Prediction of Thermal Distortion during Steel Solidification
Abstract
:1. Introduction
2. Model Description
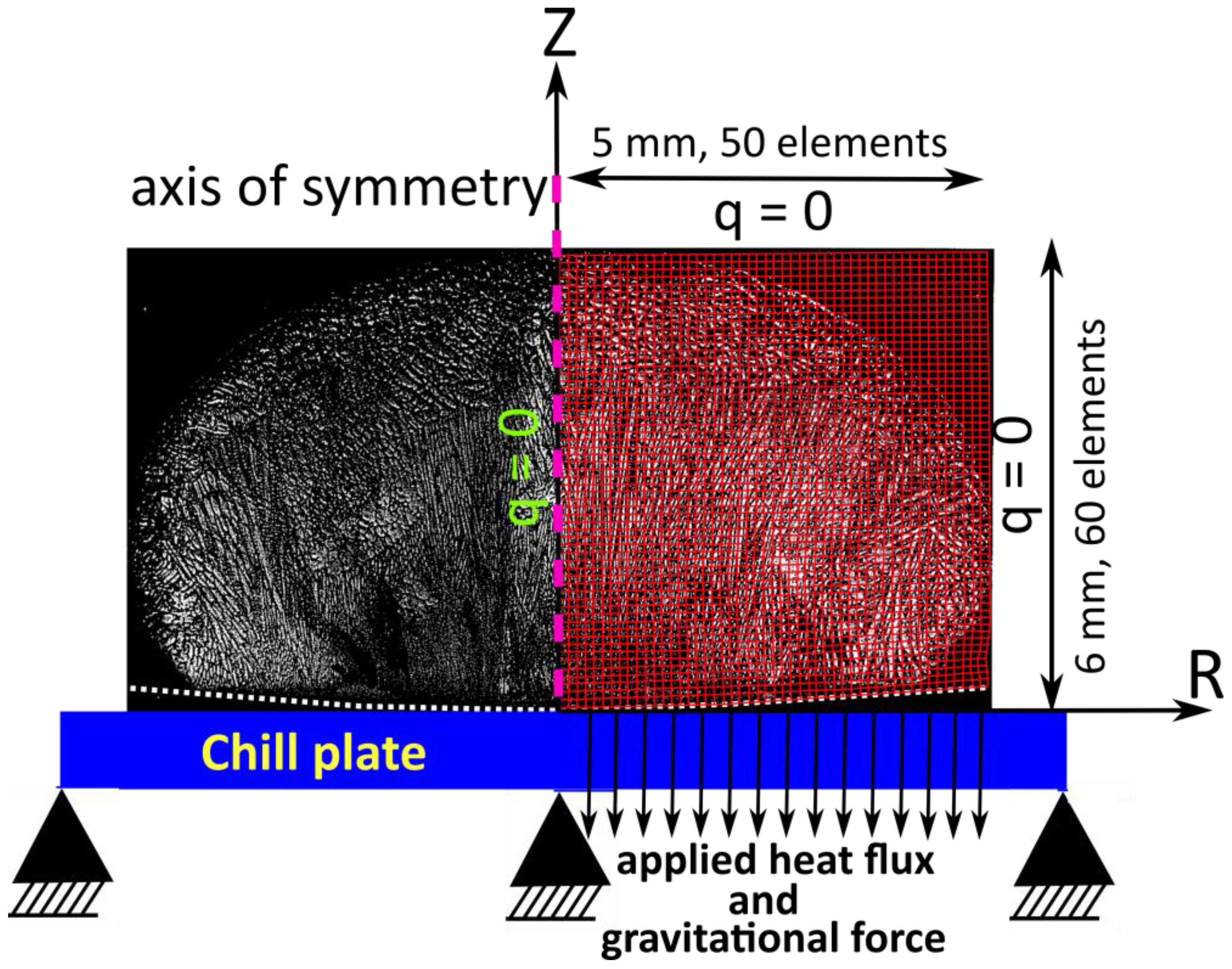
2.1. Model Domain and Boundary Conditions
2.2. Heat Transfer Model and Applied Heat Flux
2.3. Stress Model
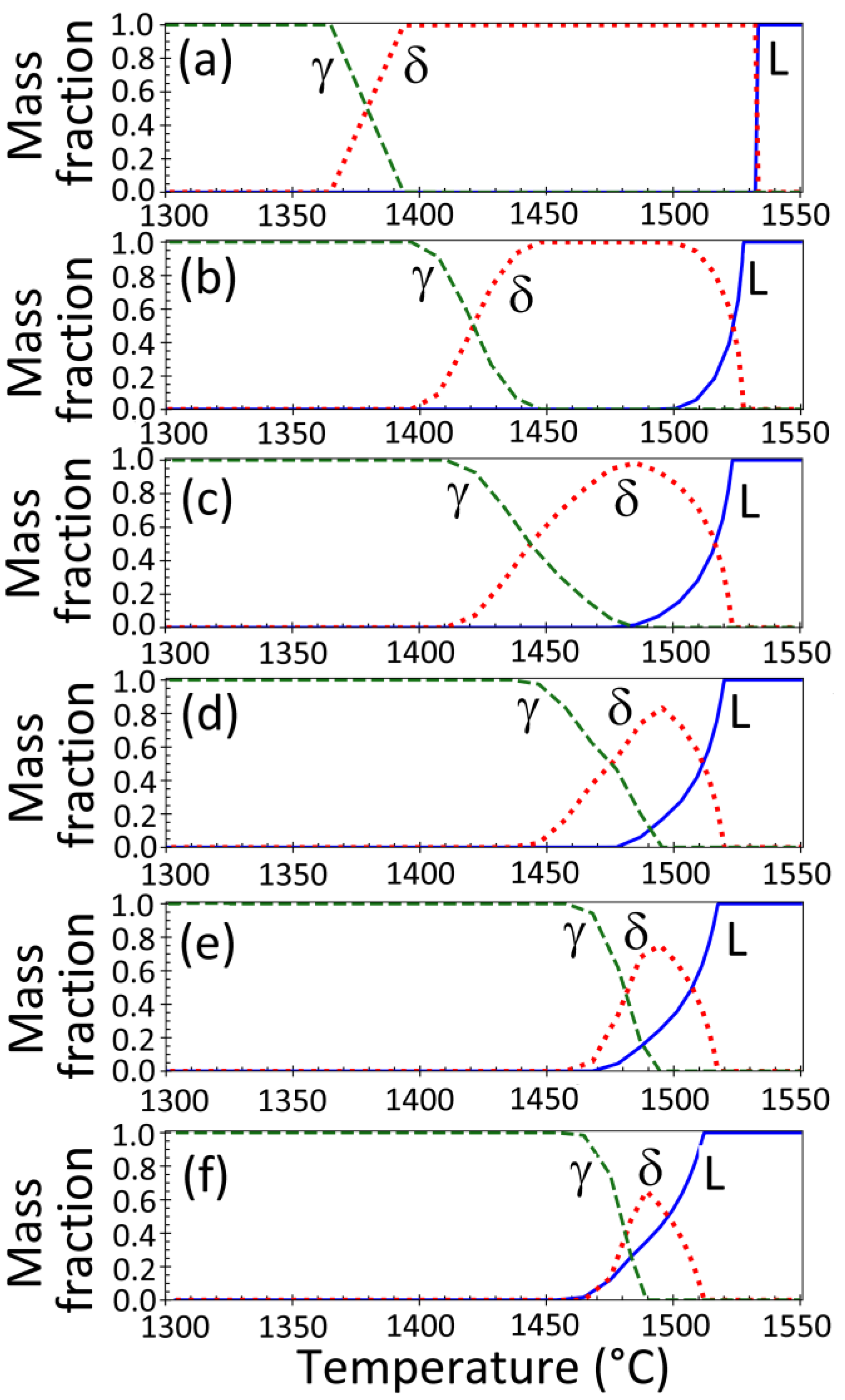
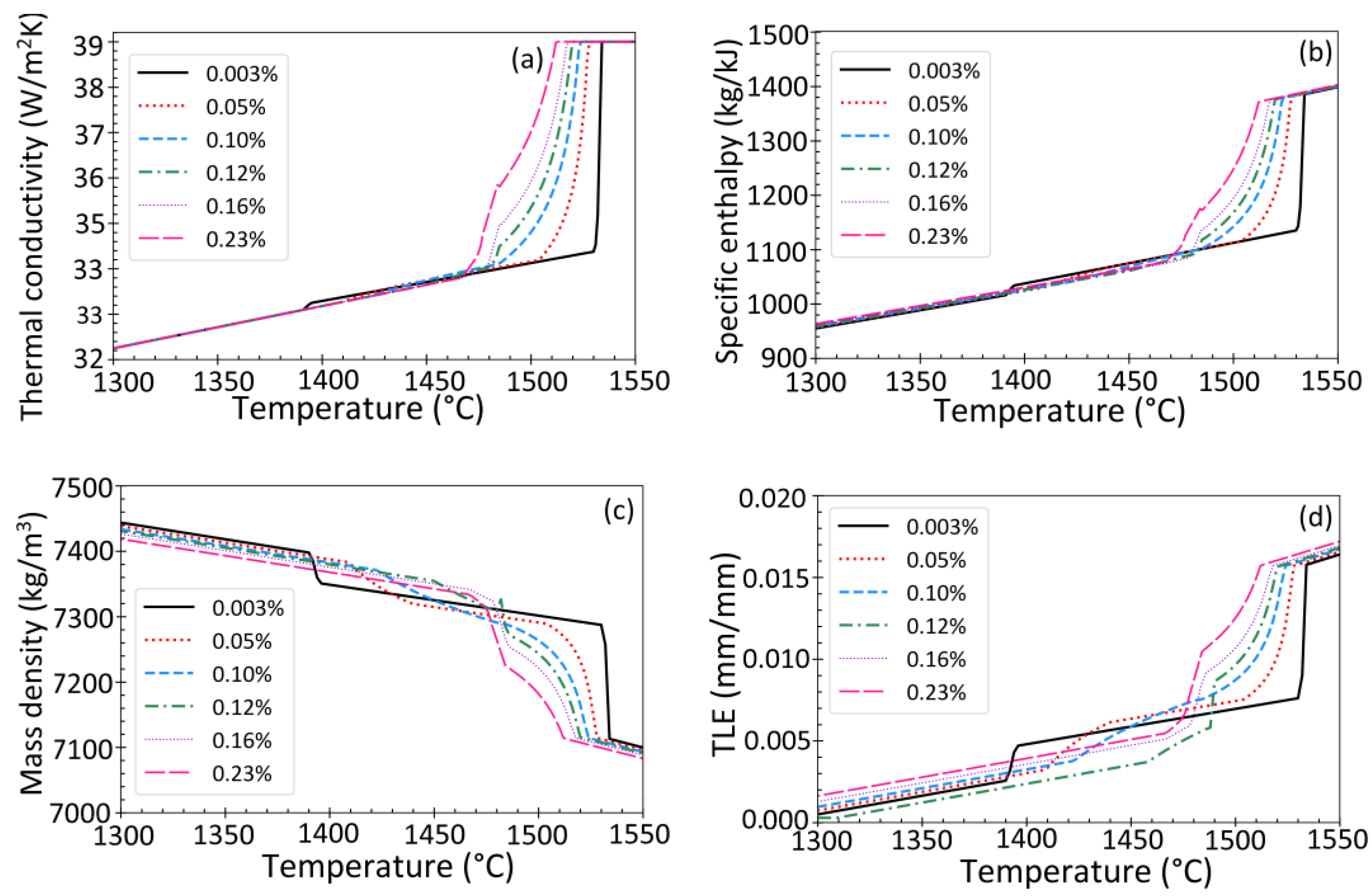
2.4. Phase Fractions and Thermal Properties
2.5. Numerical Details
2.6. Mesh Resolution study and Model Verification
3. Results
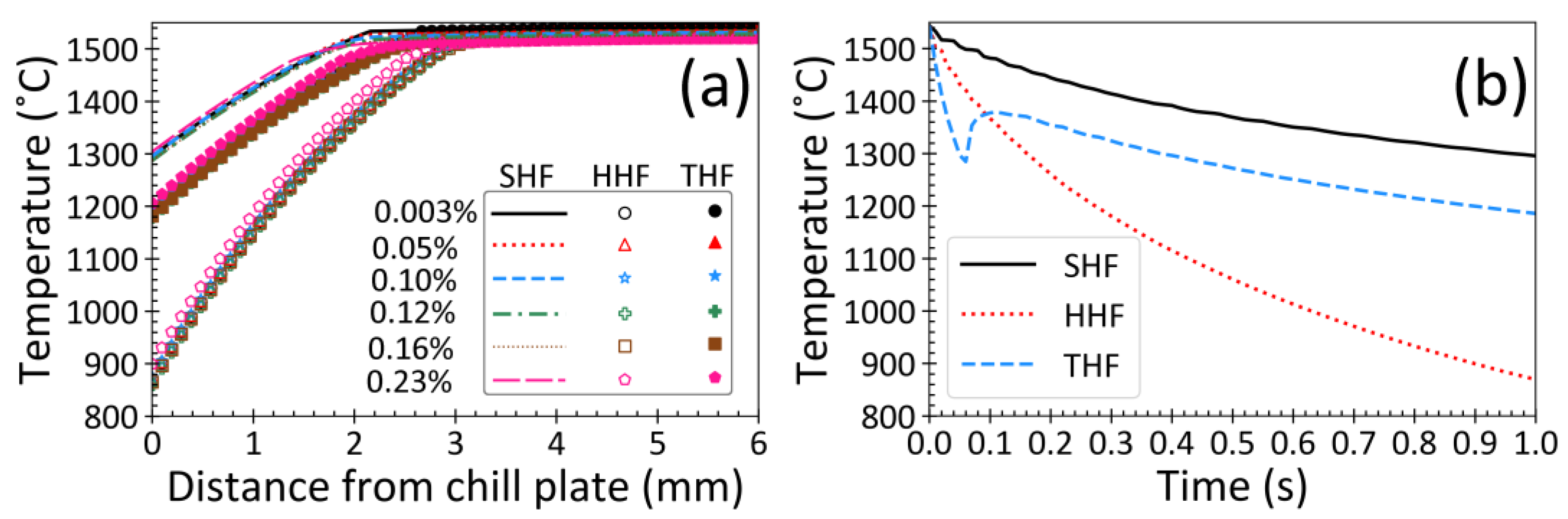
3.1. Temperature Distribution
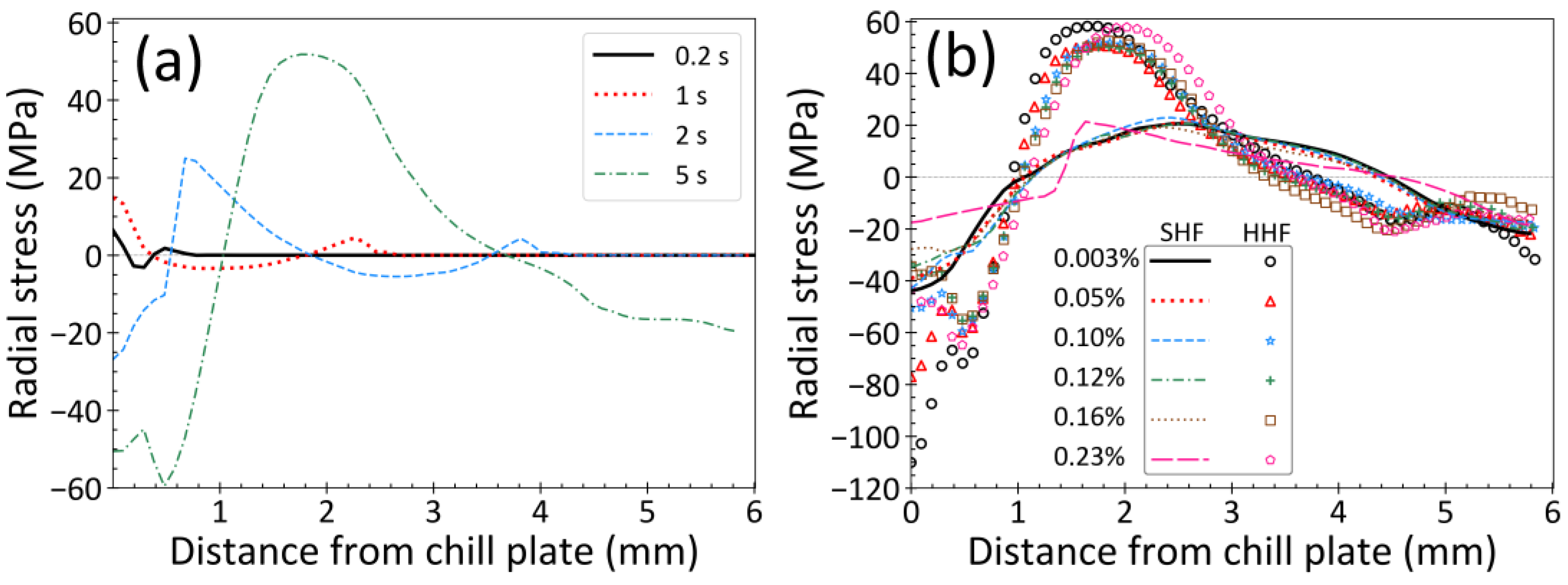
3.2. Stress Distribution
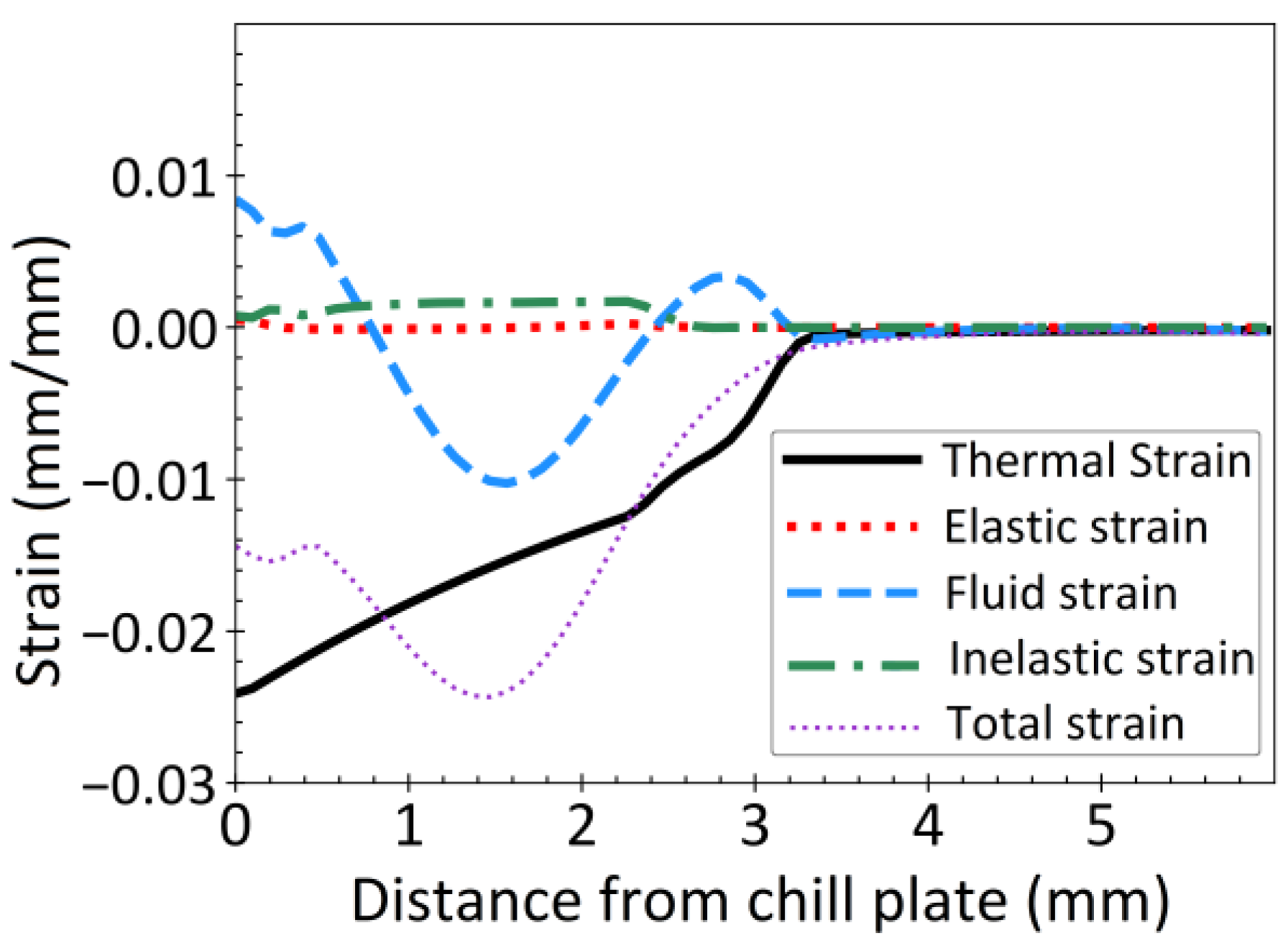
3.3. Strain Distribution
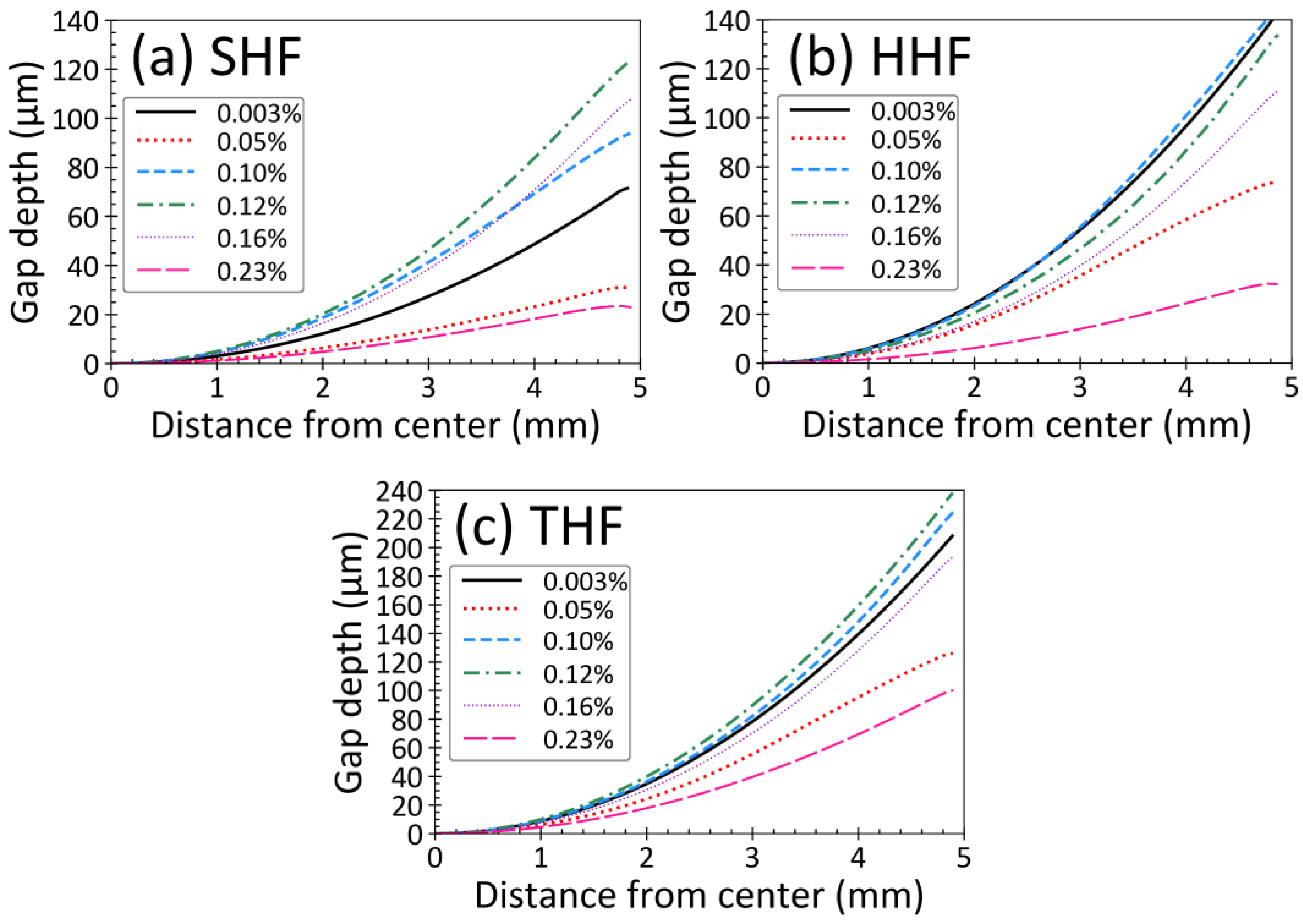
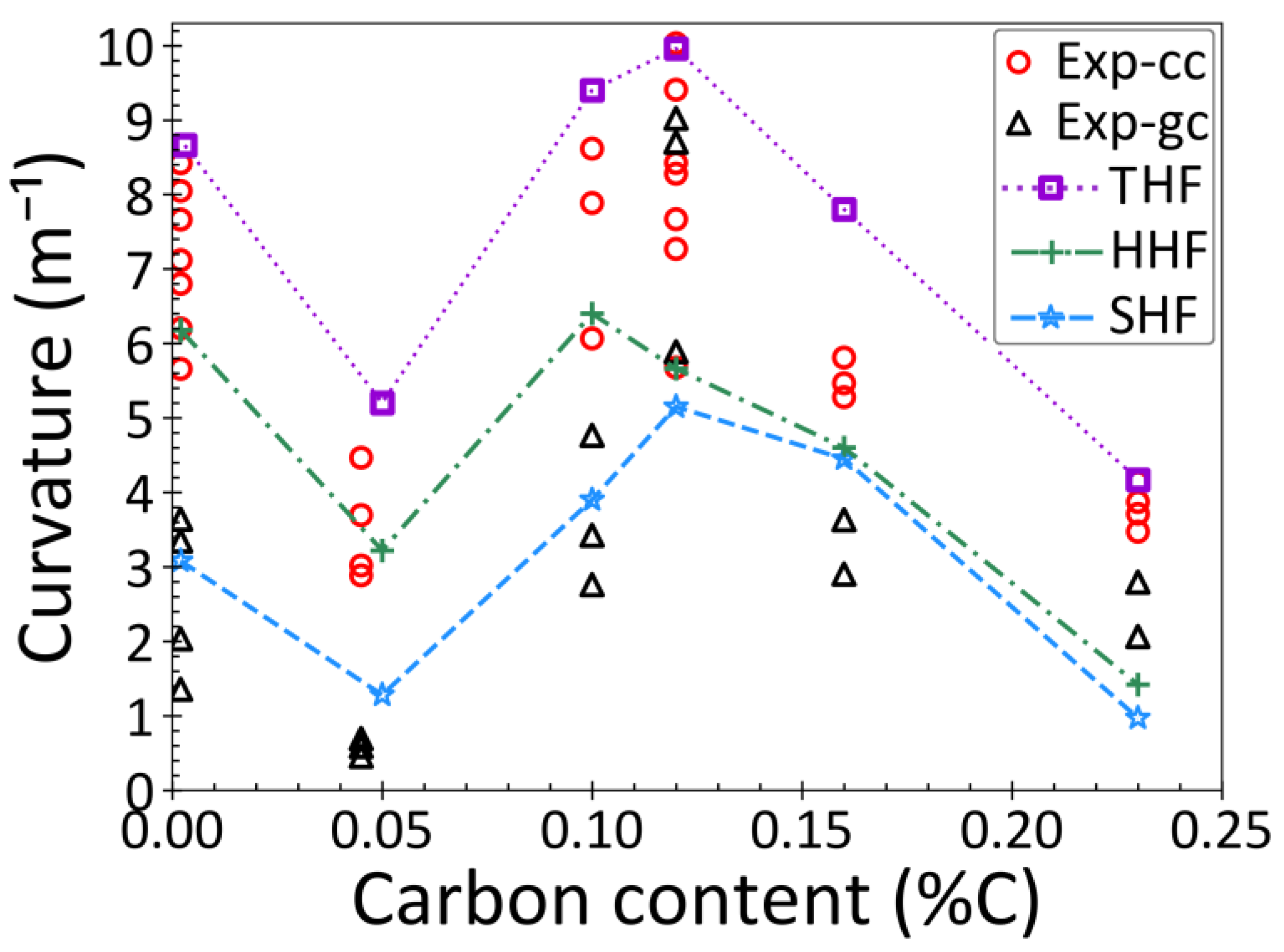
3.4. Thermal Distortion and Model Validation
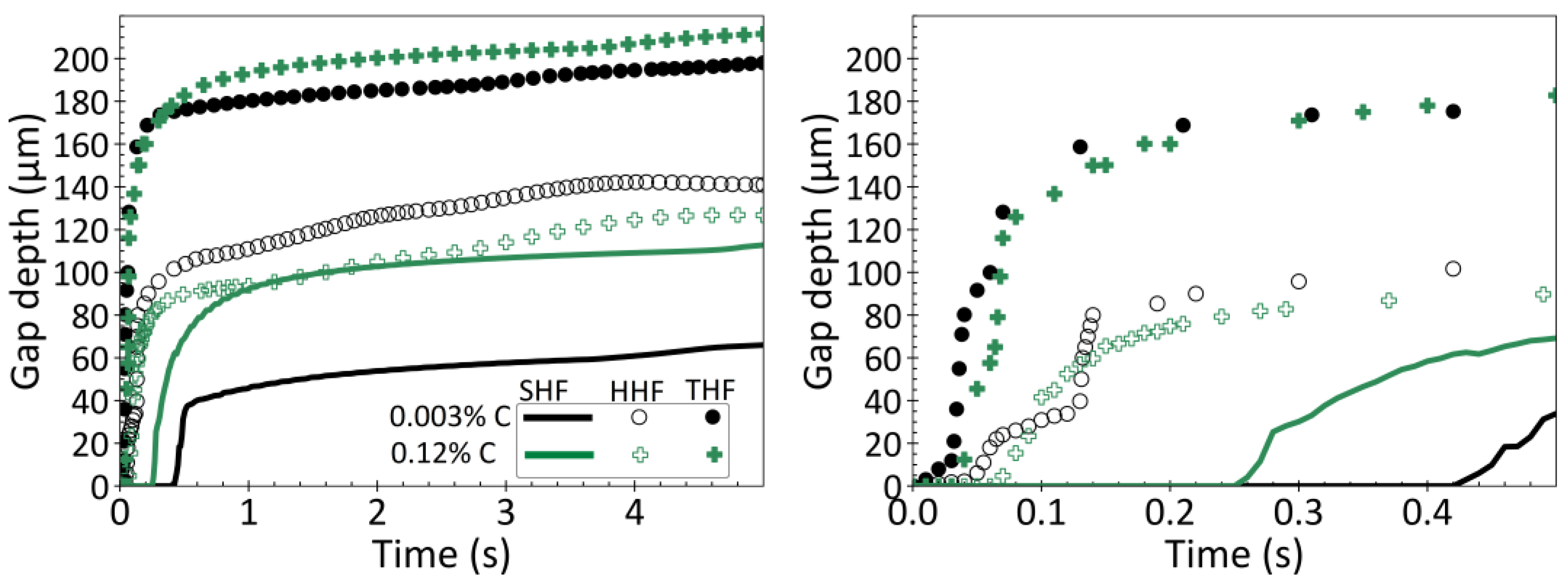
3.5. Effect of Heat Flux Condition
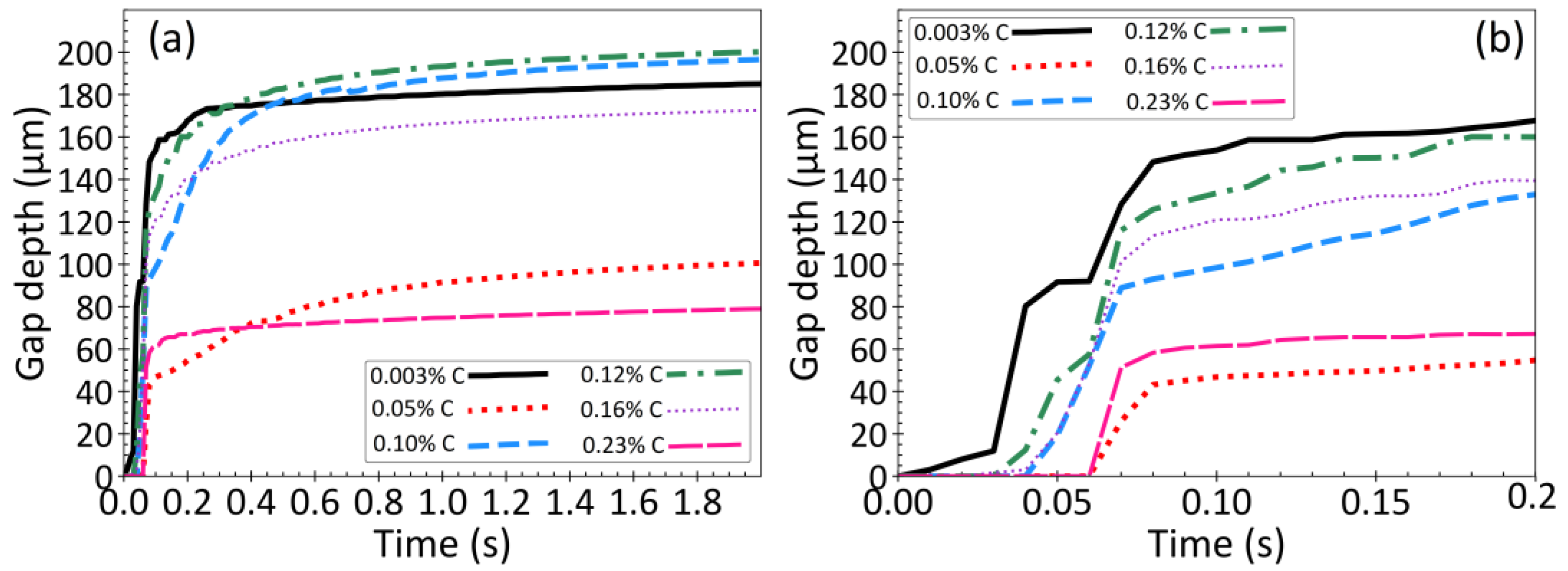
3.6. Effect of Steel Composition
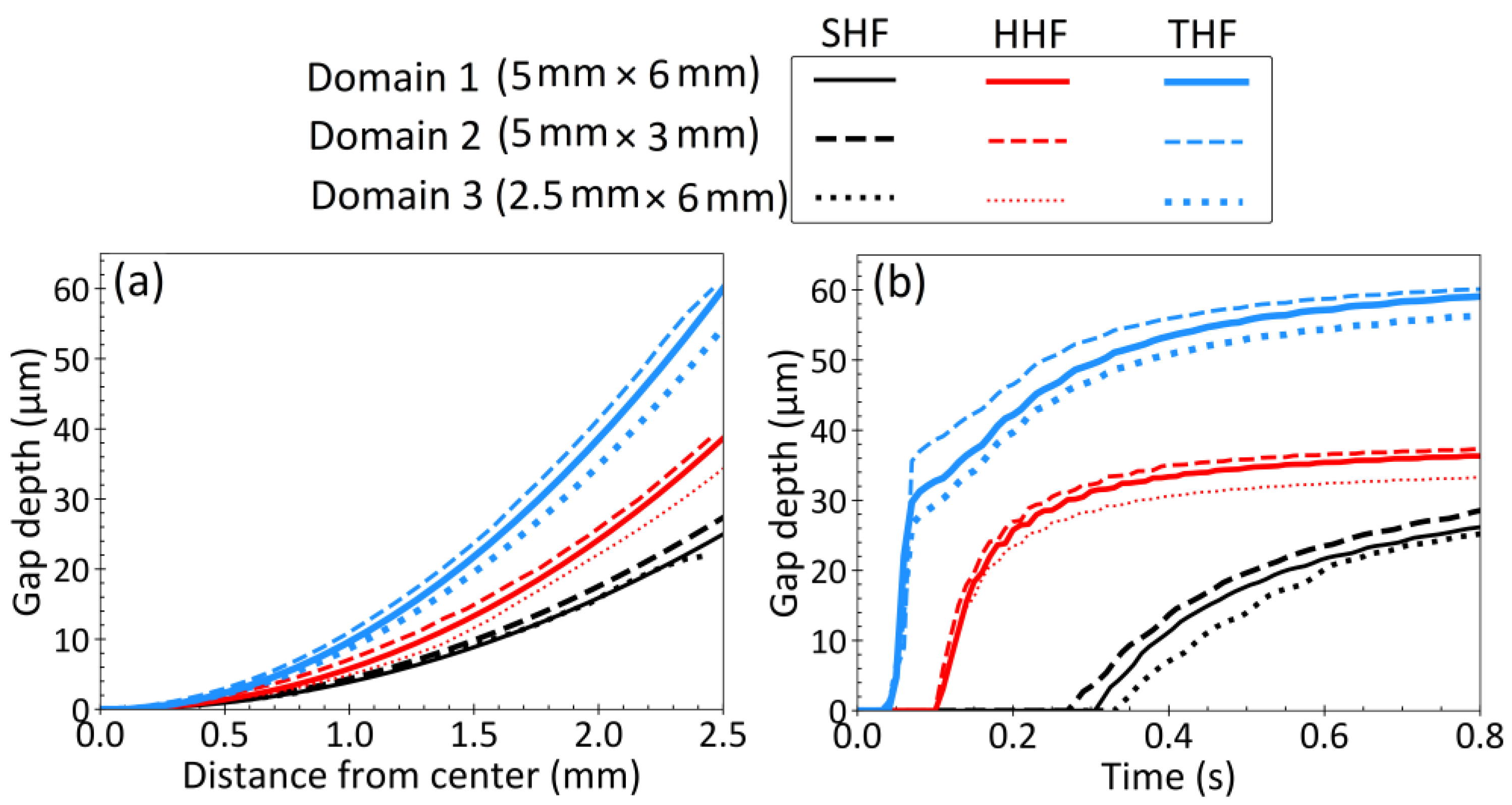
3.7. Effect of Droplet Size/Shape and Discussion
4. Conclusions
- The thermo-mechanical model captures the phenomena which govern gap formation during the initial stages of steel droplet solidification both qualitatively and quantitatively.
- Carbon content is the main factor that controls thermal distortion and bottom surface shape during steel solidification. The highest distortion, as indicated by the curvature of bottom surface of the droplet, is found in ultra-low carbon and peritectic steel grades.
- Heat flux also plays an important role in controlling thermal distortion during solidification. Increasing heat flux during the initial stages of solidification causes the gap depth (curvature of solidified droplet surface) to increase. This effect is most evident in ultralow carbon steels, and decreases with increasing carbon content, if the heat flux does not suddenly change.
- A sudden drop in heat flux typically accompanies gap formation, and is called the incubation time. This causes surface reheating, thermal expansion of the surface layer, and a very large increase in the gap depth, for all steel grades.
