Corrosion Monitoring in Atmospheric Conditions: A Review
Abstract
:1. Introduction
2. Corrosion Coupons
3. Real-Time Corrosion Monitoring
3.1. Galvanic Corrosion Sensor, Atmospheric Corrosion Monitor
3.2. Electrochemical Impedance Spectroscopy
3.3. Electrochemical Noise
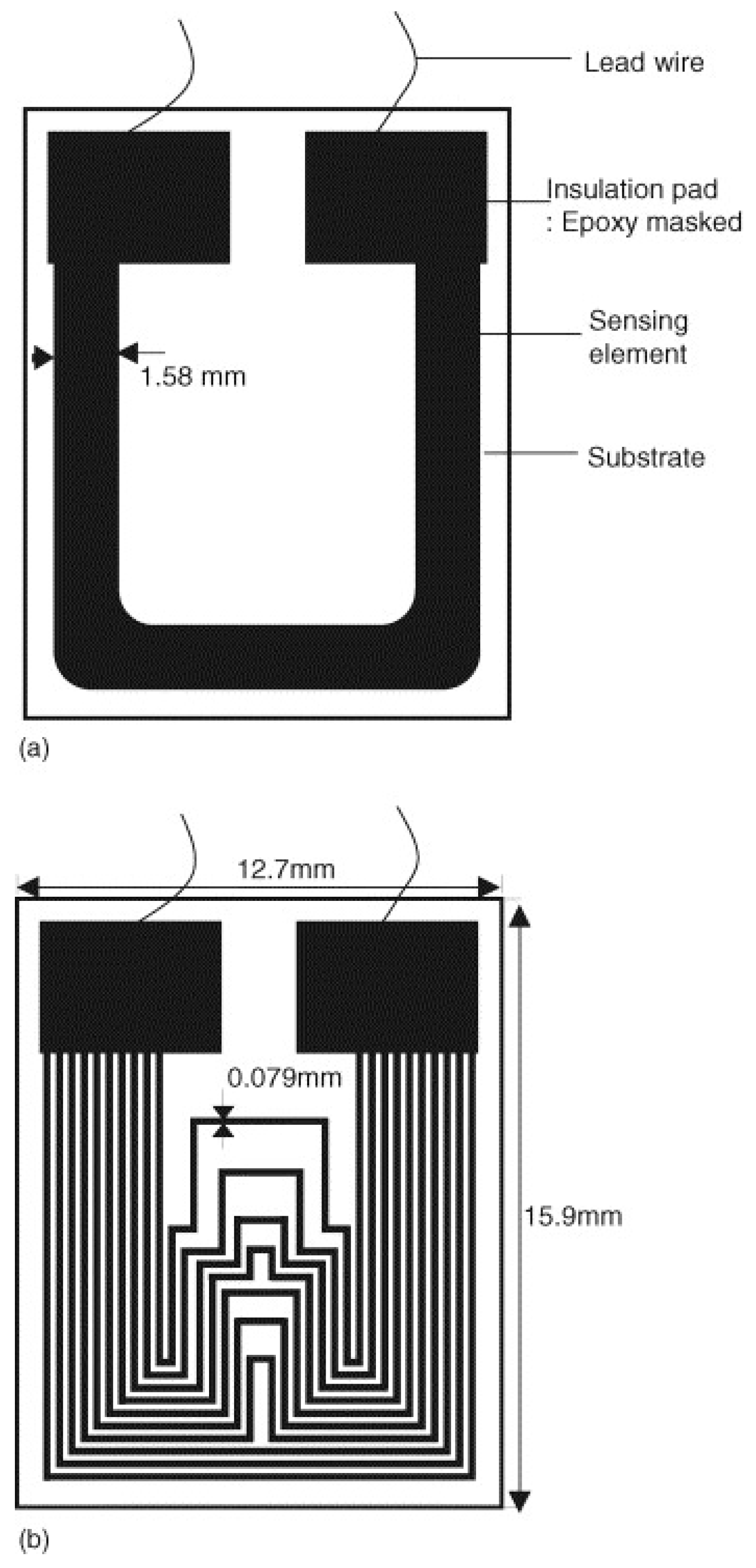
3.4. Electrical Resistance Probes
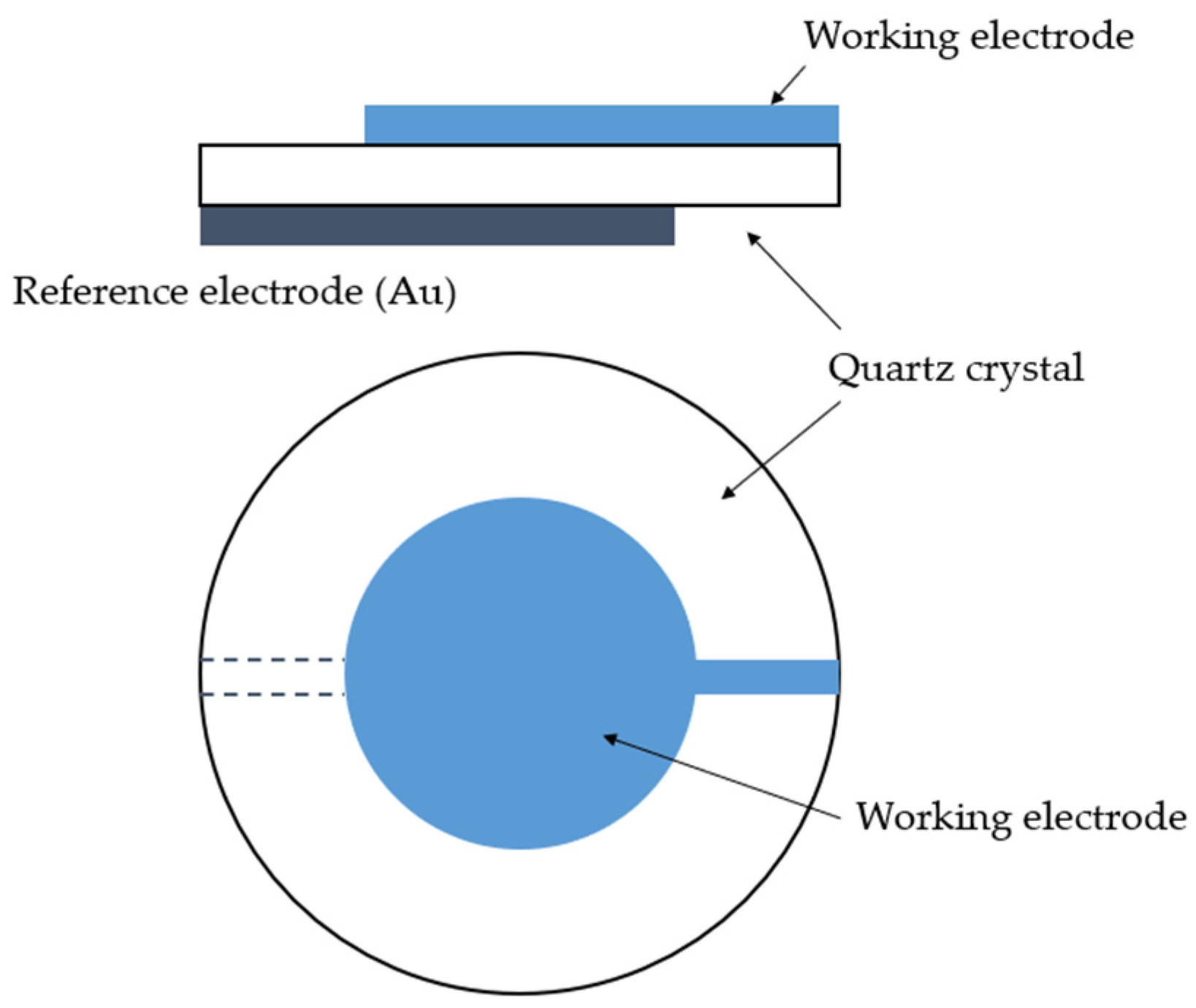
