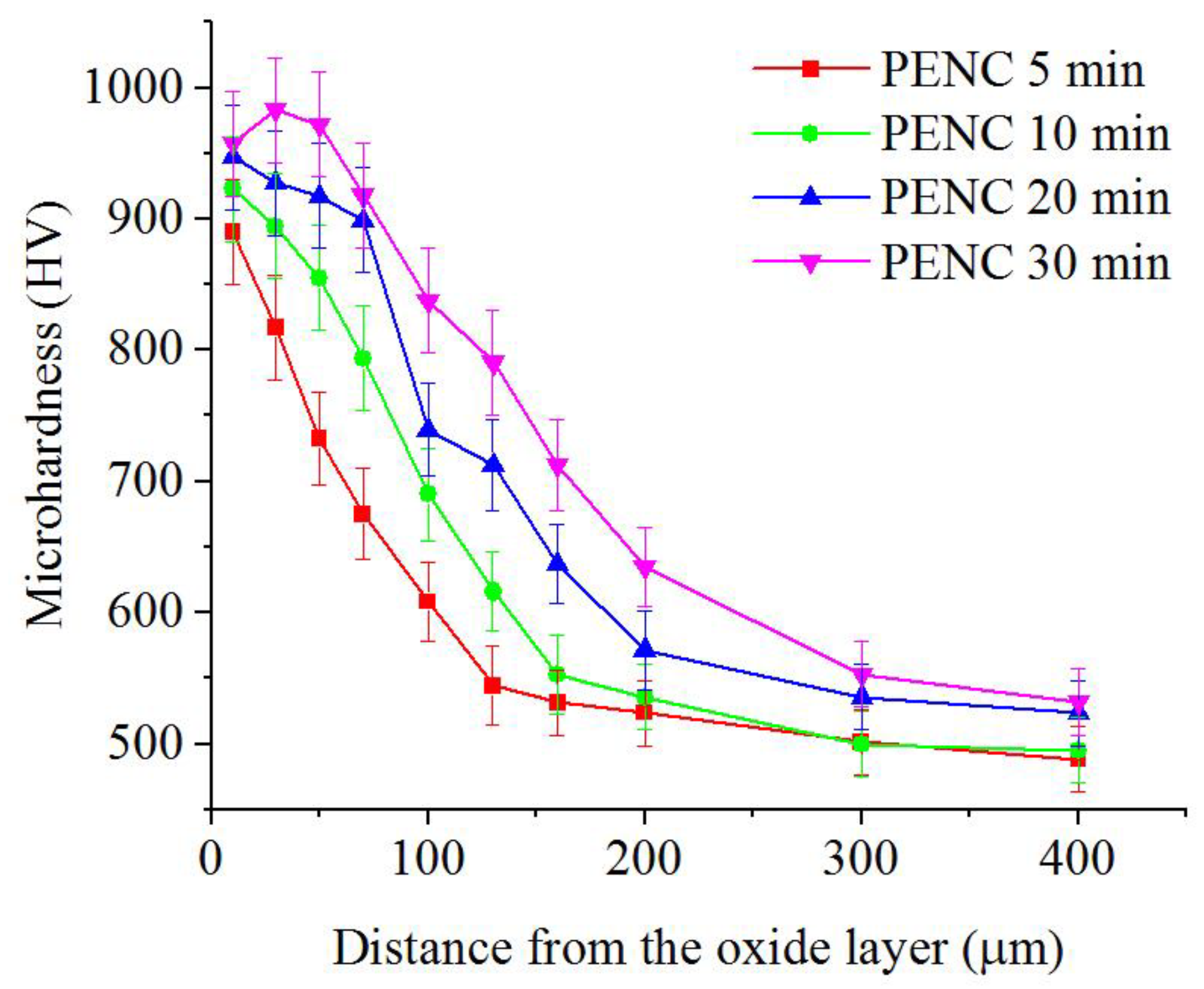
3.4. Microhardness of the Diffusion of the Nitrocarburized Layer
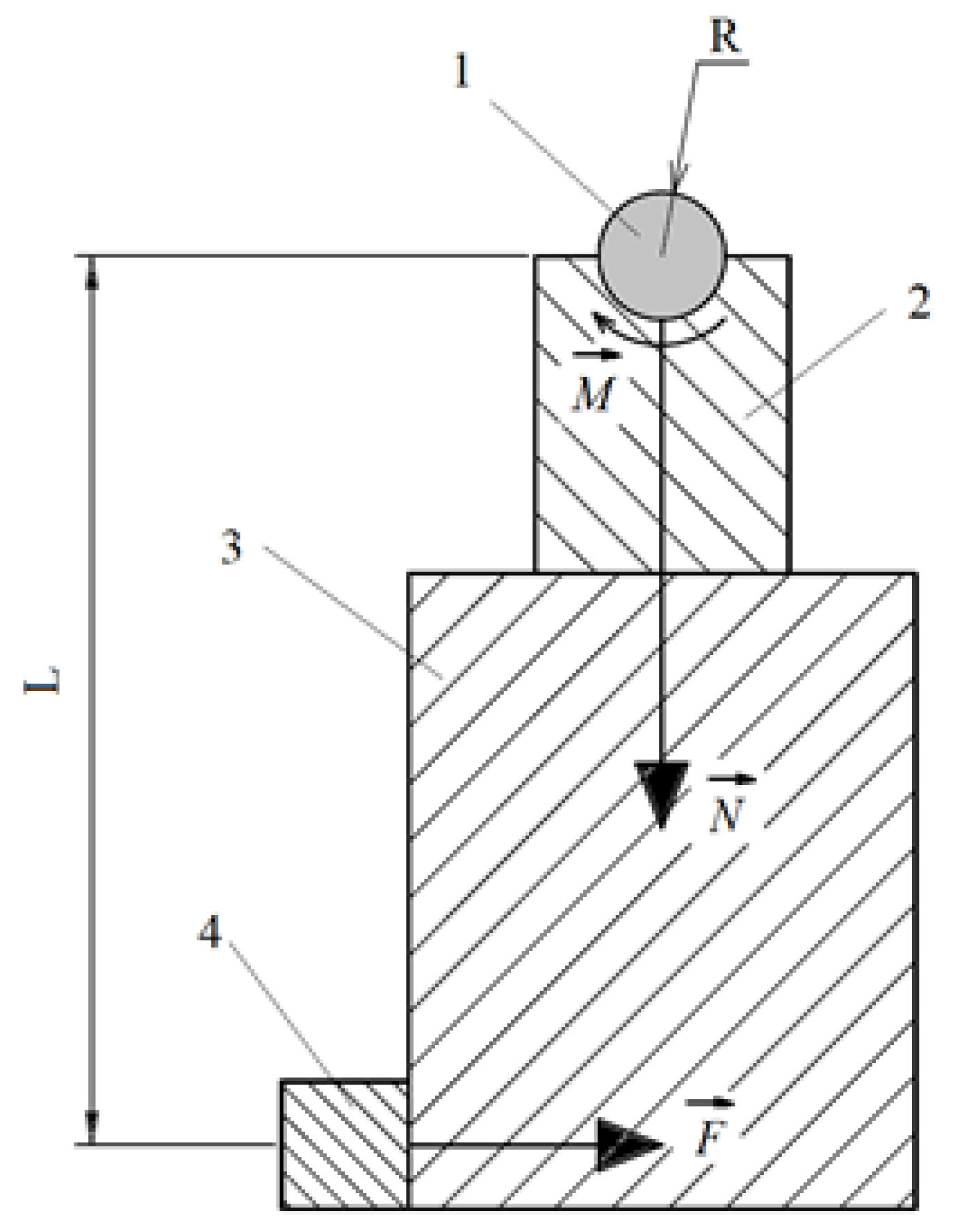
3.5. Tribological Properties of the PENC Surface
3.6. Corrosion Properties of the PENC Surface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metel, A.S.; Grigoriev, S.N.; Melnik, Y.A.; Panin, V.V. Filling the vacuum chamber of a technological system with homogeneous plasma using a stationary glow discharge. Plasma Phys. Rep. 2009, 35, 1058–1067. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Sinopalnikov, V.A.; Tereshin, M.V.; Gurin, V.D. Control of parameters of the cutting process on the basis of diagnostics of the machine tool and workpiece. Meas. Tech. 2012, 55, 555–558. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Vereschaka, A.A.; Fyodorov, S.V.; Sitnikov, N.N.; Batako, A.D. Comparative analysis of cutting properties and nature of wear of carbide cutting tools with multi-layered nano-structured and gradient coatings produced by using of various deposition methods. Int. J. Adv. Manuf. Technol. 2017, 90, 3421–3435. [Google Scholar] [CrossRef]
- Volosova, M.; Grigoriev, S.; Metel, A.; Shein, A. The Role of Thin-Film Vacuum-Plasma Coatings and Their Influence on the Efficiency of Ceramic Cutting Inserts. Coatings 2018, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Grigoriev, S.N.; Volosova, M.A.; Okunkova, A.A.; Fedorov, S.V.; Hamdy, K.; Podrabinnik, P.A.; Pivkin, P.M.; Kozochkin, M.P.; Porvatov, A.N. Electrical Discharge Machining of Oxide Nanocomposite: Nanomodification of Surface and Subsurface Layers. J. Manuf. Mater. Process. 2020, 4, 96. [Google Scholar] [CrossRef]