- Shorter incubation times lead to deeper gaps, owing to the higher temperature gradient across the thinner shell when the thermal distortion occurs.
- For every steel grade and heat flux condition investigated, the gap forms very early during solidification, within 0.06 s for fast cooling and within 0.4 s for slow cooling, and remains relatively constant after that. The heat flux rate at later times, after the first 1 s, has little effect on the final thermally-distorted shape.
- The incubation time for gap formation is usually near or after the start of the δ→γ phase transformation, which is controlled by the steel grade and initial heat flux. Thus, the gap forms sooner with faster initial cooling rates, and in peritectic steels.
- The gap depth is greatly affected by the δ→γ phase transformation. In peritectic steel grades, this transformation occurs very soon after a thin solid shell has formed, which leads to deep gaps, implying deep surface depressions in cast products.
- Increasing the cooling rate and temperature gradient during initial solidification increases the severity of δ→γ phase transformation, and results in a deeper gap.
- The findings of this fundamental model and gap formation mechanism have important implications for the formation of surface depressions and related defects in commercial steel continuous casting processes.
- Large changes in droplet/domain shape/size have only a minor effect on the thermal-mechanical behavior and final curvature of bottom surface. Thus, the above findings are expected to be similar in commercial steel casting processes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, B.G. Review on modeling and simulation of continuous casting. Steel Res. Int. 2018, 89, 1700312. [Google Scholar] [CrossRef]
- Brimacombe, J.K.; Sorimachi, K. Crack formation in the continuous casting of steel. Metall. Mater. Trans. B 1977, 8, 489–505. [Google Scholar] [CrossRef]
- Lyu, P.; Wang, W.; Zhang, H. Mold Simulator Study on the Initial Solidification of Molten Steel Near the Corner of Continuous Casting Mold. Metall. Mater. Trans. B 2017, 48, 247–259. [Google Scholar] [CrossRef]