3.5. Quartz Crystal Microbalance
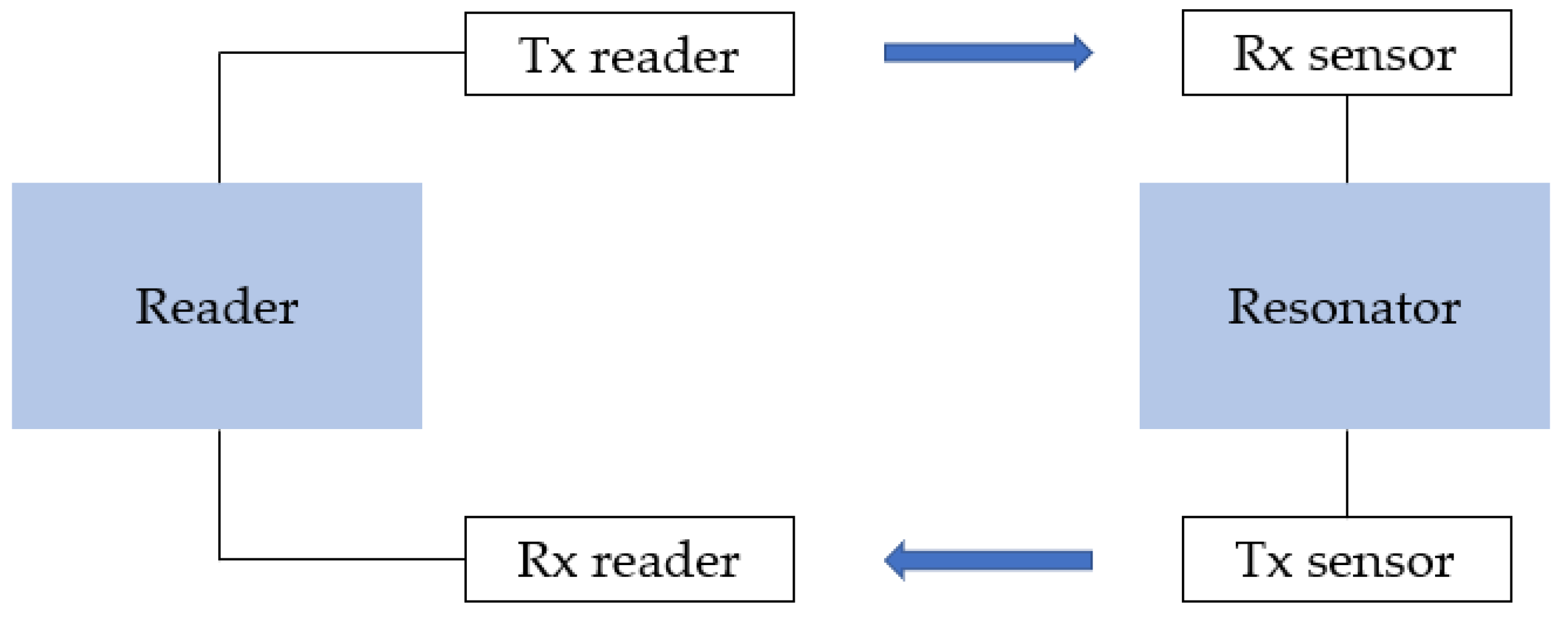
3.6. Radio-Frequency Identification Sensors
3.7. Fibre Optical Corrosion Sensors
3.8. Respirometry
4. Comparison of Atmospheric Corrosion Monitoring Techniques
5. Conclusions
- Electrochemical EIS, EN and ACM methods can be recommended for the use under outdoor conditions and in laboratory tests at higher RH when stable electric connection between electrodes in ensured.
- QCM is a powerful technique for extremely low corrosion rate detection in indoor environments.
- The ER technique is the most universal corrosion monitoring tool, which can be applied both in high and weakly corrosive environments, depending on the sensor’s thickness.