- Apelfeld, A.; Grigoriev, S.; Krit, B.; Ludin, V.; Suminov, I.; Chudinov, D. Improving the stability of the coating properties for group plasma electrolytic oxidation. Manuf. Lett. 2022, 33, 54–59. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Kondratsky, I.O.; Krit, B.L.; Ludin, V.B.; Medvetskova, V.M.; Morozova, N.V.; Suminov, I.V.; Apelfeld, A.V.; Wu, R.Z. Protective and Thermophysical Characteristics of Plasma-Electrolytic Coatings on the Ultralight Magnesium Alloy. J. Eng. Mater. Technol. 2022, 144, 021006. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. Advances 2021, 5, 100121. [Google Scholar] [CrossRef]
- Jin, S.; Ma, X.; Wu, R.; Wang, G.; Zhang, J.; Krit, B.; Betsofen, S.; Liu, B. Advances in micro-arc oxidation coatings on Mg-Li alloys. Appl. Surf. Sci. Adv. 2022, 8, 100219. [Google Scholar] [CrossRef]
- Bogdashkina, N.L.; Gerasimov, M.V.; Zalavutdinov, R.K.; Kasatkina, I.V.; Krit, B.L.; Lyudin, V.B.; Fedichkin, I.D.; Shcherbakov, A.I.; Apelfeld, A.V. Influence of Nickel Sulfate Additives to Electrolytes Subjected to Microarc Oxidation on the Structure, Composition, and Properties of Coatings Formed on Titanium. Surf. Eng. Appl. Electrochem. 2018, 54, 331–337. [Google Scholar] [CrossRef]
- Belkin, P.N.; Yerokhin, A.; Kusmanov, S.A. Plasma Electrolytic Saturation of Steels with Nitrogen and Carbon. Surf. Coat. Technol. 2016, 307, 1194–1218. [Google Scholar] [CrossRef]
- Jiang, Y.-F.; Bao, Y.-F.; Yang, K. Effect of C/N concentration fluctuation on formation of plasma electrolytic carbonitriding coating on Q235. J. Iron Steel Res. Int. Vol. 2012, 19, 39–45. [Google Scholar] [CrossRef]
- Shen, D.-J.; Wang, Y.-L.; Nash, P.; Xing, G.-Z. A novel method of surface modification for steel by plasma electrolysis carbonitriding. Mater. Sci. Eng. A 2007, 458, 240–243. [Google Scholar] [CrossRef]