- Wolf, M.; Kurz, W. The effect of carbon content on solidification of steel in the continuous casting mold. Metall. Mater. Trans. B 1981, 12, 85–93. [Google Scholar] [CrossRef]
- Sengupta, J.; Thomas, B.G.; Shin, H.J.; Lee, G.G.; Kim, S.H. A new mechanism of hook formation during continuous casting of ultra-low-carbon steel slabs. Metall. Mater. Trans. A 2006, 37, 1597–1611. [Google Scholar] [CrossRef] [Green Version]
- Grill, A.; Sorimachi, K.; Brimacombe, J.K. Heat flow, gap formation and break-outs in the continuous casting of steel slabs. Metall. Trans. B 1976, 7, 177–189. [Google Scholar] [CrossRef]
- Badri, A.; Natarajan, T.T.; Snyder, C.C.; Powers, K.D.; Mannion, F.J.; Byrne, M.; Cramb, A.W. A mold simulator for continuous casting of steel: Part II. The formation of oscillation marks during the continuous casting of low carbon steel. Metall. Mater. Trans. B 2005, 36, 373–383. [Google Scholar] [CrossRef]
- Schwerdtfeger, K.; Sha, H. Depth of oscillation marks forming in continuous casting of steel. Metall. Trans. B 2000, 31, 813–826. [Google Scholar] [CrossRef]
- Kim, K.; Han, H.N.; Yeo, T.; Lee, Y.; Oh, K.H.; Lee, D.N. Analysis of surface and internal cracks in continuously cast beam blank. Ironmak. Steelmak. 1997, 24, 249–256. [Google Scholar]
- Maehara, Y.; Yasumoto, K.; Tomono, H.; Nagamichi, T.; Ohmori, Y. Surface cracking mechanism of continuously cast low carbon low alloy steel slabs. Mater. Sci. Technol. 1990, 6, 793–806. [Google Scholar] [CrossRef]
- Singh, S.N.; Blazek, K.E. Heat transfer and skin formation in a continuous-casting mold as a function of steel carbon content. JOM 1974, 26, 17–27. [Google Scholar] [CrossRef]
- Konishi, J.; Militzer, M.; Samarasekera, I.V.; Brimacombe, J.K. Modeling the formation of longitudinal facial cracks during continuous casting of hypoperitectic steel. Metall. Mater. Trans. B 2002, 33, 413–423. [Google Scholar] [CrossRef]
- Clyne, T.W.; Wolf, M.; Kurz, W. The effect of melt composition on solidification cracking of steel, with particular reference to continuous casting. Metall. Trans. B 1982, 13, 259–266. [Google Scholar] [CrossRef]