- Further development of the state-of-the art RFID, FOCS and respirometric techniques in the field of atmospheric corrosion is expected. At the current stage, it is too early to evaluate their application potential.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACM | Atmospheric corrosion monitor |
| ACT | Accelerated corrosion test |
| AMPP | Association for Material Protection and Performance |
| CD | Corrosion depth |
| CE | Counter electrode |
| CPE | Constant phase element |
| DEFC | Dynamic electrolyte film corrosion |
| ECM | Environmental condition monitoring |
| ECN | Electrochemical current noise |
| EIS | Electrochemical impedance spectroscopy |
| EN | Electrochemical noise |
| EPN | Electrochemical potential noise |
| ER | Electrical resistance |
| FBG | Fiber Bragg grating |
| FOCS | Fibre optic corrosion sensor |
| HER | H2 evolution reaction |
| HF | High frequency |
| HFSS | High Frequency Structural Simulation |
| IMPACT | International Measures of Prevention, Application, and Economics of Corrosion Technologies |
| LF | Low frequency |
| LPFG | Long-period fibre grating |
| LPR | Linear polarization resistance |
| MIC | Maximal information coefficient |
| ORR | O2 oxidation reaction |
| PC | Personal computer |
| PCB | Printed circuit board |
| PDMS | Polydimethyl siloxane |
| QCM | Quartz crystal microbalance |
| QCM-D | Quartz crystal microbalance with dissipation monitoring |
| RE | Reference electrode |
| RFID | Radio-frequency identification sensors |
| RH | Relative humidity |
| SEM | Scanning electron microscope |
| T | Temperature |
| TREF | Thin film electrical resistance sensor |
| TML | Transmission-line model |
| TOW | Time of wetness |
| UHF | Ultrahigh frequency |
| VNA | Vector network analysis |
| WE | Working electrode |
| ZRA | Zero resistance ammeter |
References
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Prosek, T.; Kouril, M.; Hilbert, L.R.; Degres, Y.; Blazek, V.; Thierry, D.; Hansen, M. Real time corrosion monitoring in atmosphere using automated battery driven corrosion loggers. Corros. Eng. Sci. Technol. 2008, 43, 129–133. [Google Scholar] [CrossRef]
- Li, S.; Kim, Y.G.; Jung, S.; Song, H.S.; Lee, S.M. Application of steel thin film electrical resistance sensor for in situ corrosion monitoring. Sens. Actuators B Chem. 2007, 120, 368–377. [Google Scholar] [CrossRef]
- Prošek, T.; Kouřil, M.; Dubus, M.; Taube, M.; Hubert, V.; Scheffel, B.; Degres, Y.; Jouannic, M.; Thierry, D. Real-Time monitoring of indoor air corrosivity in cultural heritage institutions with metallic electrical resistance sensors. Stud. Conserv. 2013, 58, 117–128. [Google Scholar] [CrossRef]
- EN ISO 8407. Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens; EN ISO: Genève, Switzerland, 2009; p. 8407. [Google Scholar]
- Groysman, A. Corrosion Monitoring. Corros. Rev. 2009, 27, 205–343. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- ISO 11844-2:2020. Corrosion of Metals and Alloys—Classification of Low Corrosivity of Indoor Atmospheres—Part 2: Determination of Corrosion Attack in Indoor Atmospheres. ISO: Genève, Switzerland, 2020.
- ISO 9226:2012. Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Determination of Corrosion Rate of Standard Specimens for the Evaluation of Corrosivity. ISO: Genève, Switzerland, 2012.
- ISO 11844-1:2006. Corrosion of Metals and Alloys—Classification of Low Corrosivity of Indoor Atmospheres—Part 1: Determination and Estimation of Indoor Corrosivity. ISO: Genève, Switzerland, 2006.
