Tailoring the Pore Structure of Porous Ni-Sn Alloys for Boosting Hydrogen Evolution Reaction in Alkali Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Porous Ni-Sn Alloy
2.2. Instruments and Reagents for Experiments
2.3. Experiment in Electrochemical Testing
3. Results and Discussion
3.1. Characterization of Ni-Sn Electrode
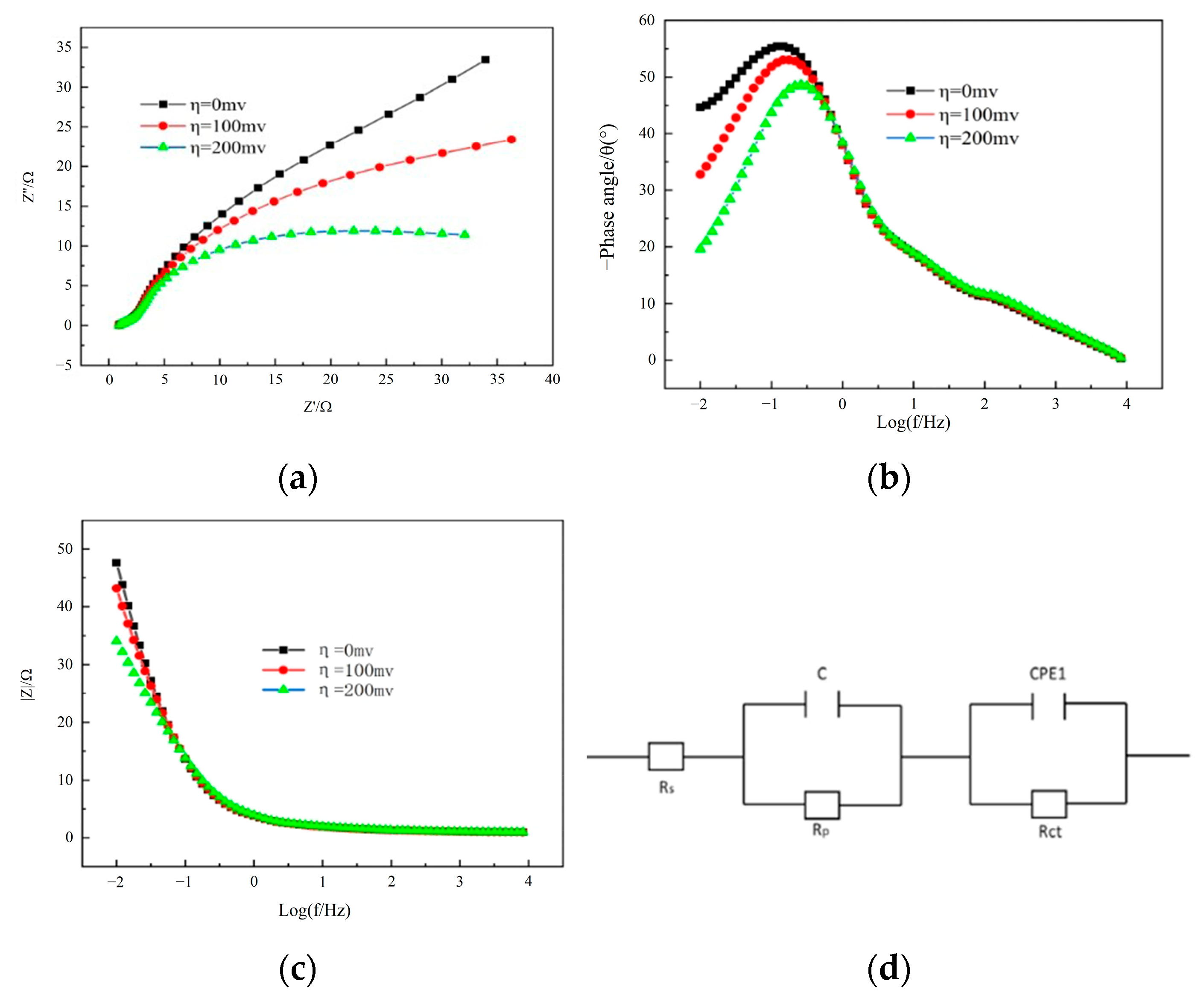
3.2. Electrochemical Characterization of Ni-Sn Electrode
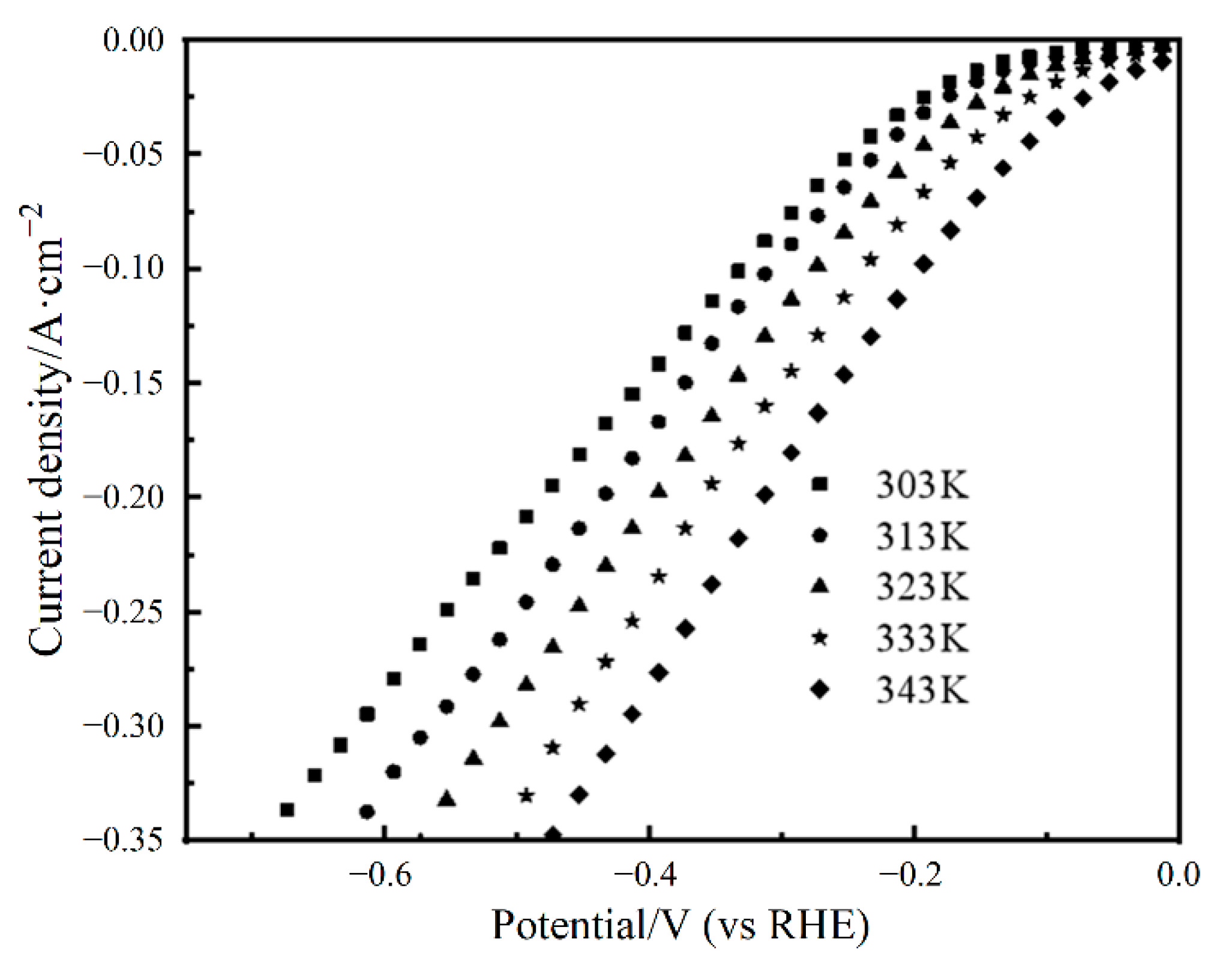
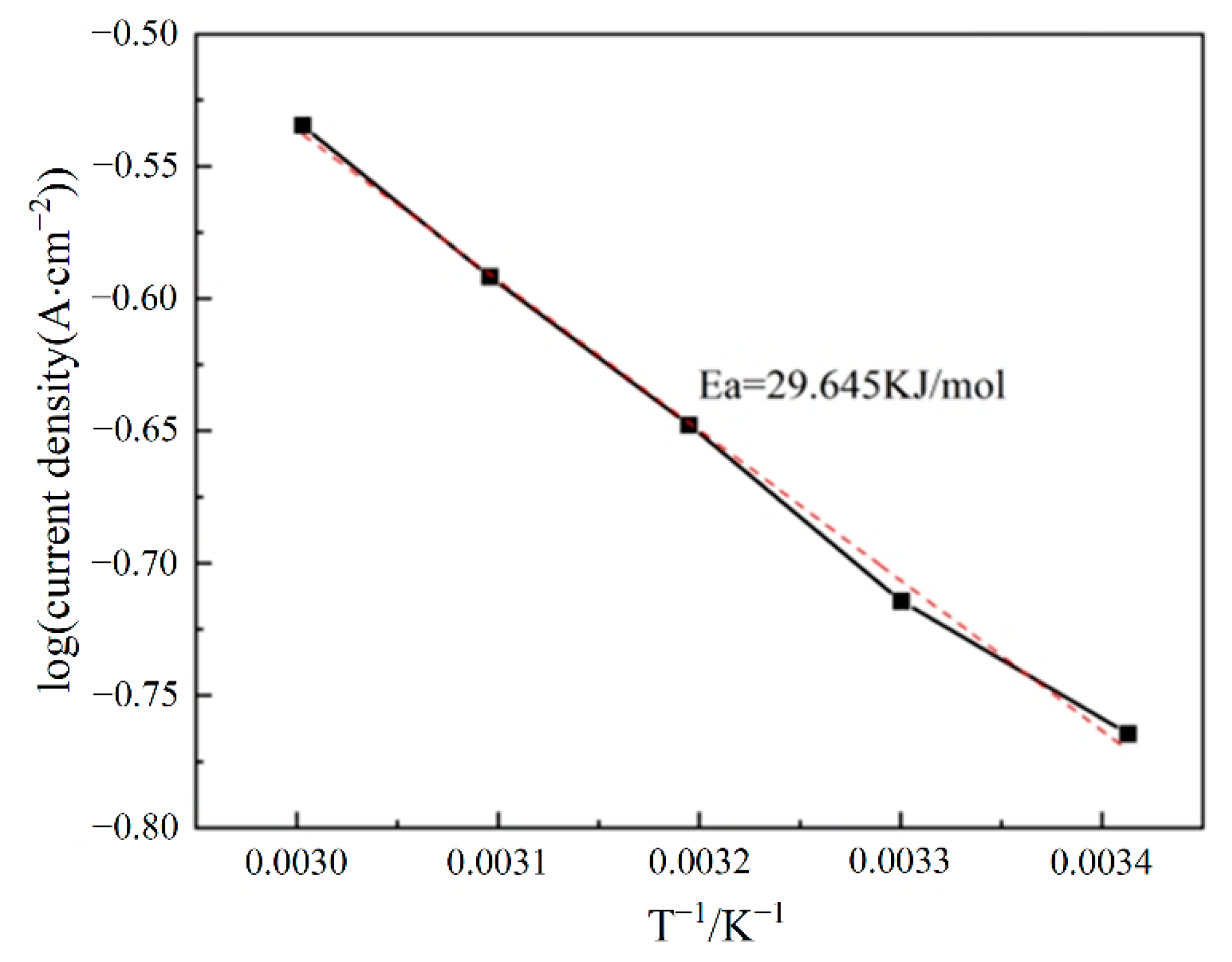
3.3. The Influence of Tested Parameters on HER Performance
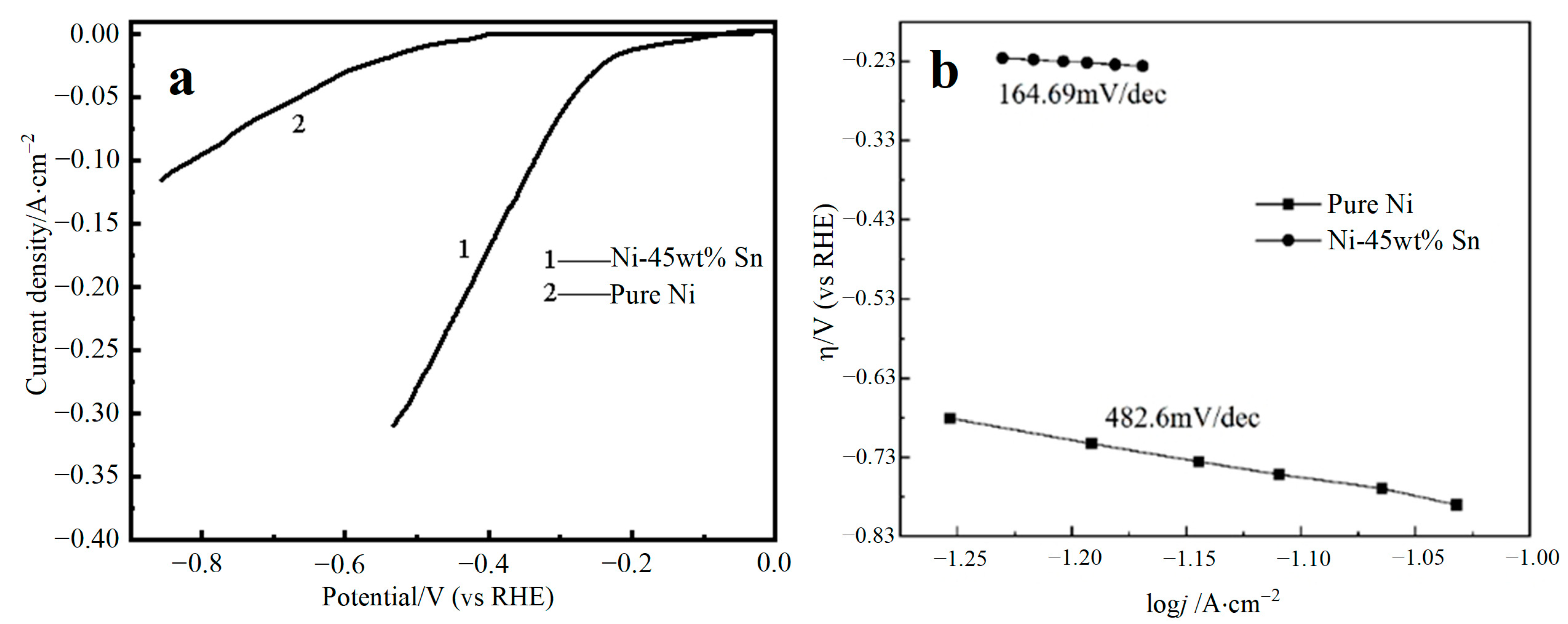
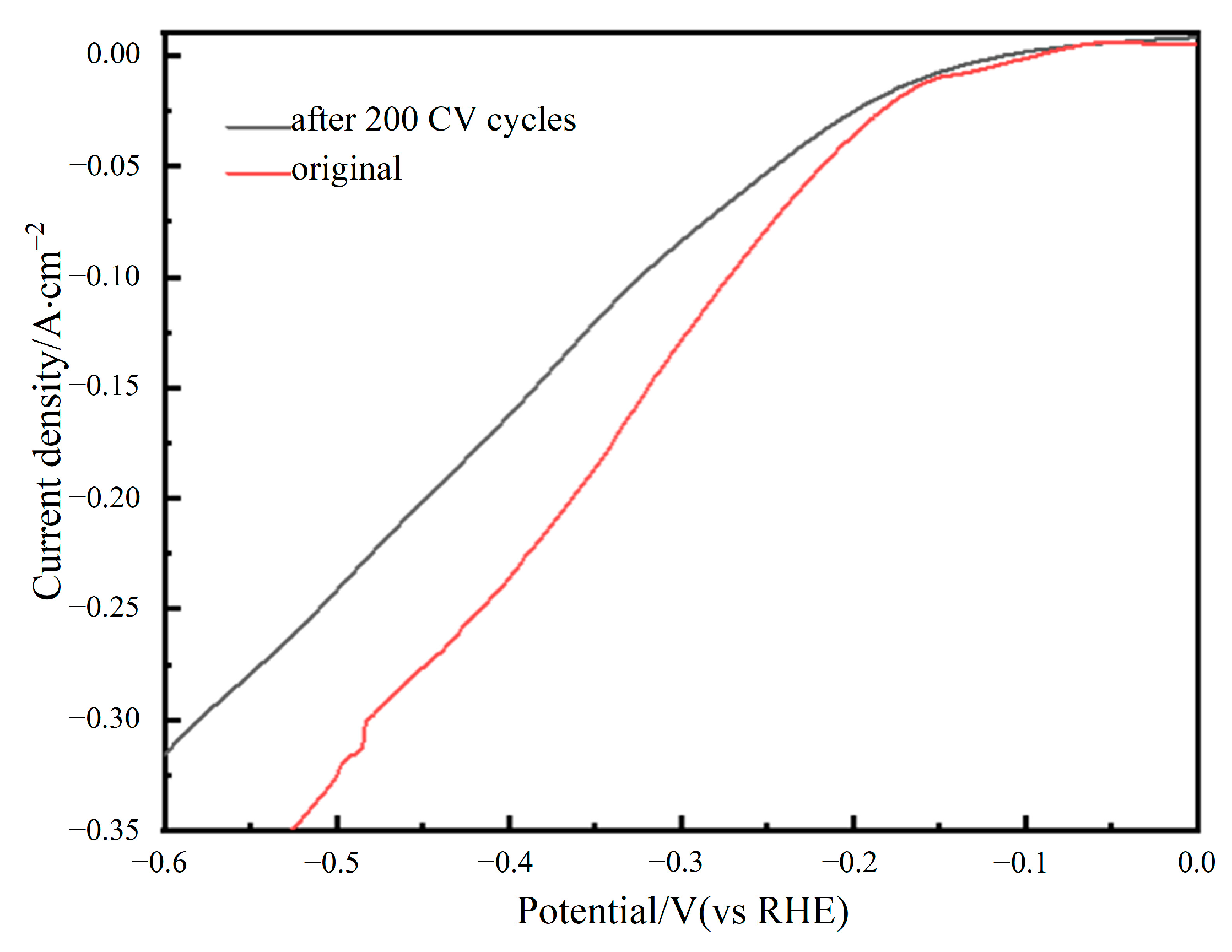
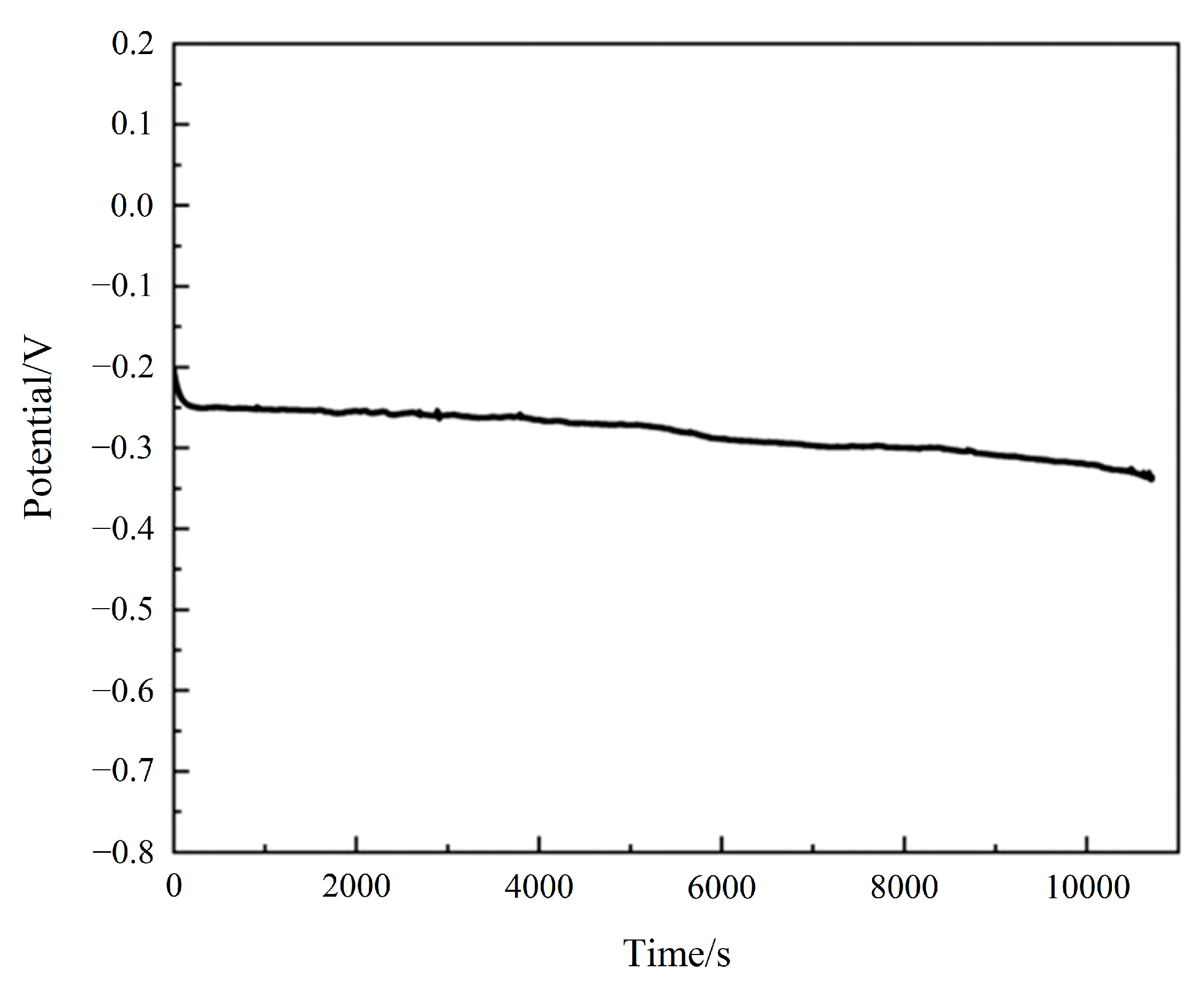
3.4. Electrocatalytic Stability of Electrode Materials
3.5. Reaction Mechanism of HER
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Acar, C.; Dincer, I. The potential role of hydrogen as a sustainable transportation fuel to combat global warming. Int. J. Hydrog. Energy 2020, 45, 3396–3406. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrog. Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Chinchilla, R.; Najera, C. Chemicals from alkynes with palladium catalysts. Chem. Rev. 2014, 114, 1783–1826. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Feng, X.; Hu, Y.; Zou, H.; Zhang, C.; Xiong, L.; Zheng, X.; Liu, Y. Electrochemical performance of porous Ni–Cr–Mo–Cu alloys for hydrogen evolution reactions in alkali solution. Mater. Res. Express 2020, 7, 095505. [Google Scholar] [CrossRef]
- Wijten, J.H.J.; Riemersma, R.L.; Gauthier, J.; Mandemaker, L.D.B.; Verhoeven, M.; Hofmann, J.P.; Chan, K.; Weckhuysen, B.M. Electrolyte Effects on the Stability of Ni-Mo Cathodes for the Hydrogen Evolution Reaction. ChemSusChem 2019, 12, 3491–3500. [Google Scholar] [CrossRef] [Green Version]
- Bau, J.A.; Kozlov, S.M.; Azofra, L.M.; Ould-Chikh, S.; Emwas, A.-H.; Idriss, H.; Cavallo, L.; Takanabe, K. Role of Oxidized Mo Species on the Active Surface of Ni–Mo Electrocatalysts for Hydrogen Evolution under Alkaline Conditions. ACS Catal. 2020, 10, 12858–12866. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.B.; Yu, Y.W.; Hua, Y.X.; Li, Y.; Dong, P. Electrochemical fabrication of porous Ni-Cu alloy nanosheets with high catalytic activity for hydrogen evolution. Electrochim. Acta 2016, 215, 609–616. [Google Scholar] [CrossRef]
