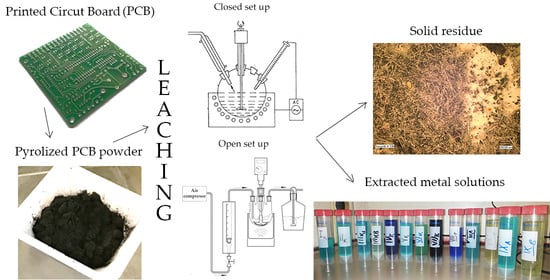
A Multifocal Study Investigation of Pyrolyzed Printed Circuit Board Leaching
Abstract
:1. Introduction
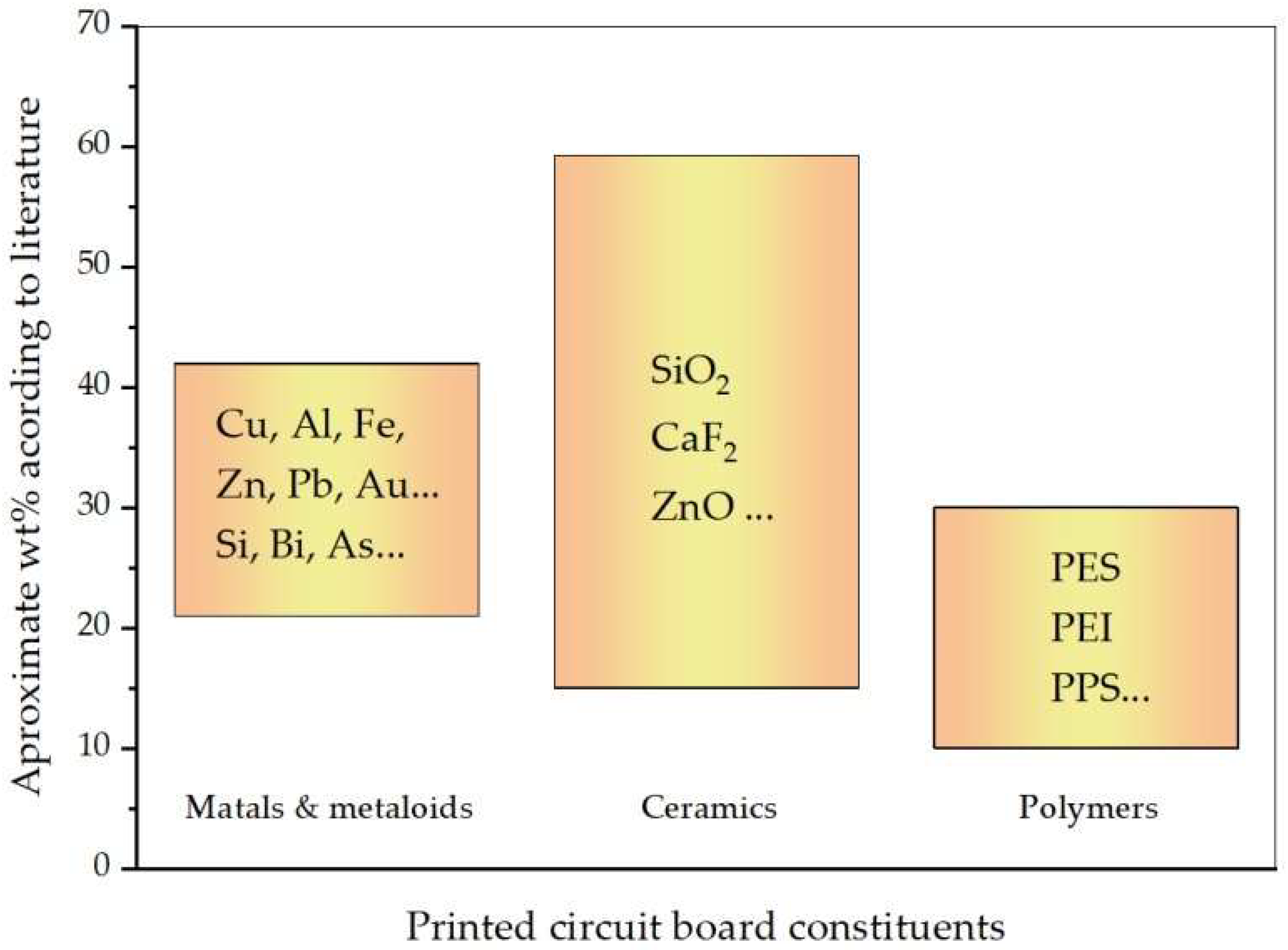
2. Materials and Methods
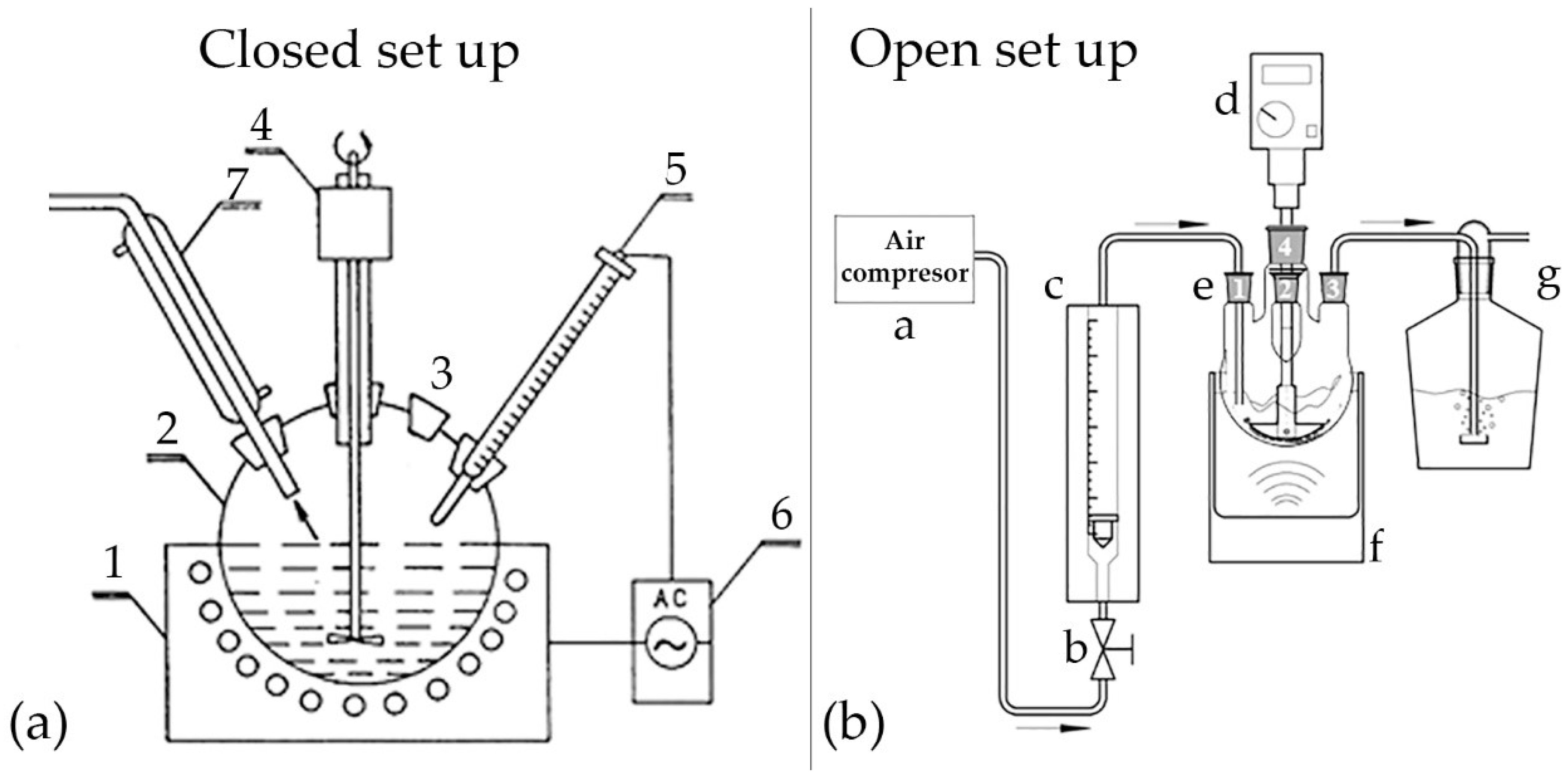
2.1. Experimental Setup
- Series 1—Solid/liquid ratio investigations on sulfuric acid leaching (high and low);
- Series 2—Influence of hydrogen peroxide concentration on sulfuric acid leaching;
- Series 3—Acid mine drainage and distilled water leaching with the aid of hydrogen peroxide.