- Yang, L. Techniques for Corrosion Monitoring; Southwest Research Institute: Boca Raton, FL, USA, 2008. [Google Scholar]
- Schindelholz, E.; Kelly, R.G. Wetting phenomena and time of wetness in atmospheric corrosion: A review. Corros. Rev. 2012, 30, 135–170. [Google Scholar] [CrossRef]
- Sereda, P.J. Atmospheric Factors Affecting the Corrosion of Steel. Ind. Eng. Chem. 1960, 52, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Sereda, P.J.; Croll, S.; Slade, H. Measurement of the time-of-wetness by moisture sensors and their calibration. In Atmospheric Corrosion of Metals; ASTM International: West Conshohocken, PA, USA, 1982. [Google Scholar]
- Shinohara, T.; Motoda, S.; Oshikawa, W. Evaluation of corrosivity in atmospheric environment by ACM (Atmospheric Corrosion Monitor) type corrosion sensor. In Proceedings of the Pricm 5: The Fifth Pacific Rim International Conference on Advanced Materials and Processing, Pts 1–5, Beijing, China, 2–5 November 2004; Volume 475–479, pp. 61–64. [Google Scholar] [CrossRef]
- Motoda, S.-I.; Suzuki, Y.; Shinohara, T.; Kojima, Y.; Tsujikawa, S.; Oshikawa, W.; Itomura, S.; Fukushima, T.; Izumo, S. ACM (Atmospheric Corrosion Monitor) type corrosion sensor to evaluate corrosivity of marine atmosphere. Zair. Kankyo 1994, 43, 550–556. [Google Scholar] [CrossRef]
- Aiello, L.; Agarwala, V. Galvanic sensor for monitoring structural damage. Corros. Rev. 2007, 25, 39–50. [Google Scholar] [CrossRef]
- To, D.; Shinohara, T.; Umezawa, O. Experimental Investigation on the Corrosivity of Atmosphere through the Atmospheric Corrosion Monitoring (ACM) Sensors. Atmos. Mar. Corros. 2017, 75, 1–10. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jeong, Y.S.; Kim, I.T.; Jeon, S.H.; Park, C.H. A Method for Estimating Time-Dependent Corrosion Depth of Carbon and Weathering Steel Using an Atmospheric Corrosion Monitor Sensor. Sensors 2019, 19, 1416. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, D.; Suzuki, S.; Fujita, S.; Hara, N. Corrosion monitoring and materials selection for automotive environments by using Atmospheric Corrosion Monitor (ACM) sensor. Corros. Sci. 2014, 83, 217–225. [Google Scholar] [CrossRef]
- Zibo Pei, X.C.; Xiaojia, Y.; Qing, L.; Chenhan, X.; Dawei, Z.; Xiaogang, L. Understanding environmental impacts on initial atmospheric corrosion based on corrosion monitoring sensors. J. Mater. Sci. Technol. 2020, 64, 214–221. [Google Scholar] [CrossRef]
- Pei, Z.B.; Zhang, D.W.; Zhi, Y.J.; Yang, T.; Jin, L.L.; Fu, D.M.; Cheng, X.Q.; Terryn, H.A.; Mol, J.M.C.; Li, X.G. Towards understanding and prediction of atmospheric corrosion of an Fe/Cu corrosion sensor via machine learning. Corros. Sci. 2020, 170, 108697. [Google Scholar] [CrossRef]
- Fuse, N.; Naganuma, A.; Fukuchi, T.; Tani, J.; Hori, Y. Methodology to Improve Corrosion Rate Estimation Based on Atmospheric Corrosion Monitoring Sensors. Corrosion 2017, 73, 199–209. [Google Scholar] [CrossRef]
- Kainuma, S.; Yamamoto, Y.; Itoh, Y.; Oshikawa, W. Prediction method for mean corrosion depth of uncoated carbon steel plate subjected to rainfall effect using Fe/Ag galvanic couple ACM-Type corrosion sensor. Zair. Kankyo Corros. Eng. 2011, 60, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Kainuma, S.; Sugitani, K.; Itoh, Y.; Kim, I.T. Evaluation Method for Time-dependent Corrosion Behavior of Carbon Steel Plate using Atmospheric Corrosion Monitoring Sensor. Adv. Fract. Damage Mech. Viii 2010, 417, 417–420. [Google Scholar] [CrossRef]