- Zarchi, M.K.; Shariat, M.H.; Dehghan, S.A.; Solhjoo, S. Characterization of nitrocarburized surface layer on AISI 1020 steel by electrolytic plasma processing in an urea electrolyte. J. Mater. Res. Technol. 2013, 2, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Rastkar, A.R.; Shokri, B. Surface modification and wear test of carbon steel by plasma electrolytic nitrocarburizing. Surf. Interface Anal. 2012, 44, 342–351. [Google Scholar] [CrossRef]
- Noori, S.M.; Dehghanian, C. Corrosion behavior of nitrocarburized coating on AISI 1045 steel deposited by Plasma Electrolytic Saturation. In Proceedings of the 5th International Conference on Materials Engineering and Metallurgy, Shiraz, Iran, 8–9 November 2016. [Google Scholar]
- Sheng, Y.; Zhang, Z.; Li, W. Effects of pulse frequency and duty cycle on the plasma discharge characteristics and surface microstructure of carbon steel by plasma electrolytic nitrocarburizing. Surf. Coat. Technol. 2017, 330, 113–120. [Google Scholar] [CrossRef]
- Tavakoli, H.; Mousavi Khoie, S.M.; Marashi, S.P.H.; Bolhasani, O. Effect of Electrolyte Composition on Characteristics of Plasma Electrolysis Nitrocarburizing. J. Mater. Eng. Perform. 2013, 22, 2351–2358. [Google Scholar] [CrossRef]
- Tsotsos, C.; Yerokhin, A.L.; Wilson, A.D.; Leyland, A.; Matthews, A. Tribological evaluation of AISI 304 stainless steel duplex treated by plasma electrolytic nitrocarburising and diamond-like carbon coating. Wear 2002, 253, 986–993. [Google Scholar] [CrossRef]
