Corrosion Study of 80S Steel under the Coexistence of CO2 and H2S
Abstract
1. Introduction
2. Materials and Methods
2.1. High Temperature and Pressure Simulation Experiments
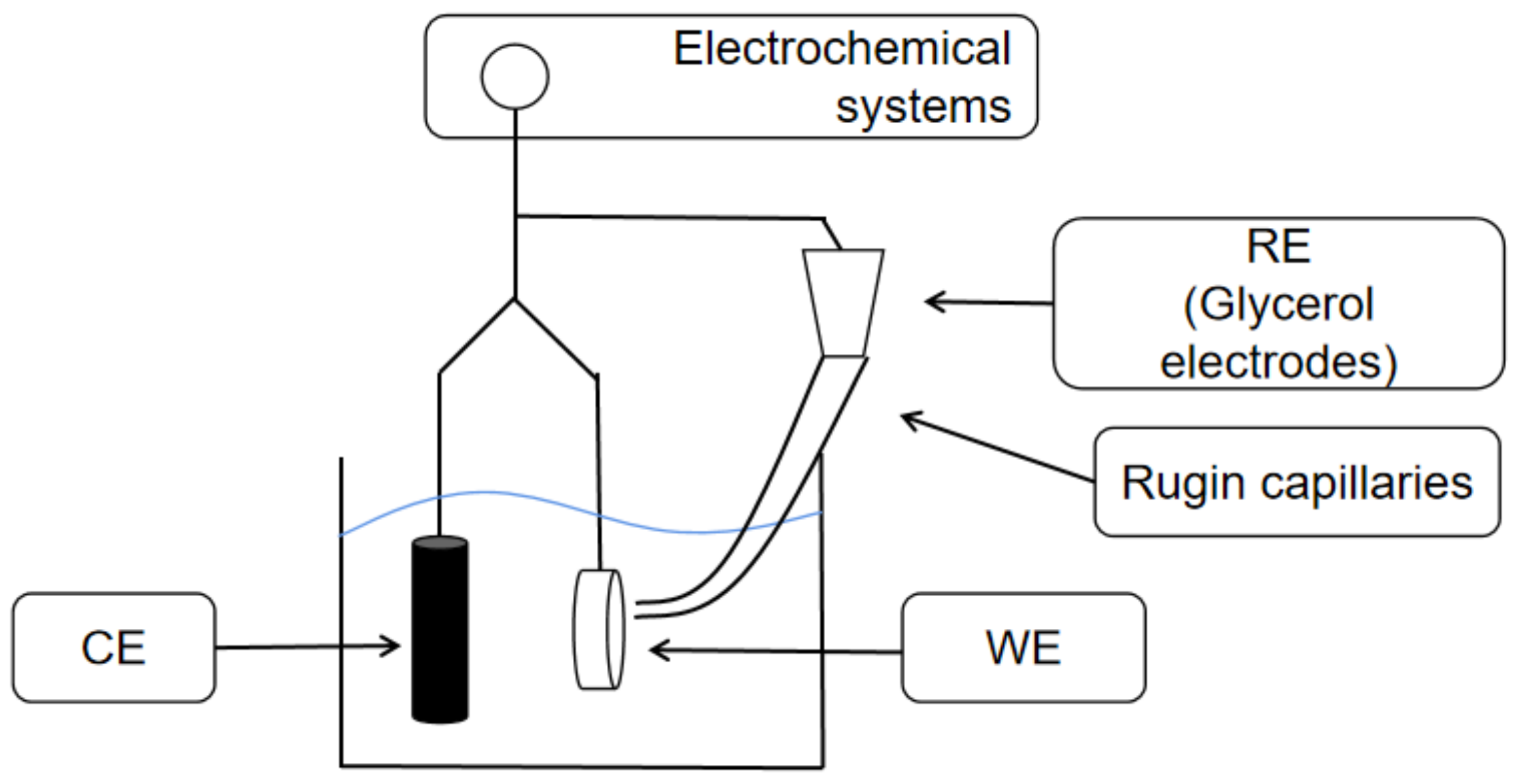