- Series 1—Influence of hydrogen peroxide on NADES and nitric acid leaching;
- Series 2—Influence of pretreatments on two-pronged leaching.
2.2. Leaching Conditions
3. Results and Discussion
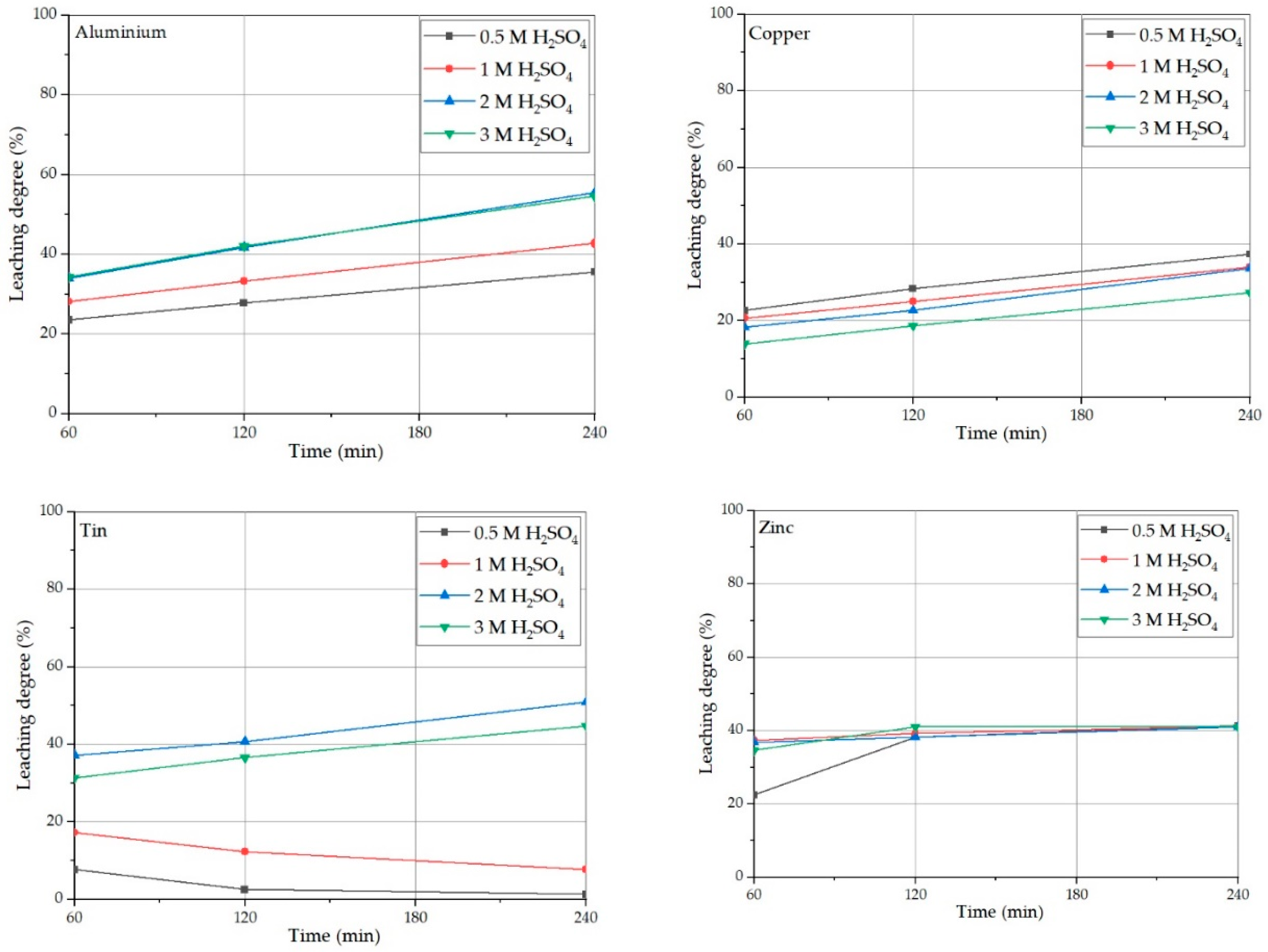
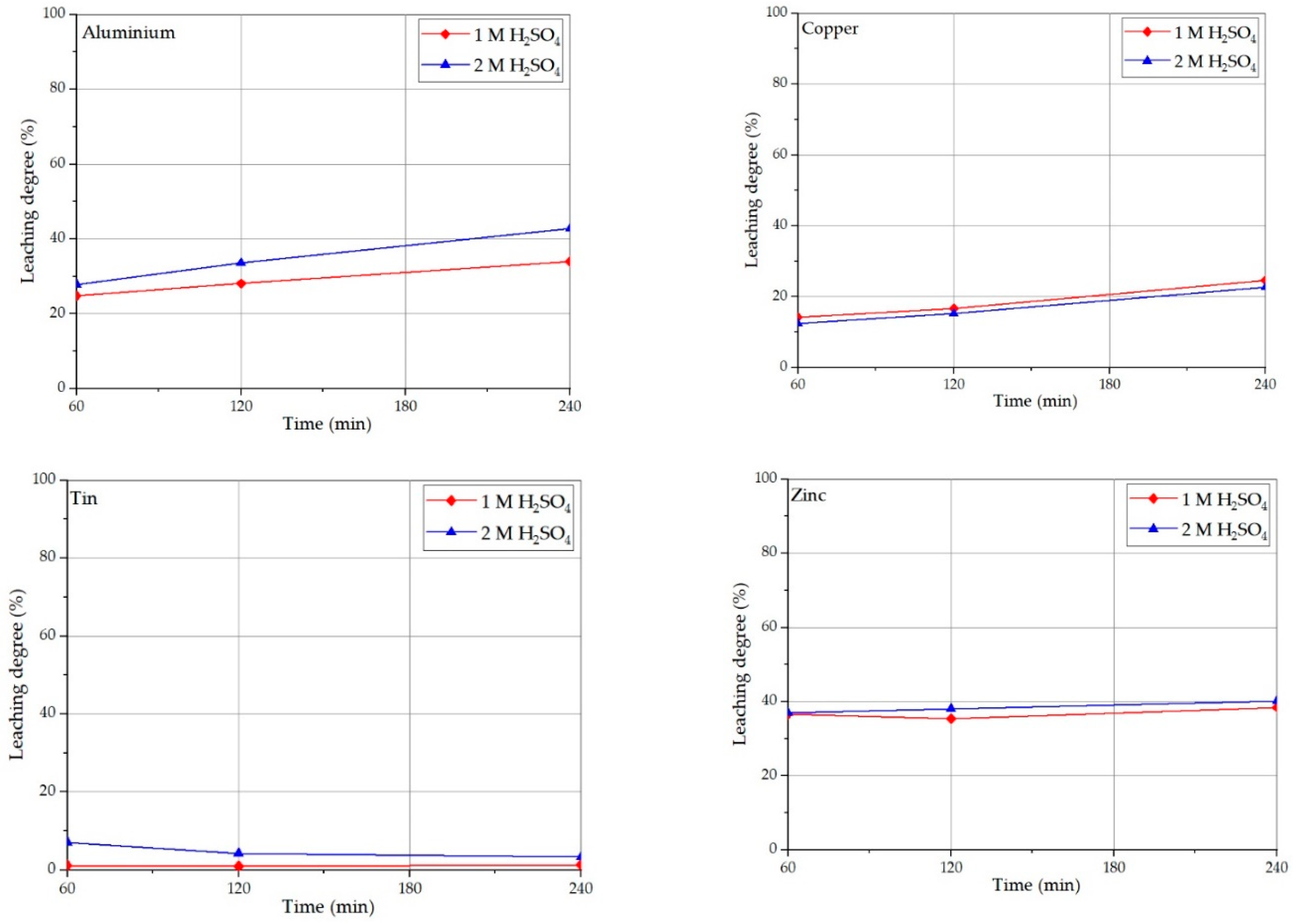
3.1. Solid/Liquid Ratio Investigations on Sulfuric Acid Leaching
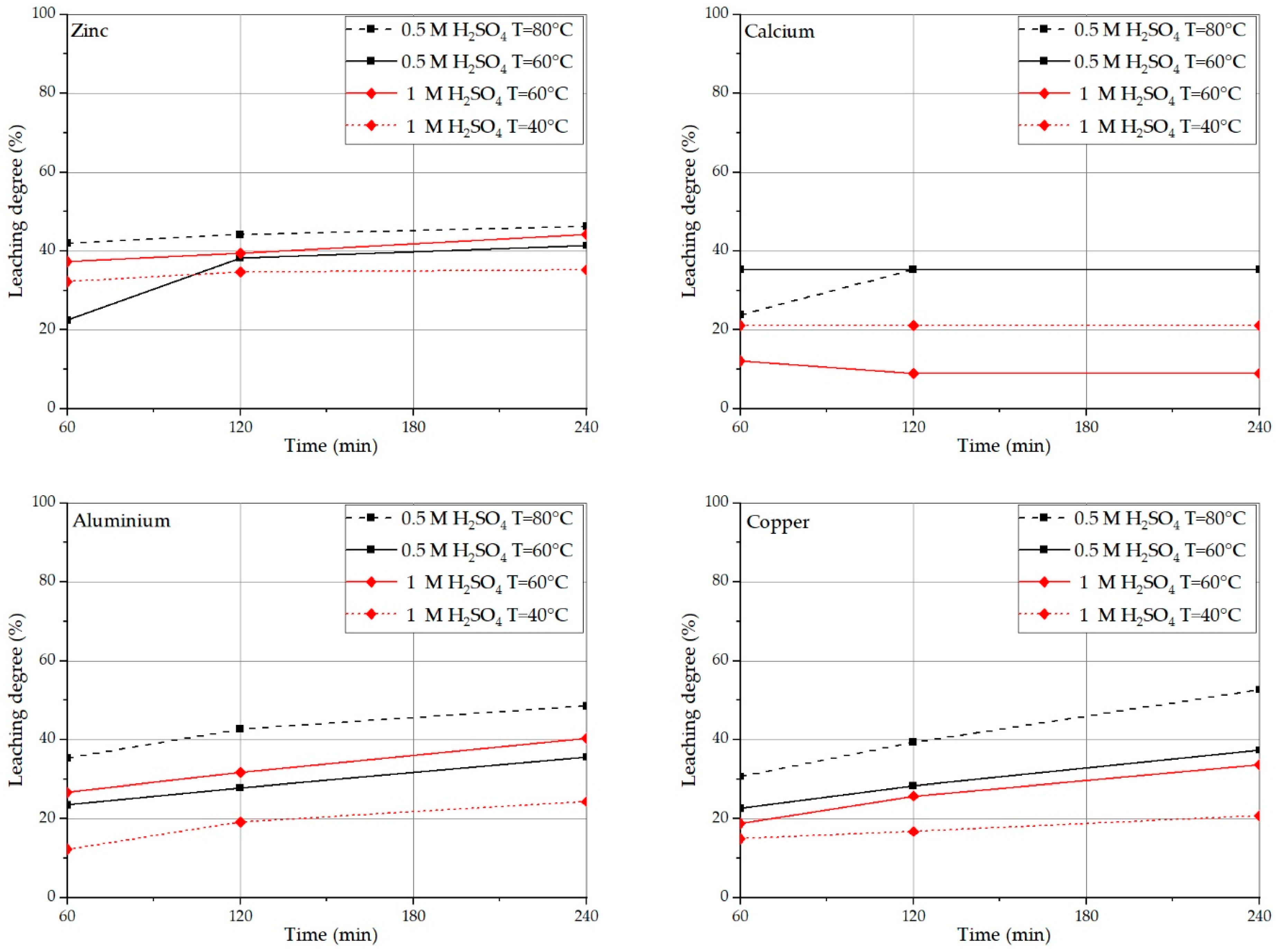
3.2. Acid Concentration Effect on PPCB Leaching