- Cardoso, D.S.P.; Eugénio, S.; Silva, T.M.; Santos, D.M.F.; Sequeira, C.A.C.; Montemor, M.F. Hydrogen evolution on nanostructured Ni–Cu foams. RSC Adv. 2015, 5, 43456–43461. [Google Scholar] [CrossRef]
- Jović, V.D.; Lačnjevac, U.; Jović, B.M.; Karanović, L.; Krstajić, N.V. Ni–Sn coatings as cathodes for hydrogen evolution in alkaline solution. Chemical composition, phase composition and morphology effects. Int. J. Hydrog. Energy 2012, 37, 17882–17891. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Song, J.; Wang, W.; Yue, F.; Ma, Q. Microstructure and hydrogen evolution catalytic properties of Ni-Sn alloys prepared by electrodeposition method. Appl. Catal. A Gen. 2015, 500, 51–57. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R.; Tahir, N.; Jamil, A.; Afzal, A. Design of advanced self-supported electrode by surface modification of copper foam with transition metals for efficient hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 33396–33406. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R.; Zahid, M.; Tahir, N.; Afzal, A.; Lin, X.-M. Bimetallic NiCo–NiCoO2 nano-heterostructures embedded on copper foam as a self-supported bifunctional electrode for water oxidation and hydrogen production in alkaline media. Int. J. Hydrog. Energy 2021, 46, 18936–18948. [Google Scholar] [CrossRef]
- Los, P.; Rami, A.; Lasia, A. Hydrogen evolution reaction on Ni-Al electrodes. J. Appl. Electrochem. 1993, 23, 135–140. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Kou, X. Electrodeposited Ni-Co-Sn alloy as a highly efficient electrocatalyst for water splitting. Int. J. Hydrog. Energy 2019, 44, 8099–8108. [Google Scholar] [CrossRef]
- Vijayakumar, J.; Mohan, S.; Anand Kumar, S.; Suseendiran, S.R.; Pavithra, S. Electrodeposition of Ni–Co–Sn alloy from choline chloride-based deep eutectic solvent and characterization as cathode for hydrogen evolution in alkaline solution. Int. J. Hydrog. Energy 2013, 38, 10208–10214. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Wang, Y.; He, Z.; Gu, Z.; You, S. Potentiostatic electrodeposited of Ni–Fe–Sn on Ni foam served as an excellent electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2021, 46, 26930–26939. [Google Scholar] [CrossRef]