2.2. Electrochemical Experiments
2.3. SEM Analysis
3. Results and Discussion
3.1. High-Temperature and High-Pressure Simulation Experiments
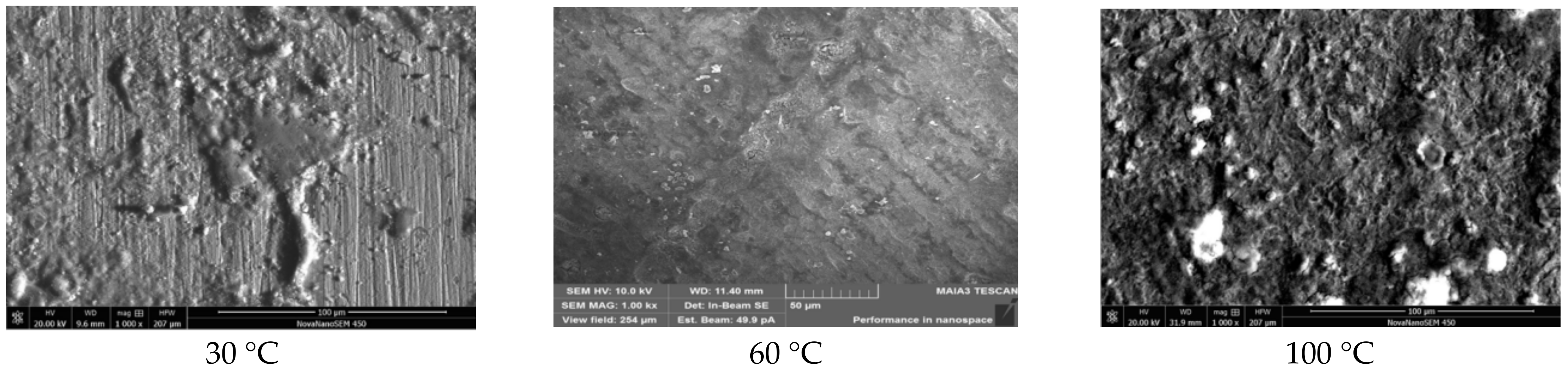