- Kim, K.H.; Han, S.; Yeo, T.J.; Oh, K.H.; Lee, D.N. Effect of carbon and sulfur in continuously cast strand on longitudinal surface cracks. ISIJ Int. 1996, 36, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Azizi, G.; Thomas, B.G.; Asle Zaeem, M. Review of Peritectic Solidification Mechanisms and Effects in Steel Casting. Metall. Mater. Trans. B 2020, 51, 1875–1903. [Google Scholar] [CrossRef]
- Won, Y.M.; Yeo, T.J.; Seol, D.J.; Oh, K.H. A new criterion for internal crack formation in continuously cast steels. Metall. Mater. Trans. B 2000, 31, 779–794. [Google Scholar] [CrossRef]
- Xia, G.; Bernhard, C.; Ilie, S.; Fuerst, C. A study about the influence of carbon content in the steel on the casting behavior. Steel Res. Int. 2011, 82, 230–236. [Google Scholar] [CrossRef]
- Suzuki, M.; Yu, C.H.; Sato, H.; Tsui, Y.; Shibata, H.; Emi, T. Origin of heat transfer anomaly and solidifying shell deformation of peritectic steels in continuous casting. ISIJ Int. 1996, 36, S171–S174. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, C.; Xia, G. Influence of alloying elements on the thermal contraction of peritectic steels during initial solidification. Ironmak. Steelmak. 2006, 33, 52–56. [Google Scholar] [CrossRef]
- Bernhard, C.; Reiter, J.; Presslinger, H. A model for predicting the austenite grain size at the surface of continuously-cast slabs. Metall. Mater. Trans. B 2008, 39, 885–895. [Google Scholar] [CrossRef]
- Badri, A.; Natarajan, T.T.; Snyder, C.C.; Powers, K.D.; Mannion, F.J.; Cramb, A.W. A mold simulator for the continuous casting of steel: Part I. The development of a simulator. Metall. Mater. Trans. B 2005, 36, 355–371. [Google Scholar] [CrossRef]
- Zhu, L.G.; Kumar, R.V. Shrinkage of carbon steel by thermal contraction and phase transformation during solidification. Ironmak. Steelmak. 2007, 34, 71–75. [Google Scholar] [CrossRef]
- Ramirez-Lopez, P.E.; Lee, P.D.; Mills, K.C. Explicit modelling of slag infiltration and shell formation during mould oscillation in continuous casting. ISIJ Int. 2010, 50, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Perrot, C.; Pontoire, N.J.; Marchionni, C.; Ridolfi, M.R.; Sancho, L.F. Several slag rims and lubrication behaviours in slab casting. Metall. Res. Technol. 2005, 102, 887–896. [Google Scholar] [CrossRef]