3.3. Temperature Influence on Leaching Effectivity
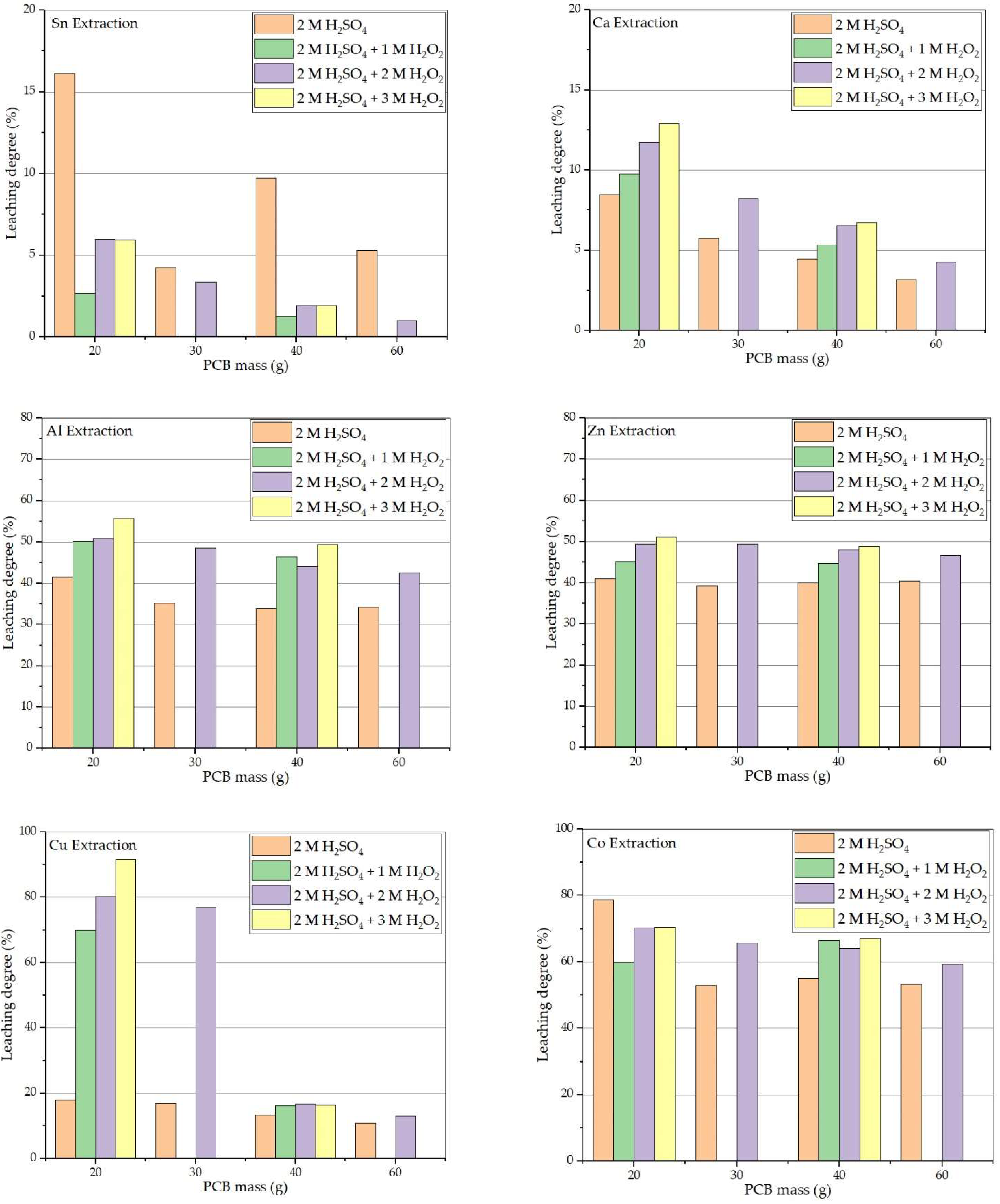
3.4. Influence of Hydrogen Peroxide Concentration on Sulfuric Acid Leaching
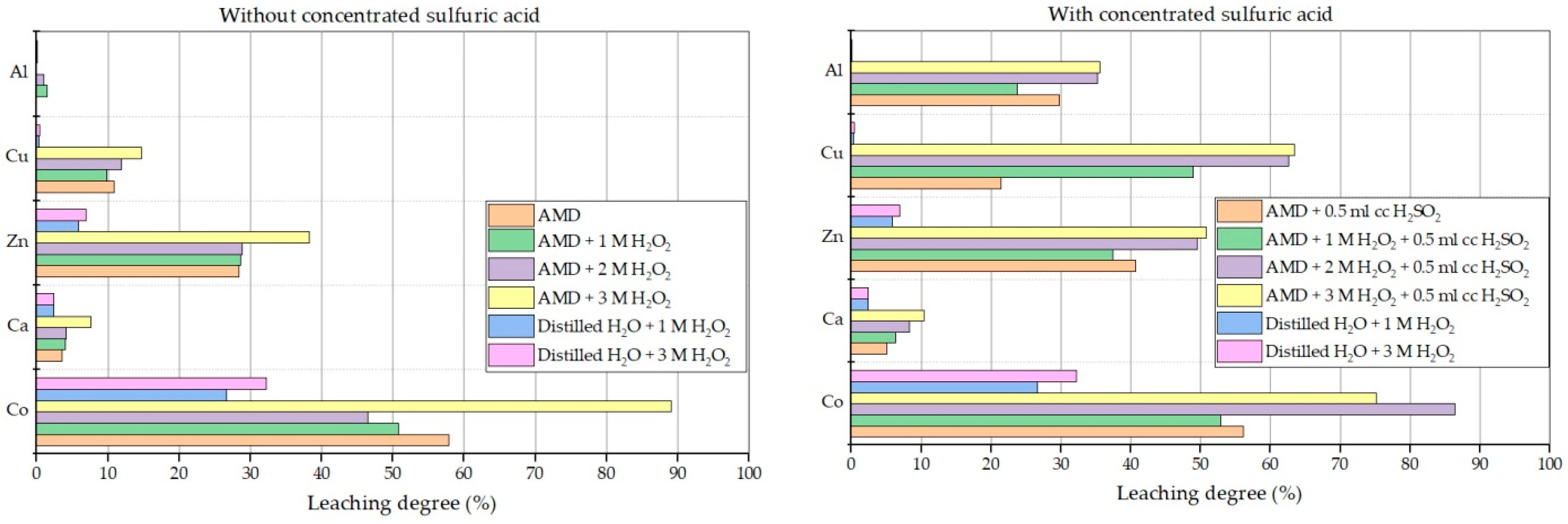
3.5. Acid Mine Drainage and Distilled Water Leaching with the Aid of Hydrogen Peroxide
3.6. Leaching with Glycine Based Lixiviant (NADES)
- Oxidation of metals present in PPCB with readily available hydroxyl radical (when H2O2 is present in solution) or superoxide and other reactive oxygen species (in the absence of H2O2 but in presence of dissolved oxygen in leaching media);
- Bonding the oxidized metal in a complex with a glycinate anion (formation of metal–glycinate complexes);
- Metal cementation from metal–glycinate complexes in the presence of easily accessible pyrolyzed polymer residue (that are carbon rich materials).