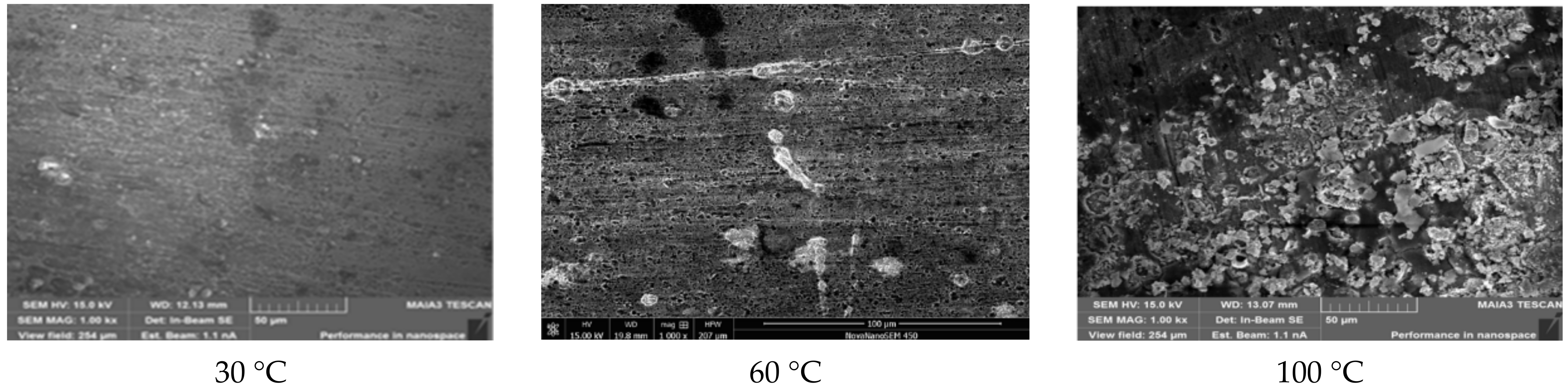
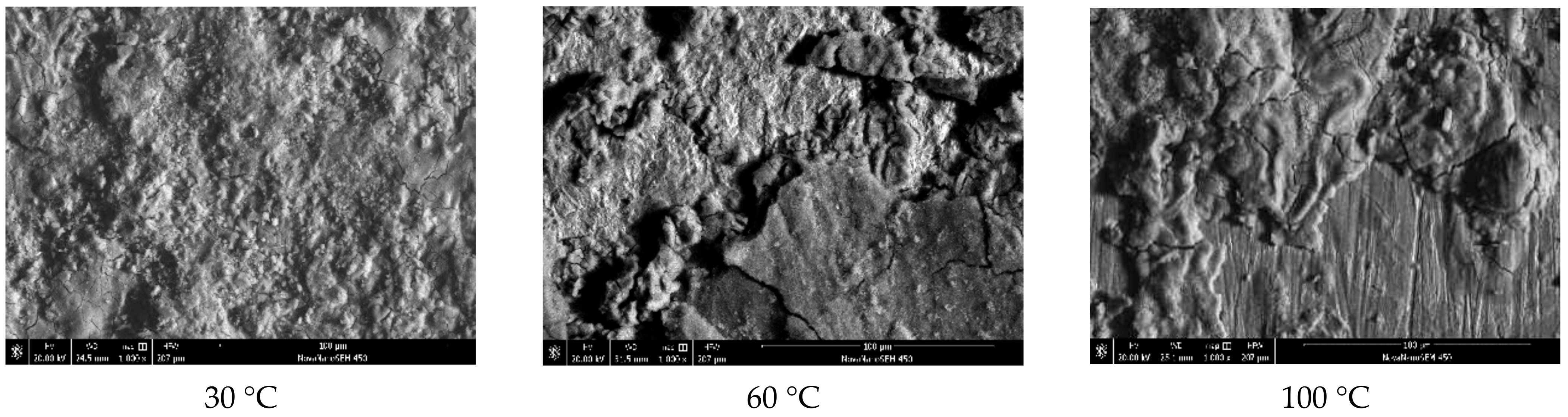
3.1.1. Experimental Results
3.1.2. Analysis of Results
3.2. Electrochemical Experiments
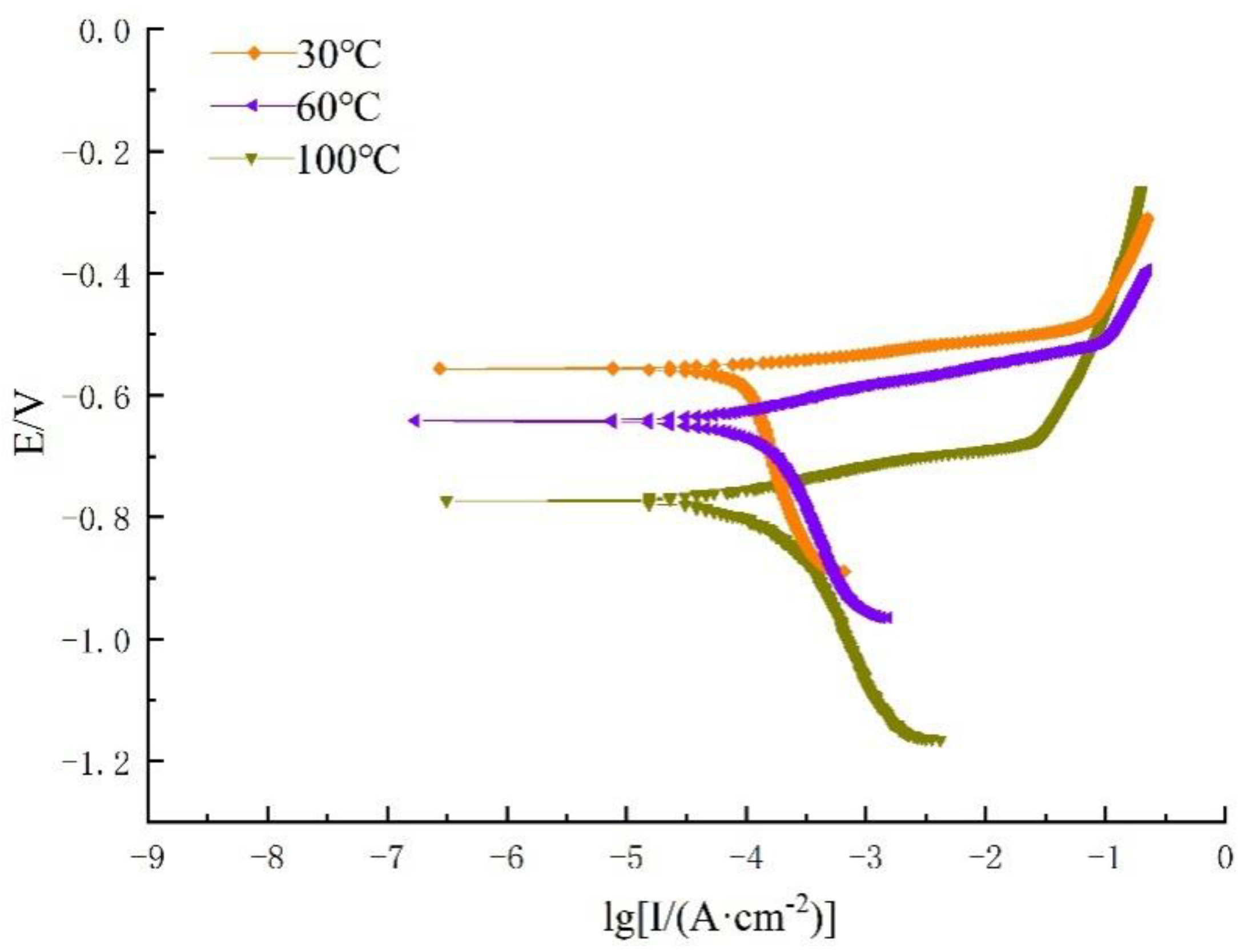