- Zhang, H.; Li, F.; Ji, S.; Yang, J.; Zhang, C.; Yang, F.; Lei, L. One-step electrodeposition of cauliflower-like Ni–Fe–Sn particles as a highly-efficient electrocatalyst for the hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 24615–24625. [Google Scholar] [CrossRef]
- Wu, L. Fabrication and Properties of Ni-Based Porous Alloys; Central South University: Changsha, China, 2013. [Google Scholar]
- Yamashita, H.; Yamamura, T.; Yoshimoto, K. The Relation Between Catalytic Ability for Hydrogen Evolution Reaction and Characteristics of Nickel-Tin Alloys. J. Electrochem. Soc. 2019, 140, 2238–2243. [Google Scholar] [CrossRef]
- Li, X.D.; Fan, Y.Q.; Liu, Y.Z.; Liu, J.L.; Yang, J.S.; Zhu, Y.; Li, J.; Liu, B. Effect of Sn Content on the Pore Structures of Porous Ni-Sn Alloys Produced by Reactive Synthesis Sintering Method. J. Mater. Eng. Perform. 2022. [Google Scholar] [CrossRef]
- Blass, P.M.; Zhou, X.L.; White, J.M. Coadsorption and reaction of water and potassium on silver(III). J. Phys. Chem. 2002, 94, 3054–3062. [Google Scholar] [CrossRef]