3.7. Two-Pronged Leaching without Pretreatment
3.8. Leaching with Two Strong Oxidizing Agents (Nitric Acid and Hydrogen Peroxide)
3.9. Two-Pronged Leaching with Two Pretreatment Approaches
4. Conclusions
- The aluminum leaching degree is limited by the increase in the S/L ratio since it is diffusion controlled. The increase of sulfuric acid concentration lowers oxygen solubility, and this promotes Al leaching. As expected, with the increase in temperature, the Al leaching degree increases, as per the corrosion approach. The addition of hydrogen peroxide in an acidic environment acts a potent etching agent for both aluminum and aluminum oxide, but in the alkali environment it hydrolyses sodium aluminate forming aluminum hydroxide.
- The copper leaching degree is obstructed with an increase in S/L ratio, similar to Al, since it is also diffusion controlled. However, an increase in sulfuric acid concentration that lowers oxygen solubility is detrimental to the Cu leaching degree, while the increase in leaching temperature is very beneficial. Additionally, the presence of an oxidation agent, be it air or hydrogen peroxide, significantly promotes Cu leaching, and this is even more pronounced under ultrasound sonification. For leaching in nitric acid this is not needed, but there is a drawback as copper (II) nitrate hydrolyses overtime. Without the presence of hydrogen peroxide, Cu leaching in an alkali environment is insignificant.
- The cobalt leaching degree is proportional to the reduction of Co3+; therefore, it is strongly related to mass transfer and the materials present in the PPCB that can be oxidized. This is why the presence of hydrogen peroxide lowers the Co leaching degree. However, it is highly dependent on the penetration of the leaching agent into the PPCB matrix, which can be promoted with ultrasound and swelling. Both sulfuric and nitric acid are very efficient in leaching Co, but the leaching in NADES is limited to the formation of cobalt glycinate, while in pure alkali media there is no leaching at all.
- The zinc leaching degree is limited by the expenditure of zinc sulfide that passivizes easily in sulfuric acid, and is elevated by a temperature increase or ultrasound sonification. Nitric acid dissolves the passive layer of sulfur, while the addition of hydrogen peroxide to sulfuric acid produced hydrogen sulfide gas instead of sulfur. The overall leaching degree of Zn is attributed to the leaching of Zn sulfide and Zn oxide, both of which are present in PPCB.
- The tin leaching degree is limited by two concurrent reactions: the production of soluble Sn (II) salts and the production of insoluble Sn (IV) compounds. At low S/L ratios, there is a critical sulfuric acid concentration between 1 M and 2 M, below which Sn (IV) oxidation is promoted. However, in nitric acid there are no concurrent reactions and only insoluble Sn (IV) compounds are produced.
- The calcium leaching degree is closely linked to the way that produced hydrofluoric acid leaves the leaching solution. Hydrofluoric acid can be either spent on fiberglass dissolution or escorted in gaseous form from the leaching solution. Fiberglass dissolution is promoted by sodium hydroxide while gas migration is promoted either by adding hydrogen peroxide or by using nitric acid.
- The neodymium leaching degree is favorable in acidic media while unfavorable in alkali media without the presence of hydrogen peroxide. The palladium leaching degree is promoted in a strong oxidation environment. This goes for platinum leaching degree as well; however, it can also be achieved in a NADES leaching solution. The lead leaching degree is the highest in nitric acid but also promoted in an alkali environment. Finally, the barium leaching degree was only seen to be promoted in nitric acid.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020; United Nations University: Bonn, Germany; United Nations Institute for Training and Research: Geneva, Switzerland; International Telecommunication Union, and International Solid Waste Association: Rotterdam, The Netherlands, 2020; ISBN 9789280891140. [Google Scholar]
- Zhang, S.; Forssberg, E. Mechanical Separation-Oriented Characterization of Electronic Scrap. Resour. Conserv. Recycl. 1997, 21, 247–269. [Google Scholar] [CrossRef]
- Sum, E.Y.L. The Recovery of Metals from Electronic Scrap. Rev. Extr. Metall. 1991, 43, 53–61. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, X.; Li, B.; Tao, D. Recovery of Copper from Waste Printed Circuit Boards. Min. Metall. Explor. 2004, 21, 99–102. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.C.; Seo, S.P.; Park, Y.K.; Sohn, H.Y. A Process for Extracting Precious Metals from Spent Printed Circuit Boards and Automobile Catalysts. JOM 2004, 56, 55–58. [Google Scholar] [CrossRef]
- Taurino, R.; Pozzi, P.; Zanasi, T. Facile Characterization of Polymer Fractions from Waste Electrical and Electronic Equipment (WEEE) for Mechanical Recycling. Waste Manag. 2010, 30, 2601–2607. [Google Scholar] [CrossRef]
- Patil, T.; Rebaioli, L.; Fassi, I. Cyber-Physical Systems for End-of-Life Management of Printed Circuit Boards and Mechatronics Products in Home Automation: A Review. Sustain. Mater. Technol. 2022, 32, e00422. [Google Scholar] [CrossRef]
- Maurice, A.A.; Dinh, K.N.; Charpentier, N.M.; Brambilla, A.; Gabriel, J.C.P. Dismantling of Printed Circuit Boards Enabling Electronic Components Sorting and Their Subsequent Treatment Open Improved Elemental Sustainability Opportunities. Sustainability 2021, 13, 10357. [Google Scholar] [CrossRef]