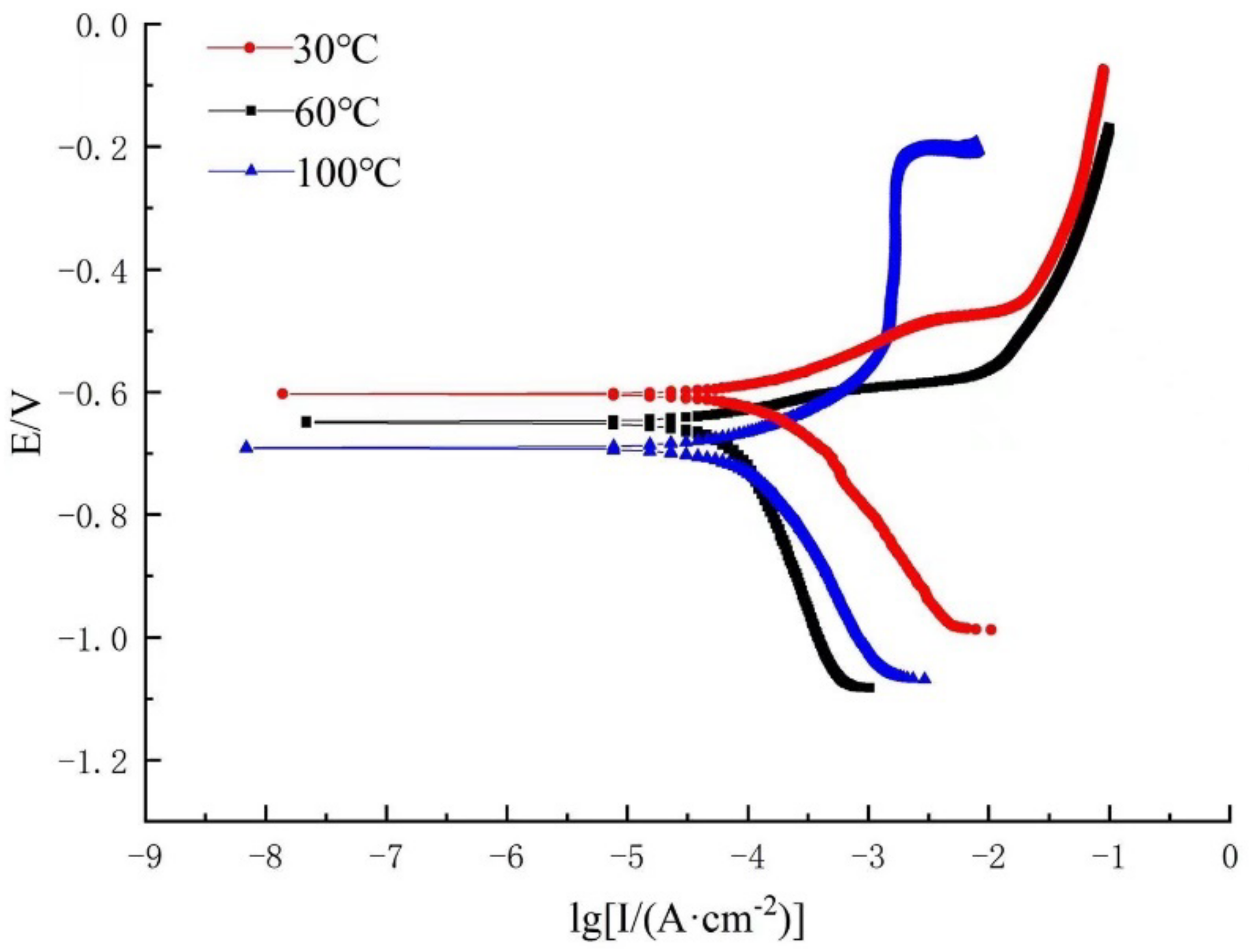
3.2.1. Dynamic Potential Polarization
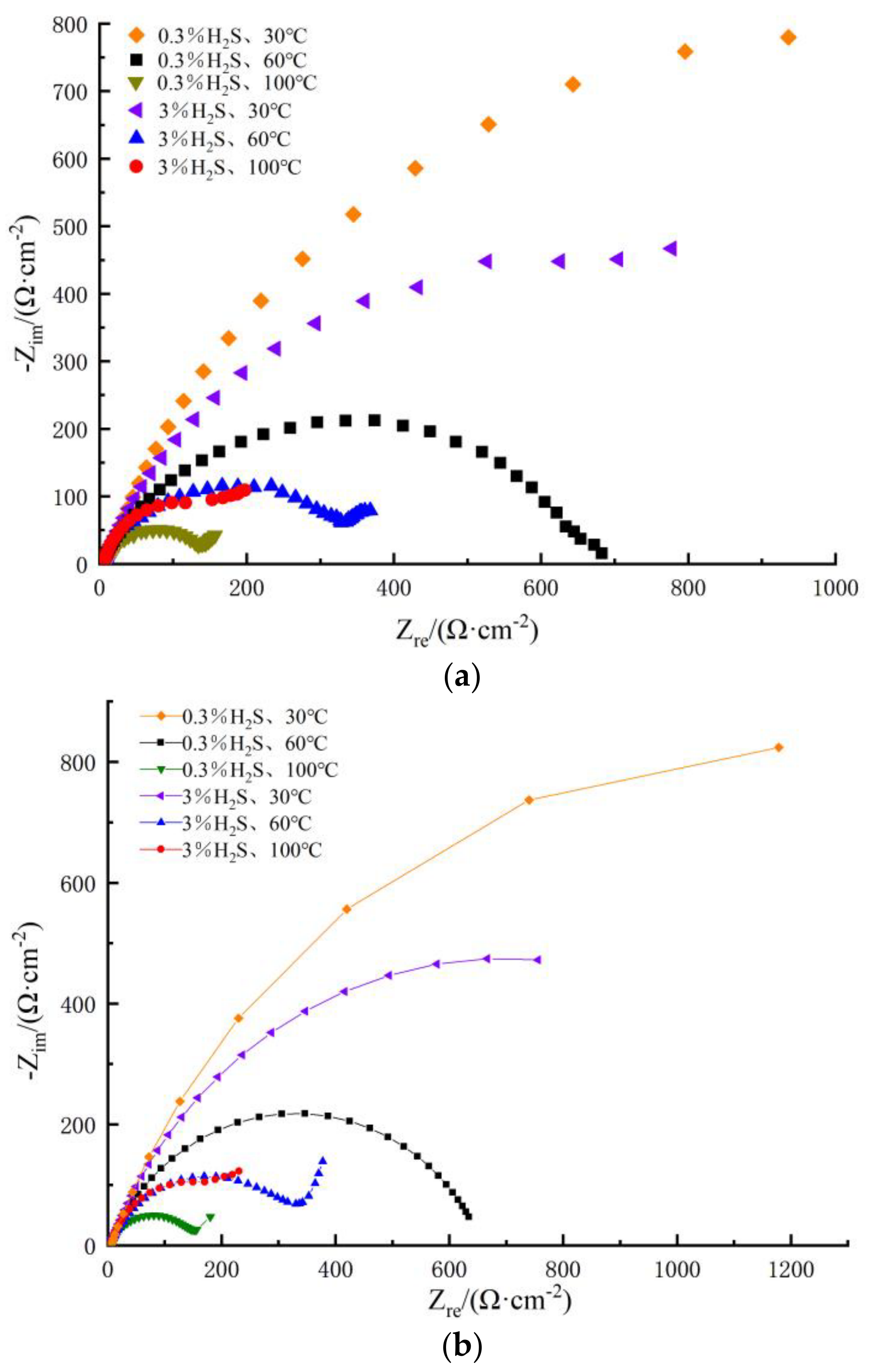
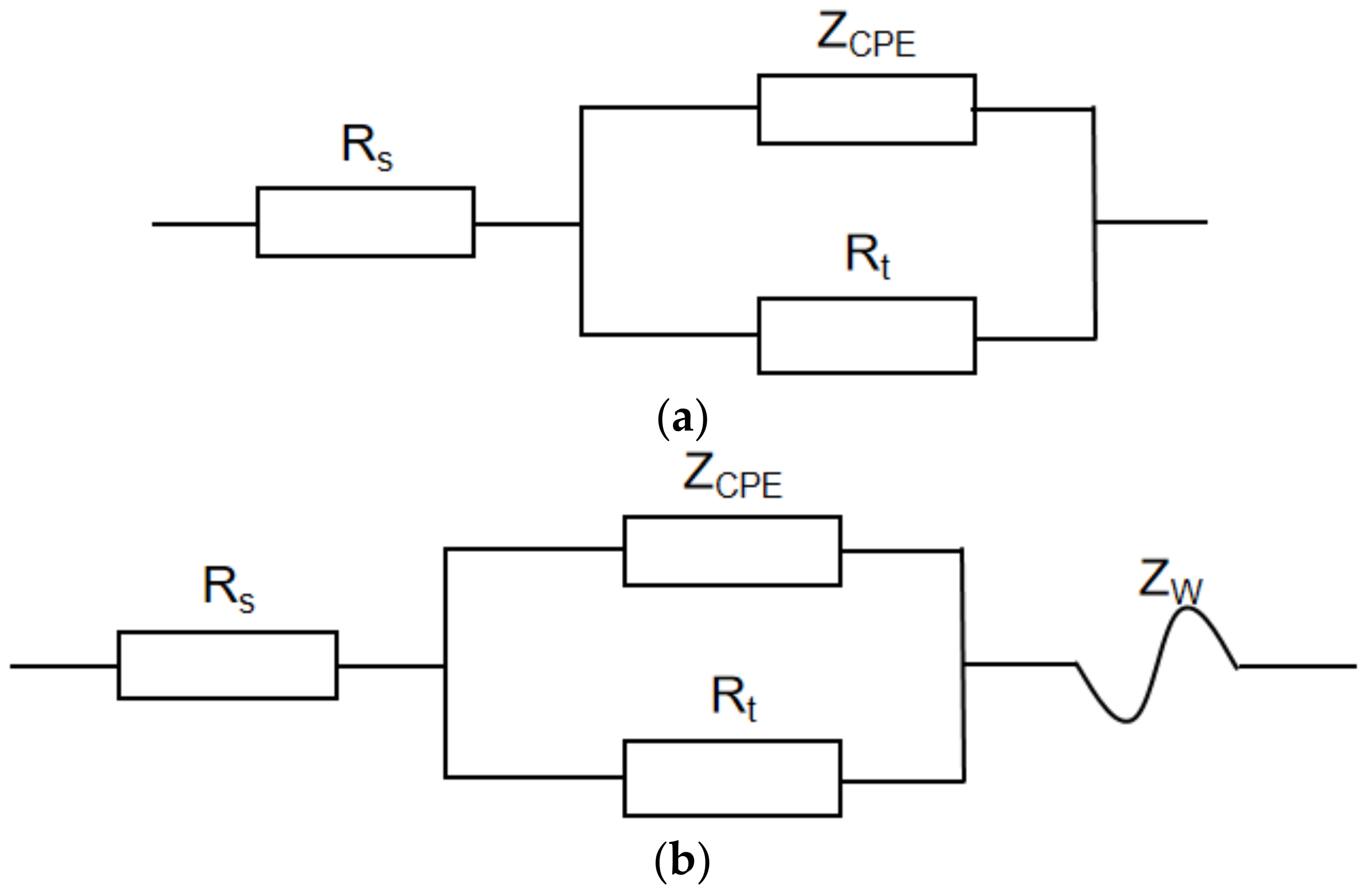
3.2.2. Electrochemical Impedance Spectroscopy
4. Conclusions
- (1)
- In the corrosive environment of CO2 and H2S coexistence, temperature is a major factor affecting the corrosion rate of 80S steel, and an increase in temperature accelerates the corrosion process.
