The Effect of Direct Strip Casting on the Kinetics of Phase Transformation of a Dual Phase Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Steel Production
2.2. Heat Treatment Equipment
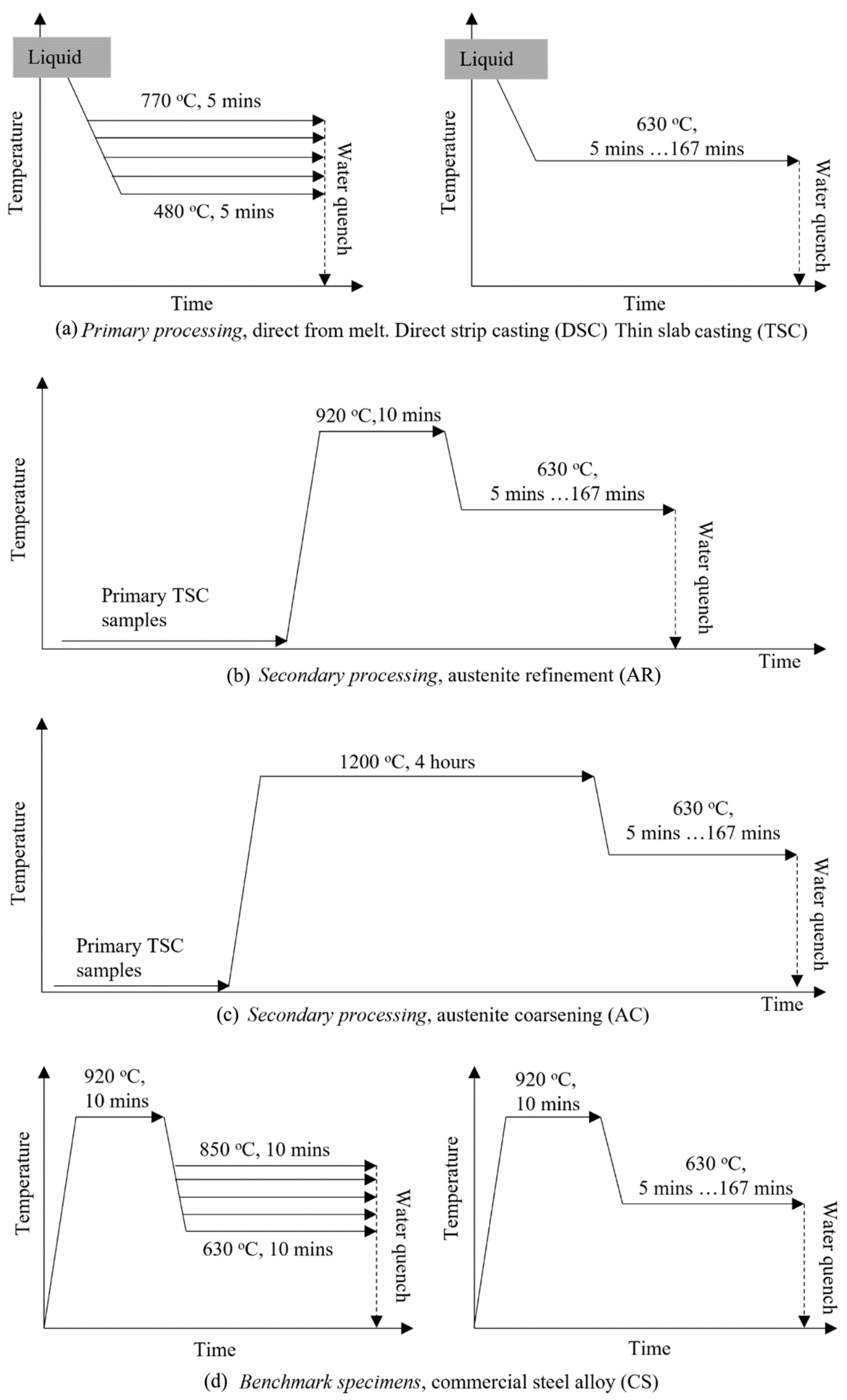
2.3. Heat Treatment Schedules
2.3.1. Primary Processed Specimens
2.3.2. Secondary Processing—Austenite Refinement (AR)
2.3.3. Secondary Processing—Austenite Coarsening (AC)
2.3.4. Benchmark Commercial Steel (CS)
2.4. Microstructural Analysis
3. Results
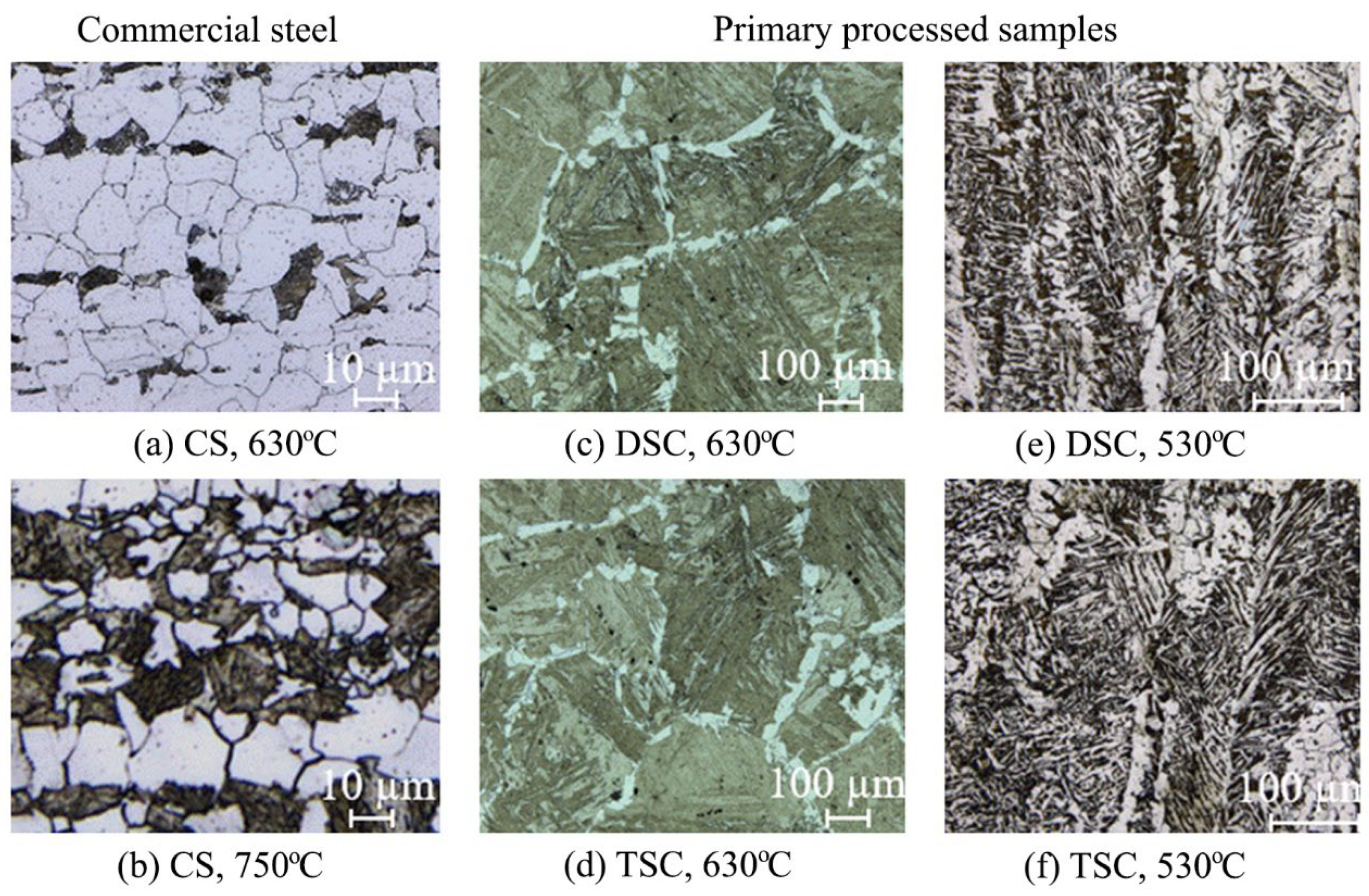
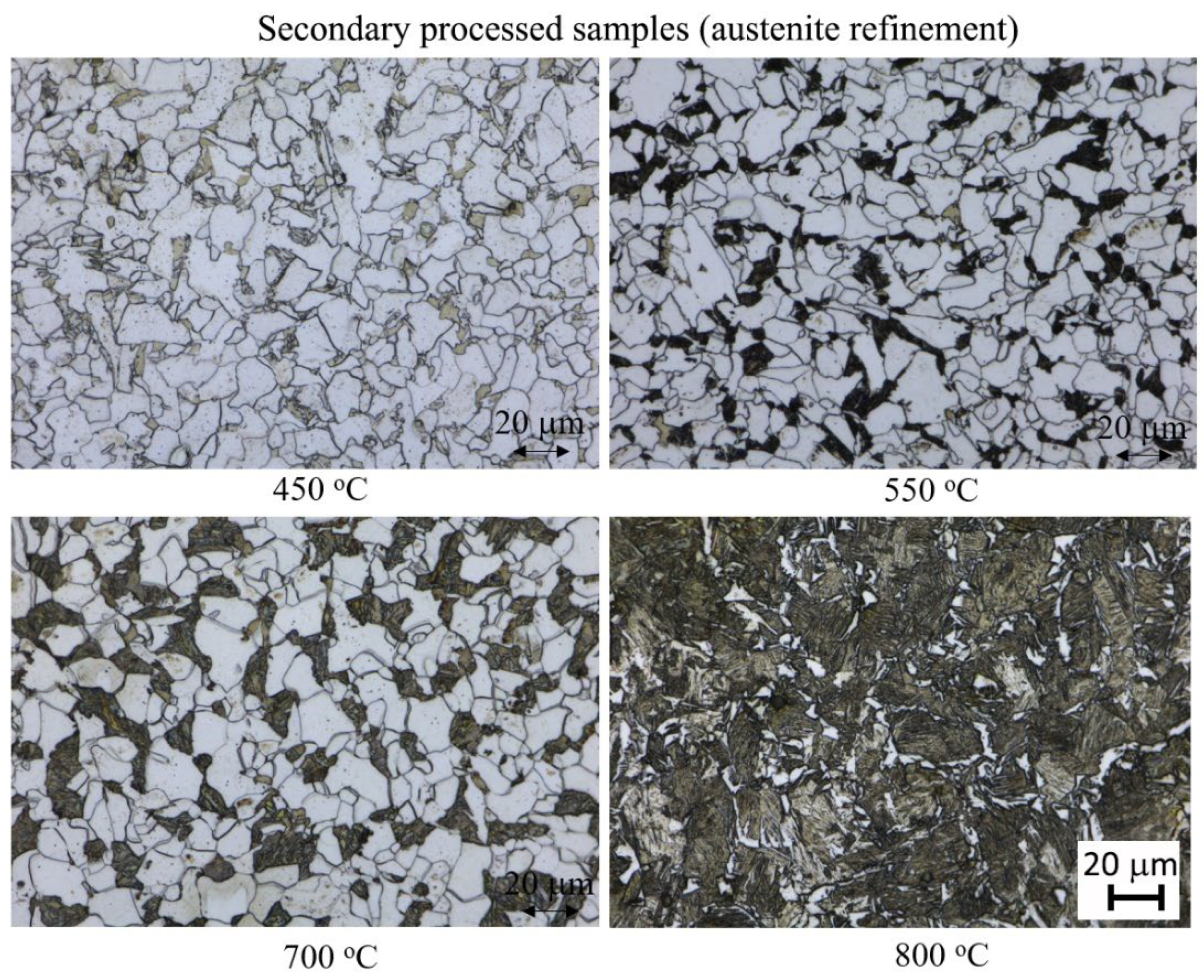
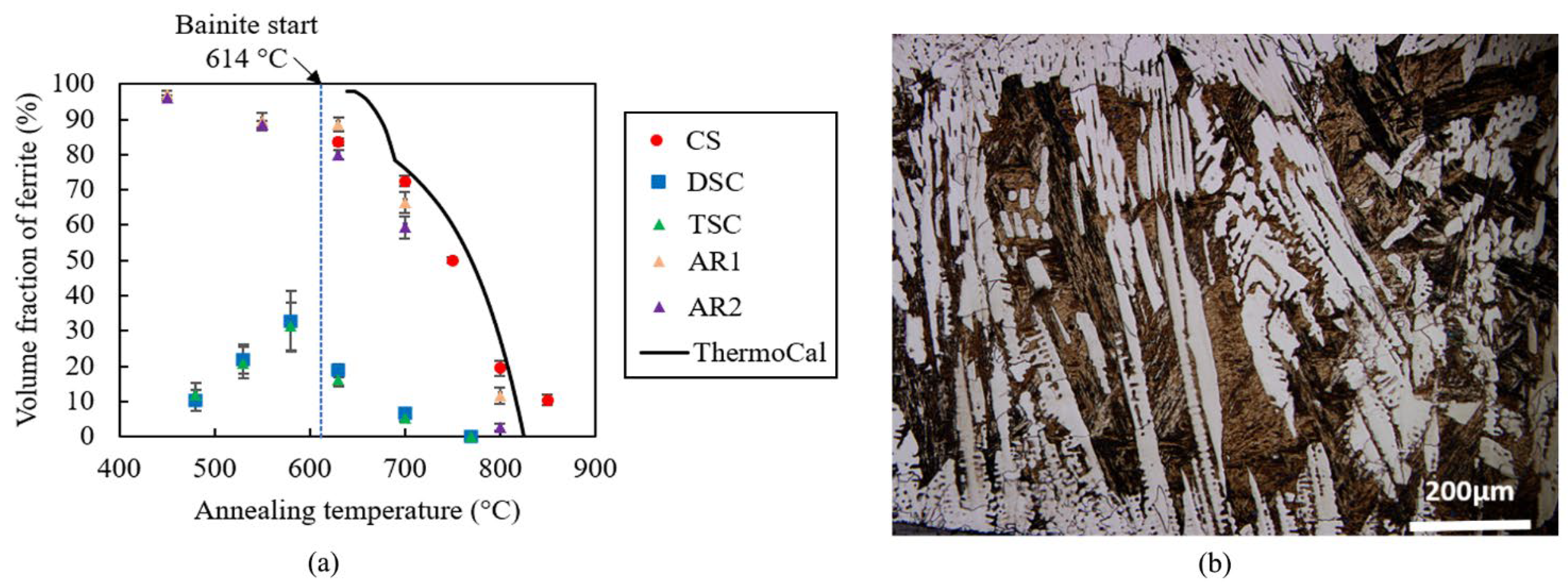
3.1. Microstructural Characterisation
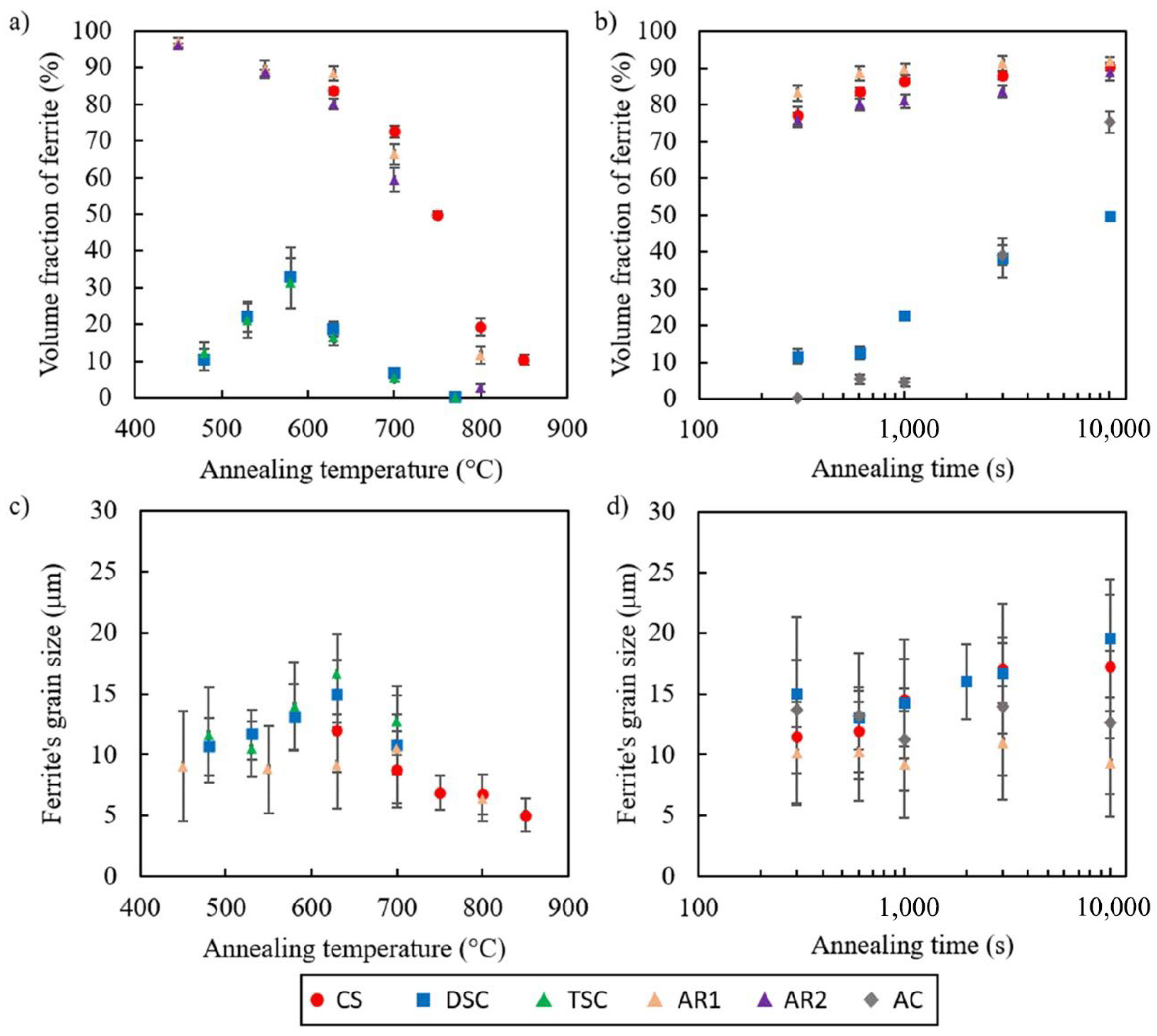
3.2. Kinetics of Transformation during Inter-Critical Annealing
3.3. Electron Microscopy
4. Discussion
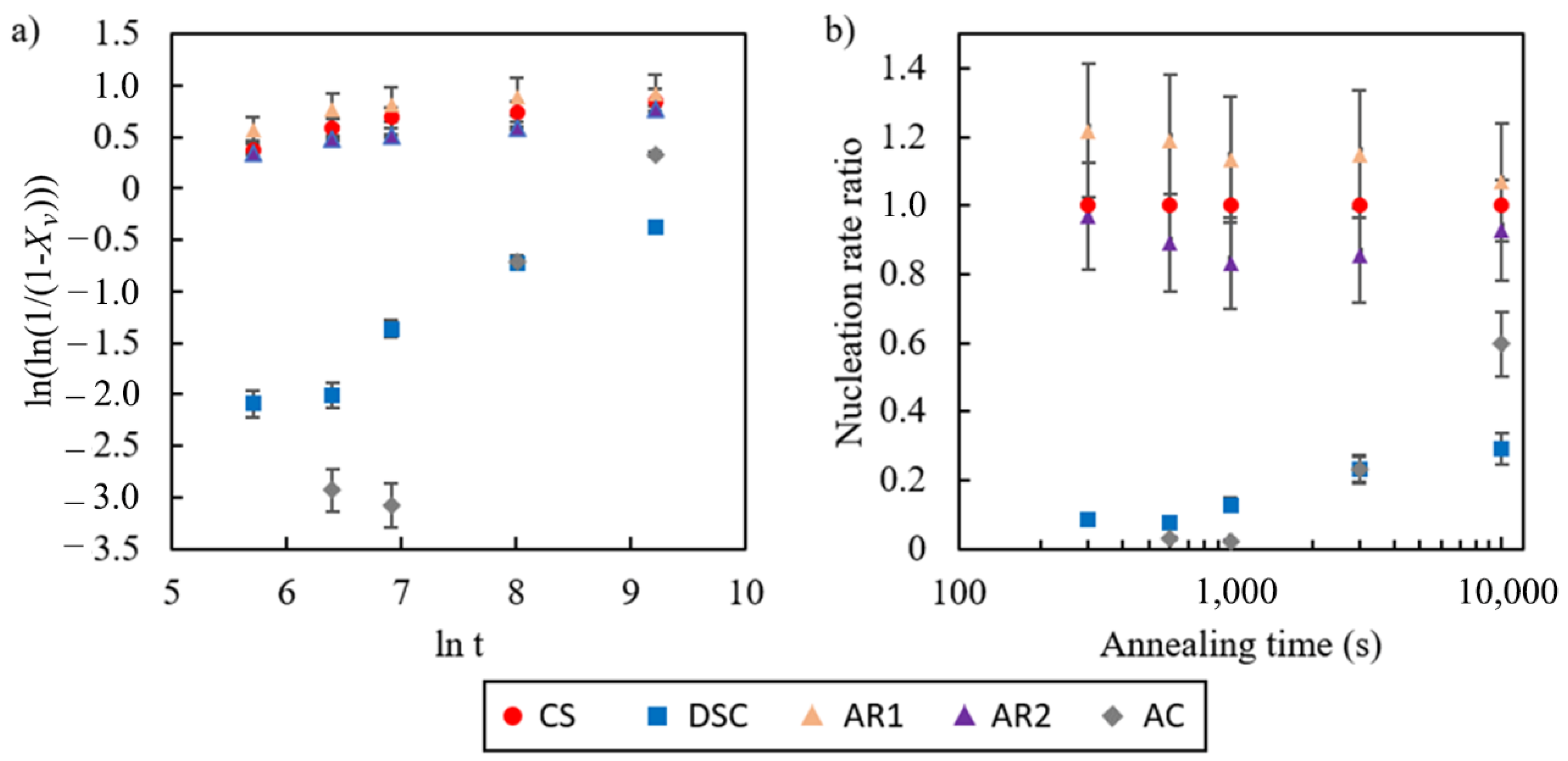
4.1. The Kinetics of Transformation during Isothermal Holding
4.2. Comparision with Literature
4.3. Effect of Nano-Precipitates
4.4. The Kinetics of Transformation during Isochronal Treatment
4.5. Summarizing Discussion
5. Conclusions
- (1)
- There was a significant delay in ferrite formation in the direct strip cast and thin slab cast samples, with the delay resulting in incomplete transformation after extended annealing times of 10,000 s;