- Thomas, B.G.; Najjar, F.M. Finite element modelling of turbulent fluid flow and heat transfer in continuous casting. Appl. Math. Model. 1991, 15, 226–243. [Google Scholar] [CrossRef]
- Sengupta, J.; Ojeda, C.; Thomas, B.G. Thermal-mechanical behaviour during initial solidification in continuous casting: Steel grade effects. Int. J. Cast Met. Res. 2009, 22, 8–14. [Google Scholar] [CrossRef]
- Harada, S.; Tanaka, S.; Misumi, H.; Mizoguchi, S.; Horiguchi, H. A formation mechanism of transverse cracks on CC slab surface. ISIJ Int. 1990, 30, 310–316. [Google Scholar] [CrossRef]
- Mukai, K.; Zeze, M. Motion of fine particles under interfacial tension gradient in relation to continuous casting process. Steel Res. Int. 2003, 74, 131–138. [Google Scholar] [CrossRef]
- Balogun, D.; Roman, M.; Gerald, R.E.; Huang, J.; Bartlett, L.; O’Malley, R. Shell Measurements and Mold Thermal Mapping Approach to Characterize Steel Shell Formation in Peritectic Grade Steels. Steel Res. Int. 2022, 93, 2100455. [Google Scholar] [CrossRef]
- Dong, S.; Niyama, E.; Anzai, K.; Matsumoto, N. Free deformation of the initial solid layer of some non-ferrous alloys. Tets Hagané 1993, 79, 1060–1066. [Google Scholar]
- Dong, S.; Niyama, E.; Anzai, K. Free deformation of initial solid shell of Fe-C alloys. ISIJ Int. 1995, 35, 730–736. [Google Scholar] [CrossRef]
- Dong, S. Free Deformation of the Initial Solid Layer of Alloys. Ph.D. Thesis, Tohoku University, Sendai, Japan, 1995. [Google Scholar]
- Thomas, B.G.; Zhu, H. Thermal Distortion of solidifying shell near meniscus in continuous casting of steel. In Proceedings of the JIM/TMS Solidification Science and Processing Conference, Honolulu, HI, USA, 13–15 December 1995; pp. 197–208. [Google Scholar]
- Thomas, B.G.; Parkman, J.T. Simulation of thermal distortion of a steel droplet solidifying on a copper chill. In Proceedings of the TMS Annual Meeting, San Antonio, TX, USA, 15–19 February 1998. [Google Scholar]
- Vynnycky, M. On the onset of air-gap formation in vertical continuous casting with superheat. Int. J. Mech. Sci. 2013, 73, 69–76. [Google Scholar] [CrossRef]