- (2)
- The corrosion rate is also affected by the CO2 and H2S partial pressure ratio; high S content at high temperatures inhibits the corrosion process, and vice versa for low temperature.
- (3)
- With an increase in the temperature, the corrosion potential decreases, corrosion current density increases, and polarization curve gradually moves to the right.
- (4)
- The shape of the cathodic branch moves in the X-negative direction by increasing S content, and the cathodic reaction is jointly controlled by activation and diffusion processes, when the temperature is 100 °C, whereas the anodic branch of the polarization curve at a 3% concentration of Na2S.9H2O significantly changes and a passivation zone appears.
- (5)
- The results of the impedance spectra showed that the impedance radius of the metal decreases significantly at increasing temperatures. In addition, the Warburg impedance showed a more pronounced diffusion phenomenon with the incerase in H2S concentration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choi, Y.S.; Nesic, S.; Ling, S. Effect of H2S on the CO2 corrosion of carbon steel in acidic solutions. Electrochim. Acta 2011, 56, 1752–1760. [Google Scholar] [CrossRef]
- He, W.; Diplas, S.; Knudsen, O.Ø. Corrosion of stainless steel 316L in simulated formation water environment with CO2–H2S–Cl−. Corros. Sci. 2009, 12, 2811–2819. [Google Scholar] [CrossRef]
- Fierro, G.; Ingo, G.M.; Mancia, F. XPS investigation on the corrosion behavior of 13Cr-Martensitic stainless steel in CO2-H2S-Cl− environments. Corrosion 1989, 10, 814–823. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, H.; Luo, J.L. Unraveling the effects of CO2 and H2S on the corrosion behavior of electroless Ni-P coating in CO2/H2S/Cl–environments at high temperature and high pressure. Corros. Sci. 2019, 148, 317–330. [Google Scholar] [CrossRef]
- Sun, J.; Sun, C.; Lin, X.; Cheng, X.; Liu, H. Effect of chromium on corrosion behavior of P110 steels in CO2-H2S environment with high pressure and high temperature. Materials 2016, 3, 200. [Google Scholar] [CrossRef]
- Asadian, M.; Sabzi, M.; Anijdan, S.H.M. The effect of temperature, CO2, H2S gases and the resultant iron carbonate and iron sulfide compounds on the sour corrosion behaviour of ASTM A-106 steel for pipeline transportation. Int. J. Press. Vessel. Pip. 2019, 171, 184–193. [Google Scholar] [CrossRef]
- Shabarchin, O.; Tesfamariam, S. Internal corrosion hazard assessment of oil & gas pipelines using Bayesian belief network model. J. Loss Prev. Process Ind. 2016, 40, 479–495. [Google Scholar]
- Prasad, A.R.; Kunyankandy, A.; Joseph, A. Corrosion inhibition in oil and gas industry: Economic considerations. Corros. Inhib. Oil Gas Ind. 2020, 135–150. [Google Scholar]
- Yin, Z.; Zhang, Y.; Chang, G. Corrosion Behavior and Characteristics of 3Cr Steel in Coexisting H2S and CO2 Containing Solutions. J. Mater. Eng. Perform. 2020, 29, 5442–5457. [Google Scholar] [CrossRef]
- Bueno, A.H.S.; Moreira, E.D.; Gomes, J.A.C.P. Evaluation of stress corrosion cracking and hydrogen embrittlement in an API grade steel. Eng. Fail. Anal. 2014, 36, 423–431. [Google Scholar] [CrossRef]
- Hu, K.; Zhuang, J.; Ding, J.; Ma, Z.; Wang, F.; Zeng, X. Influence of biomacromolecule DNA corrosion inhibitor on carbon steel. Corros. Sci. 2017, 125, 68–76. [Google Scholar] [CrossRef]
- Heakal, F.; Elkholy, A. Gemini surfactants as corrosion inhibitors for carbon steel. J. Mol. Liq. 2017, 230, 395–407. [Google Scholar] [CrossRef]
- Farsak, M.; Keleş, H.; Keleş, M. A new corrosion inhibitor for protection of low carbon steel in HCl solution. Corros. Sci. 2015, 98, 223–232. [Google Scholar] [CrossRef]
- Alvarez, P.E.; Fiori-Bimbi, M.V.; Neske, A.; Brandán, S.A. Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J. Ind. Eng. Chem. 2018, 58, 92–99. [Google Scholar] [CrossRef]