- (2)
- The kinetics of phase transformation was quantified using the JMAK model. The growth rate was found to be similar for all specimen examined, but the nucleation rate of strip and thin slab cast steels were significantly slower than commercial steel by about one order of magnitude;
- (3)
- The effect of prior austenite grain size was interrogated by producing specimens with both coarsened and refined prior austenite grain sizes. Results show that the large prior austenite grain is the main factor for slow transformation kinetics in DSC and TSC samples;
- (4)
- Small differences in reaction kinetics between the DSC and austenite coarsened specimens were observed. These were explained by investigation of MnS precipitation in the steels. It is suggested that the transformation rate is high when the solutes are in solution, and that the rate of transformation slows significantly when precipitation occurs. This results in two stages of transformation speed corresponding to the two stages of the precipitation process. In samples annealed for less than 1000 s, DSC samples show a slightly faster transformation because the sulphur (and other elements) are in solid solution. After 1000 s of annealing, nano-precipitation at the grain and phase boundaries begins and the ferrite formation is slowed. The kinetics of MnS precipitation available in literature support this hypothesis.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, S.K. Effect of martensite volume fraction on stress triaxiality and deformation behavior of dual phase steel. Mater. Des. 2013, 50, 782–789. [Google Scholar] [CrossRef]
- Bag, A.; Ray, K.K.; Dwarakadasa, E.S. Influence of martensite content and morphology on tensile and impact properties of high-martensite dual-phase steels. Metall. Mater. Trans. A 1999, 30, 1193–1202. [Google Scholar] [CrossRef]
- Zhang, J.; Di, H.; Deng, Y.; Misra, R.D.K. Effect of martensite morphology and volume fraction on strain hardening and fracture behavior of martensite–ferrite dual phase steel. Mater. Sci. Eng. A 2015, 627, 230–240. [Google Scholar] [CrossRef]
- Pierman, A.-P.; Bouaziz, O.; Pardoen, T.; Jacques, P.; Brassart, L. The influence of microstructure and composition on the plastic behaviour of dual-phase steels. Acta Mater. 2014, 73, 298–311. [Google Scholar] [CrossRef]