- Cao, X.L.; Xiao, Y.D.; Deng, H.D.; Cao, P.J.; Jia, B. Evaluation of Atmospheric Corrosivity by ACM Technique. Proc. Mater. Sci. Forum 2009, 610–613, 3–8. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.; Behnamian, Y.; Gao, Z.; Wu, Z.; Qin, Z.; Xia, D.-H. Measuring atmospheric corrosion with electrochemical noise: A review of contemporary methods. Measurement 2019, 138, 54–79. [Google Scholar] [CrossRef]
- Diler, E.; Peltier, F.; Becker, J.; Thierry, D. Real-time corrosion monitoring of aluminium alloys under chloride-contaminated atmospheric conditions. Mater. Corros. 2021, 72, 1377–1387. [Google Scholar] [CrossRef]
- Huang, Y.L.; Yang, D.; Xu, Y.; Lu, D.Z.; Yang, L.H.; Wang, X.T. Field Study of Weather Conditions Affecting Atmospheric Corrosion by an Automobile-Carried Atmospheric Corrosion Monitor Sensor. J. Mater. Eng. Perform. 2020, 29, 5840–5853. [Google Scholar] [CrossRef]
- Dara, T.; Shinohara, T.; Umezawa, O. Effects of Anion on the Corrosion Behaviors of Carbon Steel under Artificial Rainfall. In Proceedings of the 9th Pacific Rim International Conference on Advanced Materials and Processing (PRICM9), Kyoto, Japan, 1–8 August 2016; The Japan Institute of Metals and Materials: Sendai, Japan, 2016. [Google Scholar]
- Shi, Y.N.; Fu, D.M.; Zhou, X.Y.; Yang, T.; Zhi, Y.J.; Pei, Z.B.; Zhang, D.W.; Shao, L.Z. Data mining to online galvanic current of zinc/copper Internet atmospheric corrosion monitor. Corros. Sci. 2018, 133, 443–450. [Google Scholar] [CrossRef]
- Goidanich, S.; Gulotta, D.; Brambilla, L.; Beltrami, R.; Fermo, P.; Toniolo, L. Setup of Galvanic Sensors for the Monitoring of Gilded Bronzes. Sensors 2014, 14, 7066–7083. [Google Scholar] [CrossRef] [Green Version]
- Atmospheric Corrosion Monitor. Available online: https://english.uedaindustry.co.jp/product/atmospheric/ (accessed on 21 December 2021).
- Epelboin, I.; Gabrielli, C.; Keddam, M.; Takenouti, H. Alternating-current impedance measurements applied to corrosion studies and corrosion-rate determination. In Electrochemical Corrosion Testing; ASTM International: West Conshohocken, PA, USA, 1981. [Google Scholar]
- Dillmann, P.; Watkinson, D.; Angelini, E.; Adriaens, A. Corrosion and Conservation of Cultural Heritage Metallic Artefacts; Woodhead Publishing: Philadelphia, PA, USA, 2013. [Google Scholar]
- Xia, D.H.; Song, S.Z.; Qin, Z.B.; Hu, W.B.; Behnamian, Y. Review-Electrochemical Probes and Sensors Designed for Time-Dependent Atmospheric Corrosion Monitoring: Fundamentals, Progress, and Challenges. J. Electrochem. Soc. 2019, 167, 037513. [Google Scholar] [CrossRef]
- Fu, X.X.; Dong, J.H.; Han, E.H.; Ke, W. A New Experimental Method for in Situ Corrosion Monitoring Under Alternate Wet-Dry Conditions. Sensors 2009, 9, 10400–10410. [Google Scholar] [CrossRef] [PubMed]
- Grassini, S.; Corbellini, S.; Parvis, M.; Angelini, E.; Zucchi, F. A simple Arduino-based EIS system for in situ corrosion monitoring of metallic works of art. Measurement 2018, 114, 508–514. [Google Scholar] [CrossRef]
- Da-Hai Xia, C.D.; Digby, M.; Sina, J.; Douglas, M.; Jing-Li, L.; Michael, G.; Strebl, M.A.; Weixian, J.; Shizhe, S.; Wenbin, H. Electrochemical measurements used for assessment of corrosion and protection of metallic materials in the field: A critical review. J. Mater. Sci. Technol. 2021, 112, 151–183. [Google Scholar] [CrossRef]