- Kazerooni, N.A.; Bahrololoom, M.E.; Shariat, M.H.; Mahzoon, F.; Jozaghi, T. Effect of Ringer’s solution on wear and friction of stainless steel 316L after plasma electrolytic nitrocarburising at low voltages. J. Mater. Sci. Technol. 2011, 27, 906–912. [Google Scholar] [CrossRef]
- Jiang, Y.; Bao, Y.; Yang, K. Formation and friction behavior of plasma electrolytically nitrocarburized surface layers on Q235 steel. Surf. Coat. Technol. 2015, 269, 324–328. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Silkin, S.A.; Smirnov, A.A.; Belkin, P.N. Possibilities of increasing wear resistance of steel surface by plasma electrolytic treatment. Wear 2017, 386–387, 239–246. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Smirnov, A.A.; Silkin, S.A.; Belkin, P.N. Increasing wear and corrosion resistance of low-alloy steel by anode plasma electrolytic nitriding. Surf. Coat. Technol. 2016, 307, 1350–1356. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Kusmanova, Y.V.; Smirnov, A.A.; Belkin, P.N. Modification of steel surface by plasma electrolytic saturation with nitrogen and carbon. Mater. Chem. Phys. 2016, 175, 164–171. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Dyakov, I.G.; Kusmanova, Y.V.; Belkin, P.N. Surface Modification of Low-Carbon Steels by Plasma Electrolytic Nitrocarburising. Plasma Chem. Plasma Process. 2016, 36, 1271–1286. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Kusmanov, S.A.; Kusmanova, I.A.; Belkin, P.N. Effect of Electrolyte Depletion on the Characteristics of the Anodic Plasma Electrolytic Nitriding of a VT22 Titanium Alloy. Surf. Eng. Appl. Electrochem. 2017, 53, 413–418. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Tambovskiy, I.V.; Gorokhov, I.S.; Belkin, P.N. Plasma Electrolytic Polishing Effect on Steel’s Surface Roughness after Cathodic Saturation with Nitrogen and Carbon. Surf. Eng. Appl. Electrochem. 2021, 57, 513–518. [Google Scholar] [CrossRef]