- Calcagnotto, M.; Adachi, Y.; Ponge, D.; Raabe, D. Deformation and fracture mechanisms in fine-and ultrafine-grained ferrite/martensite dual-phase steels and the effect of aging. Acta Mater. 2011, 59, 658–670. [Google Scholar] [CrossRef]
- Ferry, M. Direct Strip Casting of Metals and Steels; Woodhead Publishing: Sawston, UK, 2006. [Google Scholar]
- Xiong, Z.; Saleh, A.; Kostryzhev, A.; Pereloma, E. Strain-induced ferrite formation and its effect on mechanical properties of a dual phase steel produced using laboratory simulated strip casting. J. Steels Compd. 2017, 721, 291–306. [Google Scholar] [CrossRef]
- Stanford, N.; Phelan, D. The formation of randomly textured magnesium steel sheet through rapid solidification. Acta Mater. 2010, 58, 3642–3654. [Google Scholar] [CrossRef]
- Ramajayam, M.; Stanford, N. Static recrystallisation of steels produced by direct strip casting–The effect of carbon and vanadium concentration. Mater. Sci. Eng. A 2016, 671, 147–157. [Google Scholar] [CrossRef]
- Xiong, Z.; Kostryzhev, A.; Stanford, N.; Pereloma, E. Microstructures and mechanical properties of dual phase steel produced by laboratory simulated strip casting. Mater. Des. 2015, 88, 537–549. [Google Scholar] [CrossRef]