- Florio, B.J.; Vynnycky, M.; Mitchell, S.L.; O’Brien, S. Mould-taper asymptotics and air gap formation in continuous casting. Appl. Math. Comput. 2015, 268, 1122–1139. [Google Scholar] [CrossRef]
- Niu, Z.; Cai, Z.; Zhu, M. Dynamic distributions of mold flux and air gap in slab continuous casting mold. ISIJ Int. 2019, 59, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.M.; Cai, S.W.; Xu, J.; Du, Y.Y.; Zhu, J.; Xu, J.J.; Li, T.J. Continuous casting mould for square steel billet optimised by solidification shrinkage simulation. Ironmak. Steelmak. 2010, 37, 341–346. [Google Scholar] [CrossRef]
- Florio, B.J.; Vynnycky, M.; Mitchell, S.L.; O’Brien, S. On the interactive effects of mould taper and superheat on air gaps in continuous casting. Acta Mech. 2017, 228, 233–254. [Google Scholar] [CrossRef]
- Cai, Z.Z.; Zhu, M.Y. Simulation of air gap formation in slab continuous casting mould. Ironmak. Steelmak. 2014, 41, 435–446. [Google Scholar] [CrossRef]
- Cai, Z.; Zhu, M. Simulation of Heat Transfer in SLAB Continuous Casting Mold and New Formation Mechanism of Shell Hot Spots; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Hostos, J.C.A.; Cabrera, E.S.P.; Bencomo, A.D. Stress Analysis of a Continuous Casting Process, on the Basis of the Element-Free Galerkin Formulation. Steel Res. Int. 2017, 88, 1600019. [Google Scholar] [CrossRef]
- Li, C.; Thomas, B.G. Thermomechanical finite-element model of shell behavior in continuous casting of steel. Metall. Mater. Trans. B 2004, 35, 1151–1172. [Google Scholar] [CrossRef]
- Zappulla, M.L.; Hibbeler, L.C.; Thomas, B.G. Effect of Grade on Thermal–Mechanical Behavior of Steel During Initial Solidification. Metall. Mater. Trans. A 2017, 48, 3777–3793. [Google Scholar] [CrossRef]
- Park, J.K.; Thomas, B.G.; Samarasekera, I.V. Analysis of thermomechanical behaviour in billet casting with different mould corner radii. Ironmak. Steelmak. 2002, 29, 359–375. [Google Scholar] [CrossRef]
- Thomas, B.G.; Moitra, A.; McDavid, R. Simulation of longitudinal off-corner depressions in continuously cast steel slabs. Iron Steelmak. 1996, 23, 57–70. [Google Scholar]