- Mukhacheva, T.L.; Belkin, P.N.; Dyakov, I.G.; Kusmanov, S.A. Wear mechanism of medium carbon steel after its plasma electrolytic nitrocarburising. Wear 2020, 462–463, 203516. [Google Scholar] [CrossRef]




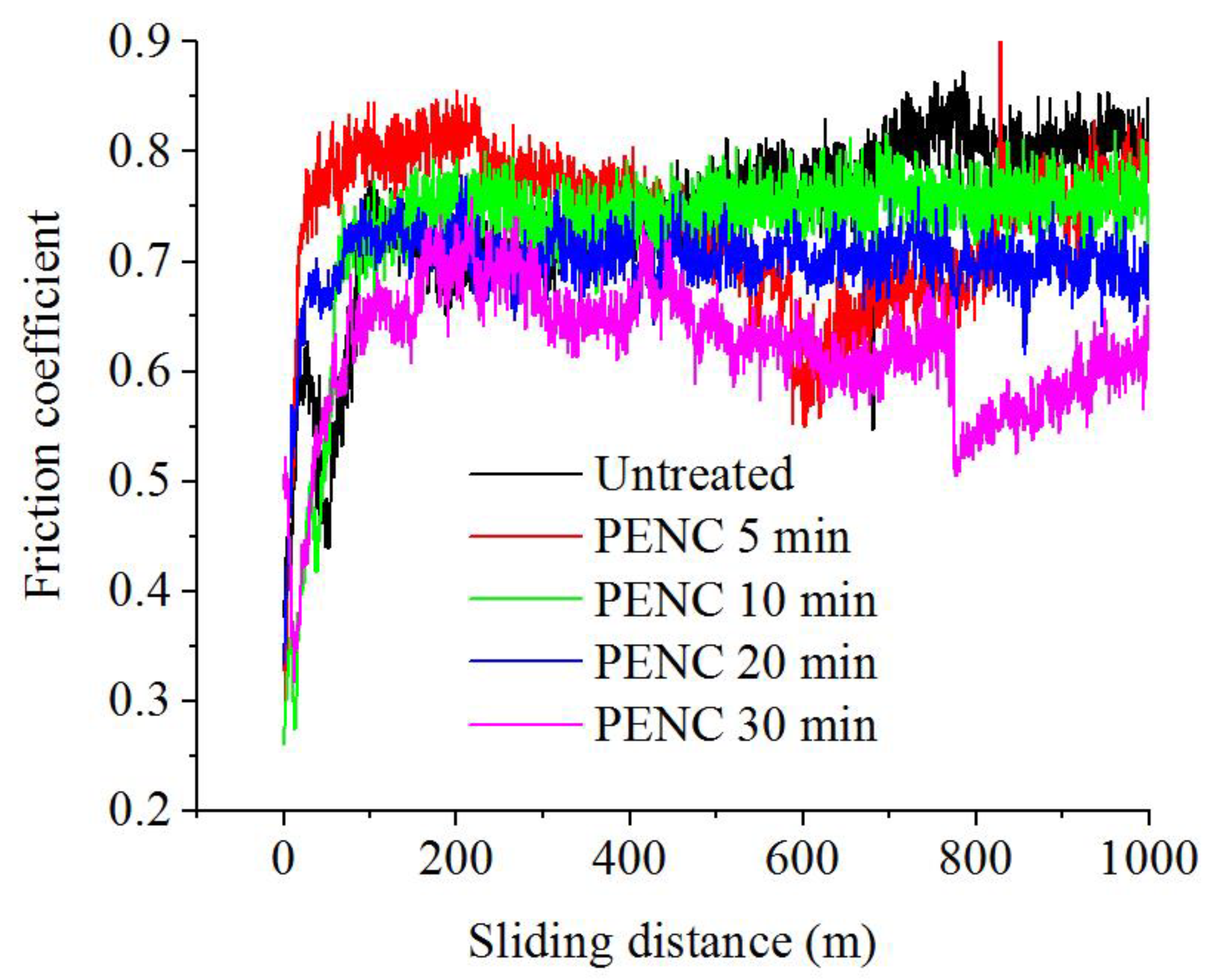

| PENC Time (min) | Weight Loss of Samples during PENC (mg) | Surface Roughness, Ra (μm) | Average Friction Coefficient for the Last 100 m of Track | Weight Loss of Samples during Tribological Test (mg) | Corrosion Current Density (μA/cm2) |
|---|---|---|---|---|---|
| Untreated | – | 1.00 ± 0.10 | 0.824 ± 0.015 | 14.0 ± 0.3 | 22.8 ± 4.0 |
| 5 | 29 ± 4 | 1.38 ± 0.32 | 0.730 ± 0.012 | 16.0 ± 0.3 | 31.9 ± 4.2 |
| 10 | 74 ± 6 | 0.94 ± 0.18 | 0.736 ± 0.012 | 16.5 ± 0.3 | 32.4 ± 4.1 |
| 20 | 177 ± 4 | 0.77 ± 0.25 | 0.694 ± 0.011 | 8.0 ± 0.2 | 16.4 ± 3.2 |
| 30 | 276 ± 8 | 0.66 ± 0.06 | 0.621 ± 0.010 | 8.0 ± 0.2 | 39.8 ± 4.4 |
| Diffuser | D (μm2/s) |
|---|---|
| Carbon | 0.866 ± 0.013 |
| Nitrogen | 0.384 ± 0.006 |
| Diffuser | D (μm2/s) | |
|---|---|---|
| Carbon | D11 | 0.768 ± 0.012 |
| D12 | 0.070 ± 0.001 | |
| Nitrogen | D21 | 0.799 ± 0.012 |
| D22 | 0.362 ± 0.005 | |
| Element | Area 1 (Oxide Layer without Friction) | Area 2 (Oxide Layer after Friction) | Area 3 (PENC Layer) | Area 4 (PENC Layer) | Area 5 (Oxide Layer after Friction) |
|---|---|---|---|---|---|
| C | 0.58 ± 0.13 | 0.36 ± 0.12 | 0.97 ± 0.16 | 0.73 ± 0.15 | 0.54 ± 0.12 |
| N | – | – | 0.26 ± 0.12 | 0.34 ± 0.14 | – |
| O | 32.72 ± 0.35 | 26.760.29 | 9.05 ± 0.19 | 2.97 ± 0.13 | 35.04 ± 0.32 |
| Si | – | 0.45 ± 0.05 | 0.57 ± 0.06 | 0.30 ± 0.06 | 0.85 ± 0.06 |