- Wan, X.; Fellman, J.; Jokilaakso, A.; Klemettinen, L.; Marjakoski, M. Behavior of Waste Printed Circuit Board (WPCB) Materials in the Copper Matte Smelting Process. Metals 2018, 8, 887. [Google Scholar] [CrossRef] [Green Version]
- Kamberović, Ž.; Ranitović, M.; Korać, M.; Andjić, Z.; Gajić, N.; Djokić, J.; Jevtić, S. Hydrometallurgical Process for Selective Metals Recovery from Waste-Printed Circuit Boards. Metals 2018, 8, 441. [Google Scholar] [CrossRef] [Green Version]
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. E-Waste Recycling and Resource Recovery: A Review on Technologies, Barriers and Enablers with a Focus on Oceania. Metals 2021, 11, 1313. [Google Scholar] [CrossRef]
- Birloaga, I.; Coman, V.; Kopacek, B.; Vegliò, F. An Advanced Study on the Hydrometallurgical Processing of Waste Computer Printed Circuit Boards to Extract Their Valuable Content of Metals. Waste Manag. 2014, 34, 2581–2586. [Google Scholar] [CrossRef]
- Lancellotti, I.; Piccolo, F.; Nguyen, H.; Mastali, M.; Alzeer, M.; Illikainen, M.; Leonelli, C. The Effect of Fibrous Reinforcement on the Polycondensation Degree of Slag-Based Alkali Activated Composites. Polymers 2021, 13, 2664. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.A.G.; Caldas, M.P.K.; de Moraes, V.T.; Tenório, J.A.S.; Espinosa, D.C.R. Recovering Metals from Motherboard and Memory Board Waste through Sulfuric Leaching. J. Environ. Chem. Eng. 2021, 9, 106789. [Google Scholar] [CrossRef]
- Dávila-Pulido, G.I.; Salinas-Rodríguez, A.; Carrillo-Pedroza, F.R.; González-Ibarra, A.A.; Méndez-Nonell, J.; Garza-García, M. Leaching Kinetics of Electronic Waste for the Recovery of Copper: Rate-Controlling Step and Rate Process in a Multisize Particle System. Int. J. Chem. Kinet. 2021, 53, 379–389. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, L.; Yu, M.; Li, J. An Innovative Method of Recycling Metals in Printed Circuit Board (PCB) Using Solutions from PCB Production. J. Hazard. Mater. 2020, 390, 121892. [Google Scholar] [CrossRef]
- Barnwal, A.; Dhawan, N. Recycling of Discarded Mobile Printed Circuit Boards for Extraction of Gold and Copper. Sustain. Mater. Technol. 2020, 25, e00164. [Google Scholar] [CrossRef]
- Havlik, T.; Orac, D.; Petranikova, M.; Miskufova, A.; Kukurugya, F.; Takacova, Z. Leaching of Copper and Tin from Used Printed Circuit Boards after Thermal Treatment. J. Hazard. Mater. 2010, 183, 866–873. [Google Scholar] [CrossRef]
- Dimitrijević, M.; Urošević, D.; Milić, S.; Sokić, M.; Marković, R. Dissolution of Copper from Smelting Slag by Leaching in Chloride Media. J. Min. Metall. Sect. B Metall. 2017, 53, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Yi, X.; Wang, R.; Huang, J.; Chen, M.; Sun, Z.; Sun, S.; Shu, J. Copper Extraction from Waste Printed Circuit Boards by Glycine. Sep. Purif. Technol. 2020, 253, 117463. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Stanković, S.; Kamberović, Ž.; Štrbac, N.; Manojlović, V.; Petronijević, N. Kinetics of Chalcopyrite Leaching by Hydrogen Peroxide in Sulfuric Acid. Metals 2019, 9, 1173. [Google Scholar] [CrossRef]
- Cui, H.; Anderson, C. Hydrometallurgical Treatment of Waste Printed Circuit Boards: Bromine Leaching. Metals 2020, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.Y.; Kim, M.S.; Lee, J.C.; Jeong, J.; Pandey, B.D. Leaching Kinetics of Copper from Waste Printed Circuit Boards by Electro-Generated Chlorine in HCl Solution. Hydrometallurgy 2011, 107, 124–132. [Google Scholar] [CrossRef]
- Pinho, S.; Ferreira, M.; Almeida, M.F. A Wet Dismantling Process for the Recycling of Computer Printed Circuit Boards. Resour. Conserv. Recycl. 2018, 132, 71–76. [Google Scholar] [CrossRef]
- Xu, M.; Alyasi, H.; Ning, C.; Hui, C.-W.; Mackey, H.; McKay, G. Removal of Toxic Cadmium Using a Binary Site Ion-exchange Material Derived from Waste Printed Circuit Boards. J. Chem. Technol. Biotechnol. 2021, 96, 3282–3292. [Google Scholar] [CrossRef]
- Stuhlpfarrer, P.; Luidold, S.; Antrekowitsch, H. Recycling of Waste Printed Circuit Boards with Simultaneous Enrichment of Special Metals by Using Alkaline Melts: A Green and Strategically Advantageous Solution. J. Hazard. Mater. 2016, 307, 17–25. [Google Scholar] [CrossRef]
- Birich, A.; Stopic, S.; Friedrich, B. Kinetic Investigation and Dissolution Behavior of Cyanide Alternative Gold Leaching Reagents. Sci. Rep. 2019, 9, 7191. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.; Tabelin, C.B.; Park, I.; Nagata, Y.; Ito, M.; Hiroyoshi, N. Ammonium Thiosulfate Extraction of Gold from Printed Circuit Boards (PCBs) of End-of-Life Mobile Phones and Its Recovery from Pregnant Leach Solution by Cementation. Hydrometallurgy 2020, 191, 105214. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Liu, W. Thiourea Leaching Gold and Silver from the Printed Circuit Boards of Waste Mobile Phones. Waste Manag. 2012, 32, 1209–1212. [Google Scholar] [CrossRef]
- Oluokun, O.O.; Otunniyi, I.O. Kinetic Analysis of Cu and Zn Dissolution from Printed Circuit Board Physical Processing Dust under Oxidative Ammonia Leaching. Hydrometallurgy 2020, 193, 105320. [Google Scholar] [CrossRef]