- Zhang, B.; He, C.; Wang, C.; Sun, P.; Li, F.; Lin, Y. Synergistic corrosion inhibition of environment-friendly inhibitors on the corrosion of carbon steel in soft water. Corros. Sci. 2015, 94, 6–20. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, B.; Bao, H.; Xie, Y.; Mou, Y.; Li, P.; Chen, D.; Shi, Y.; Li, X.; Yang, W. Evaluation of Ficus tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry 2019, 128, 49–55. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Polysaccharide from Plantago as a green corrosion inhibitor for carbon steel in 1 M HCl solution. Carbohydr. Polym. 2017, 160, 172–183. [Google Scholar] [CrossRef]
- Yang, D.; Ye, Y.; Su, Y.; Liu, S.; Gong, D.; Zhao, H. Functionalization of citric acid-based carbon dots by imidazole toward novel green corrosion inhibitor for carbon steel. J. Clean. Prod. 2019, 229, 180–192. [Google Scholar] [CrossRef]
- Ayukayeva, V.N.; Boiko, G.I.; Lyubchenko, N.P.; Sarmurzina, R.G. Polyoxyethylene sorbitan trioleate surfactant as an effective corrosion inhibitor for carbon steel protection. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123636. [Google Scholar] [CrossRef]
- Zafari, S.; Shahrak, M.N.; Ghahramaninezhad, M. New MOF-based corrosion inhibitor for carbon steel in acidic media. Met. Mater. Int. 2020, 26, 25–38. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, H.; Jiang, R. Synergistic corrosion inhibition effect of quinoline quaternary ammonium salt and Gemini surfactant in H2S and CO2 saturated brine solution. Corros. Sci. 2015, 91, 108–119. [Google Scholar] [CrossRef]
- Roy, K.; Ho Lau, H.; Fang, Z.; Masood, R.; Ting, T.C.H.; Lim, J.B.P.; Lee, V.C.C. Effects of corrosion on the strength of self-drilling screw connections in cold-formed steel structures-experiments and finite element modeling. Structures 2022, 36, 1080–1096. [Google Scholar] [CrossRef]
- Ningshen, S.; Sakairi, M.; Suzuki, K.; Ukai, S. The corrosion resistance and passive film compositions of 12% Cr and 15% Cr oxide dispersion strengthened steels in nitric acid media. Corros. Sci. 2014, 78, 322–334. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, H.; Huang, J.; Yang, J. A novel Zn-Al-Si corrosion resistant filler metal for Cu/Al brazing. Mater. Lett. 2017, 206, 201–204. [Google Scholar] [CrossRef]
- Mishra, A. Performance of corrosion-resistant alloys in concentrated acids. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 306–318. [Google Scholar] [CrossRef]
- Abbas, M.H.; Norman, R.; Charles, A. Neural network modelling of high pressure CO2 corrosion in pipeline steels. Process Saf. Environ. Prot. 2018, 119, 36–45. [Google Scholar] [CrossRef]
- Hatami, S.; Ghaderi-Ardakani, A.; Chamkalani, R.; Niknejad, M.; Ganjiazad, E.; Rasaei, M.R.; Mohammadi, A.H. On the prediction of CO2 corrosion in petroleum industry. J. Supercrit. Fluids 2016, 117, 108–112. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S. Failure analysis of carbon dioxide corrosion through wet natural gas gathering pipelines. Eng. Fail. Anal. 2019, 105, 638–646. [Google Scholar] [CrossRef]
- Barker, R.; Hua, Y.; Neville, A. Internal corrosion of carbon steel pipelines for dense-phase CO2 transport in carbon capture and storage (CCS)–A review. Int. Mater. Rev. 2017, 62, 1–31. [Google Scholar] [CrossRef]
- Qi, J.; He, M.; Yin, Z.; Hui, H. Study on the performance of 80S and 80S -3Cr sulfur-resistant oil pipe materials under corrosive environment containing H2S/CO2. Mater. Prot. 2021, 54, 63–68, 78. [Google Scholar]
- Wang, J.; Lin, Y.; Zhang, Q.; Zeng, D.; Fan, H. Effect of treatment time on the microstructure of austenitic stainless steel during low-temperature liquid nitrocarburizing. Metall. Mater. Trans. A 2014, 45, 4525–4534. [Google Scholar] [CrossRef]