| Cr | – | 0.16 ± 0.04 | 0.10 ± 0.04 | 0.06 ± 0.04 | 0.47 ± 0.04 |
| Mn | – | 0.46 ± 0.06 | 0.53 ± 0.06 | 0.61 ± 0.06 | 0.32 ± 0.05 |
| Ni | – | 0.16 ± 0.07 | 0.45 ± 0.08 | 0.15 ± 0.08 | 0.17 ± 0.07 |
| Cu | – | 0.13 ± 0.07 | 0.14 ± 0.08 | 0.11 ± 0.08 | 0.11 ± 0.07 |
| Fe | Total 100 | Total 100 | Total 100 | Total 100 | Total 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tambovskiy, I.; Mukhacheva, T.; Gorokhov, I.; Suminov, I.; Silkin, S.; Dyakov, I.; Kusmanov, S.; Grigoriev, S. Features of Cathodic Plasma Electrolytic Nitrocarburizing of Low-Carbon Steel in an Aqueous Electrolyte of Ammonium Nitrate and Glycerin. Metals 2022, 12, 1773. https://doi.org/10.3390/met12101773
Tambovskiy I, Mukhacheva T, Gorokhov I, Suminov I, Silkin S, Dyakov I, Kusmanov S, Grigoriev S. Features of Cathodic Plasma Electrolytic Nitrocarburizing of Low-Carbon Steel in an Aqueous Electrolyte of Ammonium Nitrate and Glycerin. Metals. 2022; 12(10):1773. https://doi.org/10.3390/met12101773
Chicago/Turabian StyleTambovskiy, Ivan, Tatiana Mukhacheva, Ilya Gorokhov, Igor Suminov, Sergey Silkin, Ilya Dyakov, Sergei Kusmanov, and Sergey Grigoriev. 2022. "Features of Cathodic Plasma Electrolytic Nitrocarburizing of Low-Carbon Steel in an Aqueous Electrolyte of Ammonium Nitrate and Glycerin" Metals 12, no. 10: 1773. https://doi.org/10.3390/met12101773
APA StyleTambovskiy, I., Mukhacheva, T., Gorokhov, I., Suminov, I., Silkin, S., Dyakov, I., Kusmanov, S., & Grigoriev, S. (2022). Features of Cathodic Plasma Electrolytic Nitrocarburizing of Low-Carbon Steel in an Aqueous Electrolyte of Ammonium Nitrate and Glycerin. Metals, 12(10), 1773. https://doi.org/10.3390/met12101773