- Hyk, W.; Kitka, K. Highly Efficient and Selective Leaching of Silver from Electronic Scrap in the Base-Activated Persulfate—Ammonia System. Waste Manag. 2017, 60, 601–608. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of Metal Oxides in Deep Eutectic Solvents Based on Choline Chloride. J. Chem. Eng. Data 2006, 51, 1280–1282. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, H.; Yong, W.F.; She, Q.; Esteban, J. Status and Advances of Deep Eutectic Solvents for Metal Separation and Recovery. Green Chem. 2022, 24, 1895–1929. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J.; O’Connor, G.M. Gold Leaching from Oxide Ores in Alkaline Glycine Solutions in the Presence of Permanganate. Hydrometallurgy 2020, 198, 105527. [Google Scholar] [CrossRef]
- Barani, K.; Dehghani, M.; Azadi, M.R.; Karrech, A. Leaching of a Polymetal Gold Ore and Reducing Cyanide Consumption Using Cyanide-Glycine Solutions. Miner. Eng. 2021, 163, 106802. [Google Scholar] [CrossRef]
- Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Cheshmeh, Z.A. A Comprehensive Review on Pyrolysis of E-Waste and Its Sustainability. J. Clean. Prod. 2022, 333, 130191. [Google Scholar] [CrossRef]
- Illarionov, Y.Y.; Banshchikov, A.G.; Knobloch, T.; Polyushkin, D.K.; Wachter, S.; Fedorov, V.V.; Suturin, S.M.; Stoger-Pollach, M.; Mueller, T.; Vexler, M.I.; et al. Crystalline Calcium Fluoride: A Record-Thin Insulator for Nanoscale 2D Electronics. In Proceedings of the 2020 Device Research Conference (DRC), Columbus, OH, USA, 21–24 June 2020. [Google Scholar] [CrossRef]
- Schüler, D.; Buchert, M.; Liu, R.; Dittrich, S.; Merz, C. Study on Rare Earths and Their Recycling—Final Report for the Greens/EFA Group in the European Parliament. Öko-Inst. E. V. Inst. Für Angew. Ökologie 2011, 49, 140. [Google Scholar]
- Chen, X.; Tian, W.; Li, S.; Yu, M.; Liu, J. Effect of Temperature on Corrosion Behavior of 3003 Aluminum Alloy in Ethylene Glycol–Water Solution. Chin. J. Aeronaut. 2016, 29, 1142–1150. [Google Scholar] [CrossRef] [Green Version]
- Saubestre, E.B. Copper Etching in Ferric Chloride. Ind. Eng. Chem. 1959, 51, 288–290. [Google Scholar] [CrossRef]
- Bogdanović, G.D.; Petrović, S.; Sokić, M.; Antonijević, M.M. Chalcopyrite Leaching in Acid Media: A Review. Metall. Mater. Eng. 2020, 26, 177–198. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Matković, V.; Živković, D.; Štrbac, N.; Stojanović, J. Kinetics and Mechanism of Sphalerite Leaching by Sodium Nitrate in Sulphuric Acid Solution. J. Min. Metall. Sect. B Metall. 2012, 48, 185–195. [Google Scholar] [CrossRef]
- Picazo-Rodríguez, N.G.; Soria-Aguilar, M.D.J.; Martínez-Luévanos, A.; Almaguer-Guzmán, I.; Chaidez-Félix, J.; Carrillo-Pedroza, F.R. Direct Acid Leaching of Sphalerite: An Approach Comparative and Kinetics Analysis. Minerals 2020, 10, 359. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.E.D.L.; Rivera-Santillán, R.E.; Talavera-Ortega, M.; Bautista, F. Catalytic and Galvanic Effects of Pyrite on Ferric Leaching of Sphalerite. Hydrometallurgy 2016, 163, 167–175. [Google Scholar] [CrossRef]
- Lorenzo-Tallafigo, J.; Iglesias-Gonzalez, N.; Romero, R.; Mazuelos, A.; Carranza, F. Ferric Leaching of the Sphalerite Contained in a Bulk Concentrate: Kinetic Study. Miner. Eng. 2018, 125, 50–59. [Google Scholar] [CrossRef]
- Deyab, M.A.; El-Rehim, S.S.A.; Hassan, H.H.; El-Moneim, A.A. Corrosion and Corrosion Inhibition of Aluminum Alloys A5052 and A5754 in Sulfuric Acid Solutions by Some Inorganic Inhibitors. Z. Fur Phys. Chem. 2017, 231, 1141–1157. [Google Scholar] [CrossRef]
- Lidin, R.A.; Andreeva, L.L.; Molochko, V.A. Constants of Inorganic Substances: Handbook, 2nd ed.; Drofa: Moscow, Russia, 2006; ISBN 5-7107-8085-5. [Google Scholar]
- Partridge, E.P.; White, A.H. The Solubility of Calcium Sulfate from 0 to 200°. J. Am. Chem. Soc. 1929, 51, 360–370. [Google Scholar] [CrossRef]
- Quicker, G.; Schumpe, A.; König, B.; Deckwer, W.-D. Comparison of Measured and Calculated Oxygen Solubilities in Fermentation Media. Biotechnol. Bioeng. 1981, 23, 635–650. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Slíva, A.; Paszenda, Z.; Hundáková, M.; Kratošová, G.; Holešová, S.; Majewska, J.; Kałużyński, P.; Sathish, S.K.; Martynková, G.S. Simple Approach to Medical Grade Alumina and Zirconia Ceramics Surface Alteration via Acid Etching Treatment. Crystals 2021, 11, 1232. [Google Scholar] [CrossRef]
- Aydogan, S. Dissolution Kinetics of Sphalerite with Hydrogen Peroxide in Sulphuric Acid Medium. Chem. Eng. J. 2006, 123, 65–70. [Google Scholar] [CrossRef]
- Mwewa, B.; Stopić, S.; Ndlovu, S.; Simate, G.S.; Xakalashe, B.; Friedrich, B. Synthesis of Poly-Alumino-Ferric Sulphate Coagulant from Acid Mine Drainage by Precipitation. Metals 2019, 9, 1166. [Google Scholar] [CrossRef] [Green Version]
- Rabe, A.; Büker, J.; Salamon, S.; Koul, A.; Hagemann, U.; Landers, J.; Friedel Ortega, K.; Peng, B.; Muhler, M.; Wende, H.; et al. The Roles of Composition and Mesostructure of Cobalt-Based Spinel Catalysts in Oxygen Evolution Reactions. Chem. A Eur. J. 2021, 27, 17038–17048. [Google Scholar] [CrossRef]
- Altinkaya, P.; Wang, Z.; Korolev, I.; Hamuyuni, J.; Haapalainen, M.; Kolehmainen, E.; Yliniemi, K.; Lundström, M. Leaching and Recovery of Gold from Ore in Cyanide-Free Glycine Media. Miner. Eng. 2020, 158, 106610. [Google Scholar] [CrossRef]
- Zhang, D.S.; Tan, Z.C.; Liu, B.P.; Huang, X.B. Thermodynamic Study on Complex of Neodymium with Glycine. J. Chem. Thermodyn. 2015, 83, 27–32. [Google Scholar] [CrossRef]