- Ashrafi, H.; Shamanian, M.; Emadi, R.; Saeidi, N. Examination of phase transformation kinetics during step quenching of dual phase steels. Mater. Chem. Phys. 2017, 187, 203–217. [Google Scholar] [CrossRef]
- Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Phase transformation mechanism and kinetics during step quenching of st37 low carbon steel. Mater. Res. Express 2019, 6, 1165f2. [Google Scholar] [CrossRef]
- Jia, T.; Militzer, M. General method of phase transformation modeling in advanced high strength steels. ISIJ Int. 2010, 50, 583–590. [Google Scholar] [CrossRef]
- Lusk, M.; Jou, H.-J. On the rule of additivity in phase transformation kinetics. Metall. Mater. Trans. A 1997, 28, 287–291. [Google Scholar] [CrossRef]
- Xiong, Z.; Kostryzhev, A.; Stanford, N.; Pereloma, E.V. Effect of Holding Temperature and Time on Ferrite Formation in Dual Phase Steel Produced by Strip Casting. Mater. Forum 2014, 38, 44–48. [Google Scholar]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena, 1st ed.; Elsevier Science: Oxford, UK, 1995; pp. 281–325. [Google Scholar]
- Underwood, E.E. Quantitative Stereology for Microstructural Analysis. In Microstructural Analysis: Tools and Techniques; McCall, J.L., Mueller, W.M., Eds.; Springer US: Boston, MA, USA, 1973; pp. 35–66. [Google Scholar]
- Lückl, M.; Wojcik, T.; Povoden-Karadeniz, E.; Zamberger, S.; Kozeschnik, E. Co-Precipitation Behavior of MnS and AlN in a Low-Carbon Steel. Steel Res. Int. 2018, 89, 1700342. [Google Scholar] [CrossRef]
- Lückl, M.; Zamberger, S.; Kozeschnik, E. Kinetics simulation of MnS precipitation in electrical steel. Steel Res. Int. 2016, 87, 271–275. [Google Scholar] [CrossRef]
- Liu, Z.; Kobayashi, Y.; Nagai, K.; Yang, J.; Kuwabara, M. Morphology control of copper sulfide in strip casting of low carbon steel. ISIJ Int. 2006, 46, 744–753. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Kobayashi, Y.; Nagai, K. Crystallography and precipitation kinetics of copper sulfide in strip casting low carbon steel. ISIJ Int. 2004, 44, 1560–1567. [Google Scholar] [CrossRef]
- Frawley, L.; Priestner, R. The effects of Ti and S in Direct rolled Thin Slab cast low carbon steel. Mater. Sci. Forum 1998, 284–286, 485–492. [Google Scholar] [CrossRef]