- Cao, C.N. Principles of Corrosion Electrochemistry; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Xu, X.; Yu, Z.; Cui, L.; Niu, X.; Cai, T. Microstructural characteristics of plasma nitrided layer on hot-rolled 304 stainless steel with a small amount of α-ferrite. Metall. Mater. Trans. A 2016, 47, 801–810. [Google Scholar] [CrossRef]
- Zhao, X.H.; Feng, Y.R.; Tang, S.W.; Zhang, J.X. Electrochemical Corrosion Behavior of 15Cr-6Ni-2Mo Stainless Steel with/without Stress under the coexistence of CO2 and H2S. Int. J. Electrochem. Sci. 2018, 13, 6296–6309. [Google Scholar] [CrossRef]


| Ingredients | C | Si | Mn | P | S | Cr | Mo | Ni | Cu |
|---|---|---|---|---|---|---|---|---|---|
| Content | 0.26 | 0.18 | 0.56 | 0.016 | 0.002 | 0.93 | 0.17 | 0.077 | 0.012 |
| Ions | Na+ + K+ | Ca2+ | Mg2+ | Cl− | HCO3− | SO42− |
|---|---|---|---|---|---|---|
| Concentration (mg/L) | 28,518 | 20,739 | 1521 | 90,657 | 932 | 378 |
| Gas Pressure (MPa) | Total Pressure 8 MPa CO2 Partial Pressure 0.4 H2S Partial Pressure 8 × 10−4 | Total Pressure 20 MPa CO2 Partial Pressure 0.6 H2S Partial Pressure 0.12 | Total Pressure 32 MPa CO2 Partial Pressure 0.96 H2S Partial Pressure 3.2 × 10−3 |
|---|---|---|---|
| Temperature (°C) | 30, 60, 100 | 30, 60, 100 | 30, 60, 100 |
| Other conditions | Cycle time 168 h, Produced water on site | ||
| Temperature (°C) | Experimental Conditions | Test Content |
|---|---|---|
| 30 | 0.3%Na2S.9H2O with saturation CO2 | Dynamic potential polarization and electrochemical impedance |
| 60 | ||
| 100 |
| Temperature (°C) | Experimental Conditions | Test Content |
|---|---|---|
| 30 | 3%Na2S.9H2O with saturation CO2 | Dynamic potential polarization and electrochemical impedance |
| 60 | ||
| 100 |
| Conditions | Temperature (°C) | C (%) | O (%) | S (%) | Fe (%) |
|---|---|---|---|---|---|
| Test A | 30 | 4.70 | 19.52 | 0.31 | 74.72 |
| 60 | 15.08 | 26.33 | 5.42 | 53.81 | |
| 100 | / | 52.77 | 2.43 | 44.80 | |
| Test B | 30 | 23.48 | 14.81 | 3.16 | 58.55 |
| 60 | 15.36 | 65.71 | 4.63 | 14.92 | |
| 100 | 7.12 | 9.43 | 25.08 | 58.36 | |
| Test C | 30 | 3.4 | 22.8 | 0.2 | 73.5 |
| 60 | 4.2 | 21.3 | 0.1 | 74.1 | |
| 100 | 10.27 | 20.42 | 7.96 | 53.84 |
| Temperature (°C) | Na2S.9H2O Concentration (%) | Ecorr (V) | Icorr (A·cm−2) | Ba (mV·dec−1) | Bc (mV·dec−1) | Corrosion Rate (mm/a) |
|---|---|---|---|---|---|---|
| 30 | 0.3 | −0.556 | 3.01 × 10−5 | 34.362 | 152.01 | 3.53 × 10−4 |
| 60 | −0.602 | 1.09 × 10−4 | 78.245 | 227.54 | 1.27 × 10−3 | |
| 100 | −0.773 | 5.02 × 10−4 | 143.21 | 301.75 | 5.89 × 10−3 | |
| 30 | 3 | −0.603 | 6.04 × 10−5 | 57.1 | 161.31 | 7.09 × 10−4 |
| 60 | −0.650 | 2.11 × 10−4 | 89.6 | 203.7 | 2.48 × 10−3 | |
| 100 | −0.692 | 3.28 × 10−4 | 121.6 | 209.1 | 3.85 × 10−3 |
| Parameters | 0.3%Na2S.9H2O | 3%Na2S.9H2O | ||||
|---|---|---|---|---|---|---|
| 30 °C | 60 °C | 100 °C | 30 °C | 60 °C | 100 °C | |
| RS | 5.68 | 3.75 | 1.94 | 3.92 | 3.21 | 2.95 |
| Rt | 2371 | 655 | 146 | 1391 | 288 | 194 |
| Y0 | 1.02 × 10−4 | 3.57 × 10−4 | 4.02 × 10−4 | 1.89 × 10−4 | 3.89 × 10−4 | 9.56 × 10−3 |
| n | 0.77 | 0.75 | 0.75 | 0.76 | 0.78 | 0.93 |
| ZW | / | / | 28.6 × 10−2 | / | 41.4 × 10−2 | 7.2 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, P.; Wang, W.; Jia, X. Corrosion Study of 80S Steel under the Coexistence of CO2 and H2S. Metals 2022, 12, 1923. https://doi.org/10.3390/met12111923
Song P, Wang W, Jia X. Corrosion Study of 80S Steel under the Coexistence of CO2 and H2S. Metals. 2022; 12(11):1923. https://doi.org/10.3390/met12111923
Chicago/Turabian StyleSong, Pu, Wenzhen Wang, and Xingang Jia. 2022. "Corrosion Study of 80S Steel under the Coexistence of CO2 and H2S" Metals 12, no. 11: 1923. https://doi.org/10.3390/met12111923
APA StyleSong, P., Wang, W., & Jia, X. (2022). Corrosion Study of 80S Steel under the Coexistence of CO2 and H2S. Metals, 12(11), 1923. https://doi.org/10.3390/met12111923