- Yot, P.G.; Méar, F.O. Characterization of Lead, Barium and Strontium Leachability from Foam Glasses Elaborated Using Waste Cathode Ray-Tube Glasses. J. Hazard. Mater. 2011, 185, 236–241. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, Y.; You, K.; Liu, Y.; Song, Y.; Liu, S.; Guan, S. Synthesis and Properties of Soluble Cross-Linkable Fluorinated Co-Polyimides. React. Funct. Polym. 2014, 82, 58–65. [Google Scholar] [CrossRef]

| Element * Concentration (mg/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ag | Au | Al | Ba | C | Ca | Ce | Co | Cu | Fe | Li |
| 0.180 | 0.180 | 48.1 | 8.30 | 177 | 62.4 | 0.350 | 0.042 | 115 | 11.3 | 0.023 |
| Nd | Ni | Mn | Pb | Pd | Pt | Si | Sn | Y | Zn | |
| 0.218 | 1.9 | 0.623 | 14.3 | 0.203 | 0.044 | 100 | 26.0 | 0.013 | 9.00 | |
| Element Concentration (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ag | Au | Al | Ba | Mg | Ca | Ce | Co | Cu | Fe | Li |
| <0.5 | <0.5 | 423 | <0.5 | 428 | 491 | 4.91 | 1.22 | 1750 | 2630 | <0.5 |
| Nd | Ni | Mn | Pb | Pd | Pt | Si | Sn | Y | Zn | La |
| 1.1 | 1.26 | 8.88 | 0.76 | <0.5 | <0.5 | 44.9 | <0.5 | 1.54 | 8.61 | <0.5 |
| without H2O2 | ORP (mV) | Time (h) | Leaching Degree (%) | ||||||||||
| Al | Cu | Zn | Sn | Nd | Pd | Ca | Pb | Ba | Co | Pt | |||
| −388.5 | 2 | 16.9 | 1.6 | 25.6 | 3.6 | n.d. | n.d. | 0.3 | 26.2 | 0.1 | n.d. | n.d. | |
| −375.6 | 4 | 15.9 | 2.5 | 28.9 | 1.5 | n.d. | n.d. | 0.3 | 27.1 | 0.5 | 27.6 | 22.7 | |
| −389.3 | 6 | 14.8 | 5.1 | 28.9 | 1.1 | n.d. | n.d. | 0.2 | 21.0 | 1.0 | 29.0 | n.d. | |
| −418.7 | 8 | 12.5 | 4.5 | 24.3 | 0.8 | n.d. | n.d. | 0.1 | 14.4 | 1.0 | 25.7 | n.d. | |
| with H2O2 | ORP | Time (h) | Leaching Degree (%) | ||||||||||
| Al | Cu | Zn | Sn | Nd | Pd | Ca | Pb | Ba | Co | Pt | |||
| −317.1 | 2 | 23.5 | 21.9 | 39.3 | 4.3 | 19.9 | n.d. | 2.0 | 28.0 | 1.8 | 48.3 | 98.2 | |
| −277.3 | 4 | 6.8 | 17.2 | 29.2 | 3.2 | 11.9 | 5.6 | 0.5 | 17.1 | 0.8 | 44.7 | 95.3 | |
| −230.4 | 6 | 2.2 | 33.7 | 25.8 | 3.3 | 12.6 | 5.8 | 0.4 | 12.5 | 1.0 | 40.9 | 86.3 | |
| −83.5 | 8 | 0.6 | 12.9 | 16.1 | 1.8 | / | n.d. | 0.2 | 7.3 | 0.4 | 25.7 | 27.5 | |
| Leaching Agent | ORP (mV) | Time (h) | Leaching Degree (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Cu | Zn | Sn | Nd | Pd | Ca | Pb | Ba | Co | Pt | |||
| Sulfuric Acid | 336.2 | 2 | 30.3 | 53.6 | 53.1 | 1.5 | 73.8 | n.d. | 12.9 | 0.5 | n.d. | 94.4 | n.d. |
| 416 | 4 | 38.5 | 77.6 | 54.2 | 1.7 | 61.5 | 4.9 | 10.9 | 0.6 | 0.1 | 93.8 | 22.7 | |
| Nitric acid | 874.2 | 2 | 9 | 17.5 | 6.1 | 2 | 17.6 | 23.7 | 12.2 | 23.4 | n.d. | n.d. | n.d. |
| 891.4 | 4 | 13.4 | 14.3 | 4.1 | 1.3 | 15.8 | 33 | 19.6 | 49.5 | 5.4 | n.d. | n.d. | |
| Summed LD * (4 h + 4 h) | 51.9 | 91.9 | 58.3 | 3.0 | 77.3 | 37.9 | 30.5 | 50.1 | 5.5 | 93.8 | 22.7 | ||
| ORP (mV) | Time (h) | Leaching Degree (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Cu | Zn | Sn | Nd | Pd | Ca | Pb | Ba | Co | Pt | ||
| 779.5 | 2 | 46.8 | 92.6 | 76.3 | 1.2 | 94.3 | 26.5 | 30.1 | 65.5 | 20.9 | 99.2 | 87.3 |
| 770.7 | 4 | 41.9 | 94.7 | 74.5 | 0.8 | 93.7 | 64.3 | 37.1 | 78.8 | 22.9 | 95.1 | 93.4 |
| 798.2 | 6 | 48.7 | 98.9 | 70.1 | 0.8 | 86.4 | 87.9 | 29.8 | 81.9 | 26.1 | 92.7 | 98.1 |
| 859.4 | 8 | 36.7 | 71.9 | 54.3 | 0.4 | 75.0 | 97.7 | 29.8 | 94.9 | 19.7 | 80.7 | 99.6 |
| Element | NaOH Pretreatment | Swelling Pretreatment | |||||||
| Sodium Hydroxide Treatment | Nitric Acid Leaching | NaGly Leaching | Nitric Acid Leaching | NaGly Leaching | |||||
| ORP (mV) | ORP (mV) | ||||||||
| 865.7 | 851.7 | −335 | −306.3 | 866 | 854.4 | −243 | −291 | ||
| Time (h) | Time (h) | ||||||||
| 2 | 4 | 2 | 4 | 2 | 4 | 2 | 4 | ||
| Leaching Degree (%) | |||||||||
| Al | 23.1 | 0.031 | 0.030 | n.d. | n.d. | 39.8 | 43.9 | n.d. | n.d. |
| Cu | 6.0 | 86.6 | 77.8 | 0.1 | 0.5 | 93.4 | 95.8 | 1.1 | 0.3 |
| Zn | 33.4 | 36.4 | 29.8 | 0.3 | 0.3 | 68.3 | 65.3 | 1.9 | 0.3 |
| Sn | 11.3 | 7.6 | 6.7 | 0.1 | n.d. | 1.4 | 2.4 | 0.1 | 0.1 |
| Nd | n.d. | 92.2 | 78.4 | n.d. | n.d. | 95.3 | 93.8 | n.d. | n.d. |
| Pd | n.d. | 6.9 | 5.7 | 22.5 | 25.3 | 27.3 | 27.5 | 45.9 | 31.1 |
| Ca | 0.03 | 55.0 | 53.1 | 0.2 | 0.5 | 31.8 | 24.5 | 0.1 | 0.2 |
| Pb | 59.3 | 41.5 | 29.4 | n.d. | n.d. | 97.1 | 86.9 | 0.4 | n.d. |
| Ba | n.d. | 24.2 | 34.3 | n.d. | n.d. | 23.3 | 21.6 | 0.7 | n.d. |
| Co | n.d. | 97.7 | 93.8 | n.d. | n.d. | 99.5 | 99.1 | n.d. | n.d. |
| Pt | n.d. | 88.3 | 94.2 | n.d. | n.d. | 89.1 | 93.2 | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, G.; Bugarčić, M.; Petronijević, N.; Stopic, S.R.; Friedrich, B.; Marković, B.; Stanković, S.; Sokić, M. A Multifocal Study Investigation of Pyrolyzed Printed Circuit Board Leaching. Metals 2022, 12, 2021. https://doi.org/10.3390/met12122021
Jovanović G, Bugarčić M, Petronijević N, Stopic SR, Friedrich B, Marković B, Stanković S, Sokić M. A Multifocal Study Investigation of Pyrolyzed Printed Circuit Board Leaching. Metals. 2022; 12(12):2021. https://doi.org/10.3390/met12122021
Chicago/Turabian StyleJovanović, Gvozden, Mladen Bugarčić, Nela Petronijević, Srecko R. Stopic, Bernd Friedrich, Branislav Marković, Srđan Stanković, and Miroslav Sokić. 2022. "A Multifocal Study Investigation of Pyrolyzed Printed Circuit Board Leaching" Metals 12, no. 12: 2021. https://doi.org/10.3390/met12122021
APA StyleJovanović, G., Bugarčić, M., Petronijević, N., Stopic, S. R., Friedrich, B., Marković, B., Stanković, S., & Sokić, M. (2022). A Multifocal Study Investigation of Pyrolyzed Printed Circuit Board Leaching. Metals, 12(12), 2021. https://doi.org/10.3390/met12122021