- Badji, R.; Bouabdallah, M.; Bacroix, B.; Kahloun, C.; Bettahar, K.; Kherrouba, N. Effect of solution treatment temperature on the precipitation kinetic of σ-phase in 2205 duplex stainless steel welds. Mater. Sci. Eng. A 2008, 496, 447–454. [Google Scholar] [CrossRef]
- Dorin, T.; Wood, K.; Taylor, A.; Hodgson, P.; Stanford, N. Effect of coiling treatment on microstructural development and precipitate strengthening of a strip cast steel. Acta Mater. 2016, 115, 167–177. [Google Scholar] [CrossRef]
- Sun, W.; Militzer, M.; Jonas, J. Strain-induced nucleation of MnS in electrical steels. Metall. Mater. Trans. A 1992, 23, 821–830. [Google Scholar] [CrossRef]
- Sun, W.P. Measurement and Analysis of MnS Precipitation in Electrical Steels. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1991. [Google Scholar]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]






| C | Si | Mn | Cr | Al | N | P | S |
|---|---|---|---|---|---|---|---|
| 0.13 | 0.87 | 1.98 | 0.035 | 0.014 | 0.014 | 0.005 | <0.0005 |
| Specimen/Study | PAGS (µm) | GBA (μm2/μm3) | n | k |
|---|---|---|---|---|
| Commercial Steel benchmark | 30 | 0.07 | 0.12 | 0.80 |
| DSC | 300 | 0.007 | 0.53 | 0.005 |