- Niu, Z.; Cai, Z.; Zhu, M. Formation Mechanism of a Wide-Face Longitudinal Off-Corner Depression during Thick Slab Continuous Casting. Metall. Mater. Trans. B 2021, 52, 2737–2752. [Google Scholar] [CrossRef]
- Risso, J.M.; Huespe, A.E.; Cardona, A. Thermal stress evaluation in the steel continuous casting process. Int. J. Numer. Methods Eng. 2006, 65, 1355–1377. [Google Scholar] [CrossRef]
- Kristiansson, J.O. Thermal stresses in the early stage of solidification of steel. J. Therm. Stress. 1982, 5, 315–330. [Google Scholar] [CrossRef]
- Zuo, X.J.; Lin, R.G.; Wang, N.; Yang, J.; Meng, X.N.; Zhu, M.Y. Formation of Initial Defects at Meniscus in Oscillating Mold. Steel Res. Int. 2016, 87, 413–423. [Google Scholar] [CrossRef]
- Hebi, Y.; Man, Y.; Huiying, Z.; Dacheng, F. 3D stress model with friction in and of mould for round billet continuous casting. ISIJ Int. 2006, 46, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.Z.; Zhu, M.Y. Thermo-mechanical behavior of peritectic steel solidifying in slab continuous casting mold and a new mold taper design. ISIJ Int. 2013, 53, 1818–1827. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.G.; Ojeda, C. Ideal taper prediction for slab casting. In Proceedings of the ISSTech Steelmaking Conference, Indianapolis, IN, USA, 27–30 April 2003. [Google Scholar]
- Kozlowski, P.F.; Thomas, B.G.; Azzi, J.A.; Wang, H. Simple constitutive equations for steel at high temperature. Metall. Mater. Trans. A 1992, 23, 903. [Google Scholar] [CrossRef]
- Jablonka, A.; Harste, K.; Schwerdtfeger, K. Thermomechanical properties of iron and iron-carbon alloys: Density and thermal contraction. Steel Res. 1991, 62, 24–33. [Google Scholar] [CrossRef]
- Jimbo, I.; Cramb, A.W. The density of liquid iron-carbon alloys. Metall. Trans. B 1993, 24, 5–10. [Google Scholar] [CrossRef]
- DS SIMULIA Corp. ABAQUS: ABAQUS 6.13 Theory Manual; DS SIMULIA Corp.: Providence, RI, USA, 2013. [Google Scholar]
- DS SIMULIA Corp. ABAQUS: ABAQUS 6.13 Subroutine Manual; DS SIMULIA Corp.: Providence, RI, USA, 2013. [Google Scholar]
- Weiner, J.H.; Boley, B.A. Elasto-plastic thermal stresses in a solidifying body. J. Mech. Phys. Solids 1963, 11, 145–154. [Google Scholar] [CrossRef]