- Solymosi, F.; Kiss, J.; Kovacs, I. Adsorption and decomposition of formic acid on potassium-promoted rhodium(111) surfaces. J. Phys. Chem. 2002, 92, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Krstajic, N.; Jovic, V.; Gajickrstajic, L.; Jovic, B.; Antozzi, A.; Martelli, G. Electrodeposition of Ni–Mo alloy coatings and their characterization as cathodes for hydrogen evolution in sodium hydroxide solution. Int. J. Hydrog. Energy 2008, 33, 3676–3687. [Google Scholar] [CrossRef]
- Liu, Y. Investigation on The Fabrication and Properties of The Porous Ni-Cr-Fe Materials. Master’s Thesis, Xiangtan University, Xiangtan, China, 2016. [Google Scholar]
- Qu, Y. MO-MOSE2 Core-Shell Nanostructures for Hydrogen Evolution Reaction. Master’s Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2016. [Google Scholar]
- Xia, M.; Lei, T.; Lv, N.; Li, N. Synthesis and electrocatalytic hydrogen evolution performance of Ni–Mo–Cu alloy coating electrode. Int. J. Hydrog. Energy 2014, 39, 4794–4802. [Google Scholar] [CrossRef]
- Giner-Sanz, J.J.; Ortega, E.M.; Pérez-Herranz, V. Hydrogen crossover and internal short-circuit currents experimental characterization and modelling in a proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2014, 39, 13206–13216. [Google Scholar] [CrossRef] [Green Version]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions—A Review. Int. J. Hydrog. Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]



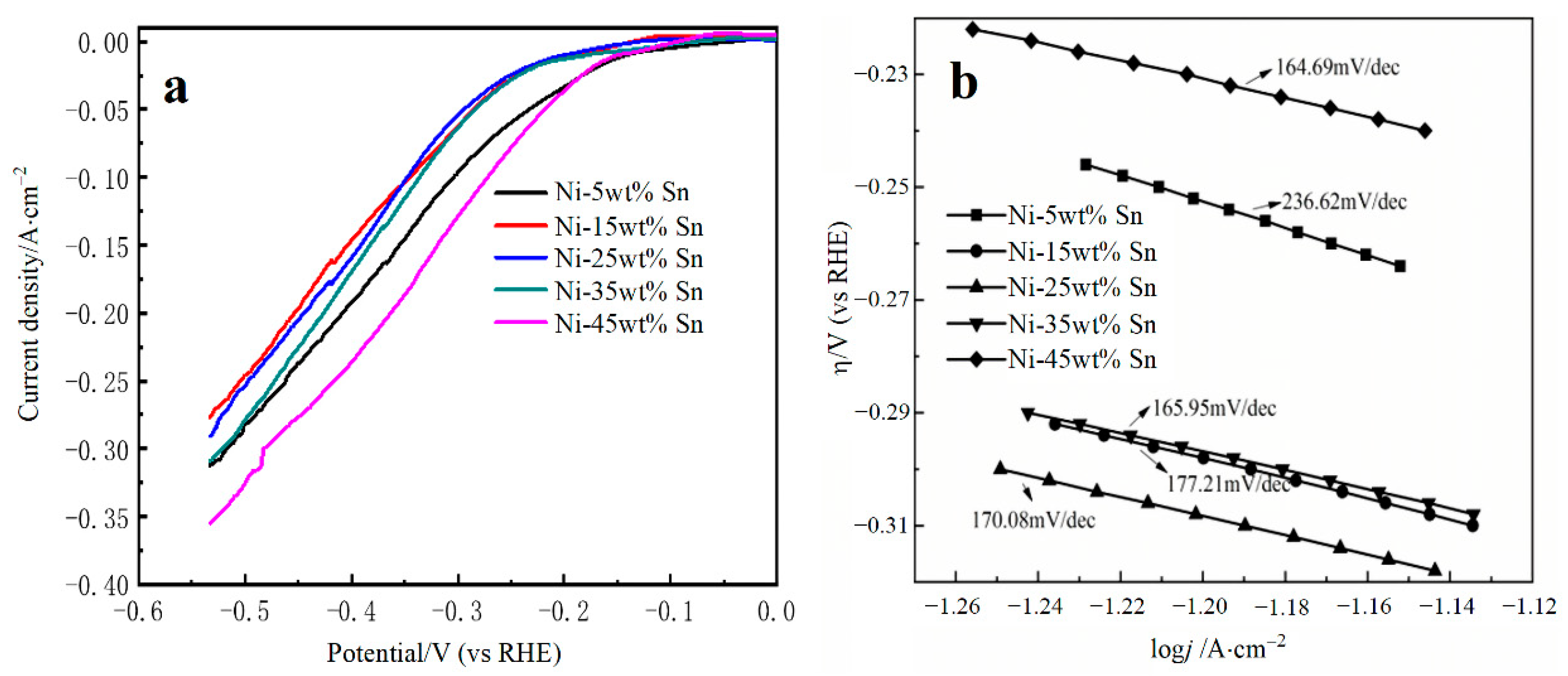
| Sn Content | −b (mV·dec−1) | j0 (A·cm−2) | ECSA (cm2) | j0/ECSA (A·cm−2) | Overpotential@20 mA·cm−2 (V vs. RHE) |
|---|---|---|---|---|---|
| 5% | 236.62 | 0.03165 | 424 | 7.46 × 10−5 | −0.149 |
| 15% | 177.21 | 0.03305 | 345.5 | 9.57 × 10−5 | −0.218 |
| 25% | 170.08 | 0.03293 | 175.5 | 1.88 × 10−4 | −0.218 |
| 35% | 165.95 | 0.03419 | 238.9 | 1.43 × 10−4 | −0.217 |
| 45% | 164.69 | 0.03994 | 494.7 | 8.07 × 10−5 | −0.151 |
| Electrolysts | −b (mV·dec−1) | j0 (A·cm−2) | ECSA (cm2) | Overpotential@20 mA·cm−2 (V vs. RHE) |
|---|---|---|---|---|
| Ni-45 wt% Sn | 164.69 | 0.039 | 494.7 | −0.151 |
| Pure Ni | 482.6 | 0.006 | 30.49 | −0.463 |
| η/V | Rs(Ω/cm2) | Rp(Ω/cm2) | Rct(Ω/cm2) |
|---|---|---|---|
| 0 | 1.24 | 2.832 | 141.9 |
| 100 | 1.068 | 1.191 | 36.43 |