| Secondary processed (austenite refined) | 20 | 0.1 | 0.09 | 1.18 |
| Secondary Processed (austenite coarsened) | 200 | 0.01 | 1.3 | 9.8 × 10−6 |
| Ashrafi et al. [11] | 15 | 0.13 | 0.68 | 0.47 |
| Soleimani et al. [12] | 40 | 0.05 | 0.2 | 0.58 |
| Xiong et al. [15] | 100 | 0.02 | 0.7 | 0.07 |
| Xiong et al. [10] | 120 | 0.017 | 1.8 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, N.; Schulz, C.; Stanford, N. The Effect of Direct Strip Casting on the Kinetics of Phase Transformation of a Dual Phase Steel. Metals 2022, 12, 170. https://doi.org/10.3390/met12020170
Mai N, Schulz C, Stanford N. The Effect of Direct Strip Casting on the Kinetics of Phase Transformation of a Dual Phase Steel. Metals. 2022; 12(2):170. https://doi.org/10.3390/met12020170
Chicago/Turabian StyleMai, Nam, Christiane Schulz, and Nikki Stanford. 2022. "The Effect of Direct Strip Casting on the Kinetics of Phase Transformation of a Dual Phase Steel" Metals 12, no. 2: 170. https://doi.org/10.3390/met12020170
APA StyleMai, N., Schulz, C., & Stanford, N. (2022). The Effect of Direct Strip Casting on the Kinetics of Phase Transformation of a Dual Phase Steel. Metals, 12(2), 170. https://doi.org/10.3390/met12020170