- Wray, P.J. Tensile failure behavior of plain carbon steels at elevated temperatures. Metall. Trans. A 1984, 15, 2059–2073. [Google Scholar] [CrossRef]

| Carbon Content (wt%) | Liquidus Temp. (°C) | Solidus Temp. (°C) | δ→γ Transformation Start Temp. (°C) | Heat Flux Case | Time Bottom Surface Reaches Solidus Temp. (s) | Time Bottom Surface Reaches δ→γ Temp.(s) | Incubation Time (s) | Time Top Surface Reaches Solidus Temp. (s) |
|---|---|---|---|---|---|---|---|---|
| 0.003%C | 1532 | 1532 | 1393 | SHF | 0.01 | 0.38 | 0.40 | 4.3 |
| HHF | <0.01 | 0.08 | 0.05 | 2.4 | ||||
| THF | <0.01 | 0.02 | 0.01 | 3.2 | ||||
| 0.05%C | 1528 | 1500 | 1445 | SHF | 0.05 | 0.19 | 0.52 | 4.7 |
| HHF | 0.01 | 0.04 | 0.15 | 2.7 | ||||
| THF | <0.01 | 0.02 | 0.06 | 3.3 | ||||
| 0.10%C | 1525 | 1480 | 1481 | SHF | 0.09 | 0.09 | 0.30 | 4.8 |
| HHF | 0.02 | 0.02 | 0.10 | 2.7 | ||||
| THF | 0.01 | 0.01 | 0.04 | 3.6 | ||||
| 0.12%C | 1521 | 1479 | 1494 | SHF | 0.08 | 0.05 | 0.26 | 4.8 |
| HHF | 0.02 | 0.01 | 0.07 | 2.7 | ||||
| THF | 0.01 | <0.01 | 0.03 | 3.7 | ||||
| 0.16%C | 1519 | 1470 | 1494 | SHF | 0.09 | 0.04 | 0.28 | 4.8 |
| HHF | 0.03 | 0.01 | 0.08 | 2.8 | ||||
| THF | 0.01 | <0.01 | 0.04 | 3.8 | ||||
| 0.23%C | 1514 | 1463 | 1491 | SHF | 0.12 | 0.03 | 0.57 | 5.8 |
| HHF | 0.03 | 0.01 | 0.21 | 2.9 | ||||
| THF | 0.01 | <0.01 | 0.06 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizi, G.; Thomas, B.G.; Asle Zaeem, M. Prediction of Thermal Distortion during Steel Solidification. Metals 2022, 12, 1807. https://doi.org/10.3390/met12111807
Azizi G, Thomas BG, Asle Zaeem M. Prediction of Thermal Distortion during Steel Solidification. Metals. 2022; 12(11):1807. https://doi.org/10.3390/met12111807
Chicago/Turabian StyleAzizi, Ghavam, Brian. G. Thomas, and Mohsen Asle Zaeem. 2022. "Prediction of Thermal Distortion during Steel Solidification" Metals 12, no. 11: 1807. https://doi.org/10.3390/met12111807
APA StyleAzizi, G., Thomas, B. G., & Asle Zaeem, M. (2022). Prediction of Thermal Distortion during Steel Solidification. Metals, 12(11), 1807. https://doi.org/10.3390/met12111807