| 200 | 1.157 | 1.456 | 19.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, J.; Wang, Y.; Dong, S.; Fan, Y.; Liu, W.; Kuang, Y.; Tan, S.; Xiao, G.; Wang, B.; et al. Tailoring the Pore Structure of Porous Ni-Sn Alloys for Boosting Hydrogen Evolution Reaction in Alkali Solution. Metals 2022, 12, 2123. https://doi.org/10.3390/met12122123
Yang J, Li J, Wang Y, Dong S, Fan Y, Liu W, Kuang Y, Tan S, Xiao G, Wang B, et al. Tailoring the Pore Structure of Porous Ni-Sn Alloys for Boosting Hydrogen Evolution Reaction in Alkali Solution. Metals. 2022; 12(12):2123. https://doi.org/10.3390/met12122123
Chicago/Turabian StyleYang, Junsheng, Jie Li, Ying Wang, Shijie Dong, Yiquan Fan, Wenkang Liu, Yijian Kuang, Siwei Tan, Gan Xiao, Baogang Wang, and et al. 2022. "Tailoring the Pore Structure of Porous Ni-Sn Alloys for Boosting Hydrogen Evolution Reaction in Alkali Solution" Metals 12, no. 12: 2123. https://doi.org/10.3390/met12122123
APA StyleYang, J., Li, J., Wang, Y., Dong, S., Fan, Y., Liu, W., Kuang, Y., Tan, S., Xiao, G., Wang, B., & Yu, Z. (2022). Tailoring the Pore Structure of Porous Ni-Sn Alloys for Boosting Hydrogen Evolution Reaction in Alkali Solution. Metals, 12(12), 2123. https://doi.org/10.3390/met12122123