- Cano, E.; Lafuente, D.; Bastidas, D.M. Use of EIS for the evaluation of the protective properties of coatings for metallic cultural heritage: A review. J. Solid State Electrochem. 2010, 14, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.-N.; Cao, F.-H.; Chen, A.-N.; Liu, W.-J.; Zhang, J.-Q.; Cao, C.-N. In-situ investigation of atmospheric corrosion behavior of bronze under thin electrolyte layers using electrochemical technique. Trans. Nonferrous Met. Soc. China 2012, 22, 1239–1249. [Google Scholar] [CrossRef]
- Pan, C.; Lv, W.; Wang, Z.; Su, W.; Wang, C.; Liu, S. Atmospheric corrosion of copper exposed in a simulated coastal-industrial atmosphere. J. Mater. Sci. Technol. 2017, 33, 587–595. [Google Scholar] [CrossRef]
- Ma, X.M.; Cheng, Q.L.; Zheng, M.; Cui, F.Y.; Hou, B.R. Monitoring Marine Atmospheric Corrosion by Electrochemical Impedance Spectroscopy under Various Relative Humidities. Int. J. Electrochem. Sci. 2015, 10, 10402–10421. [Google Scholar]
- Nishikata, A.; Yamashita, Y.; Katayama, H.; Tsuru, T.; Usami, A.; Tanabe, K.; Mabuchi, H. An Electrochemical Impedance Study on Atmospheric Corrosion of Steels in a Cyclic Wet-Dry Condition. Corros. Sci. 1995, 37, 2059–2069. [Google Scholar] [CrossRef]
- Nishikata, A.; Ichihara, Y.; Tsuru, T. An application of electrochemical impedance spectroscopy to atmospheric corrosion study. Corros. Sci. 1995, 37, 897–911. [Google Scholar] [CrossRef]
- Nishikata, A.; Suzuki, F.; Tsuru, T. Corrosion monitoring of nickel-containing steels in marine atmospheric environment. Corros. Sci. 2005, 47, 2578–2588. [Google Scholar] [CrossRef]
- Shi, Y.; Tada, E.; Nishikata, A. A method for determining the corrosion rate of a metal under a thin electrolyte film. J. Electrochem. Soc. 2015, 162, C135–C139. [Google Scholar] [CrossRef]
- Nishikata, A.; Ichihara, Y.; Tsuru, T. Electrochemical impedance spectroscopy of metals covered with a thin electrolyte layer. Electrochim. Acta 1996, 41, 1057–1062. [Google Scholar] [CrossRef]
- Nishikata, A.; Ichihara, Y.; Hayashi, Y.; Tsuru, T. Influence of electrolyte layer thickness and pH on the initial stage of the atmospheric corrosion of iron. J. Electrochem. Soc. 1997, 144, 1244. [Google Scholar] [CrossRef]
- Thee, C.; Hao, L.; Dong, J.H.; Mu, X.; Wei, X.; Li, X.F.; Ke, W. Atmospheric corrosion monitoring of a weathering steel under an electrolyte film in cyclic wet-dry condition. Corros. Sci. 2014, 78, 130–137. [Google Scholar] [CrossRef]
- Thee, C.; Hao, L.; Dong, J.H.; Mu, X.; Ke, W. Numerical Approach for Atmospheric Corrosion Monitoring Based on EIS of a Weathering Steel. Acta Met. Sin. Engl. 2015, 28, 261–271. [Google Scholar] [CrossRef]
- Thee, C.; Dong, J.; Ke, W. Corrosion monitoring of weathering steel in a simulated coastal-industrial environment. Int. J. Environ. Ecol. Eng. 2015, 9, 587–593. [Google Scholar]
- Cruz, R.V.; Nishikata, A.; Tsuru, T. AC impedance monitoring of pitting corrosion of stainless steel under a wet-dry cyclic condition in chloride-containing environment. Corros. Sci. 1996, 38, 1397–1406. [Google Scholar] [CrossRef]
- Cruz, R.V.; Nishikata, A.; Tsuru, T. Pitting corrosion mechanism of stainless steels under wet-dry exposure in chloride-containing environments. Corros. Sci. 1998, 40, 125–139. [Google Scholar] [CrossRef]
- Katayama, H.; Tay, Y.-C.; AS, V.; Nishikata, A.; Tsuru, T. Corrosion monitoring of Zn and Zn–Al coated steels under wet-dry cyclic conditions using AC impedance method. Mater. Trans. JIM 1997, 38, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- El-Mahdy, G.A.; Nishikata, A.; Tsuru, T. AC impedance study on corrosion of 555%Al-Zn alloy-coated steel under thin electrolyte layers. Corros. Sci. 2000, 42, 1509–1521. [Google Scholar] [CrossRef]
- El-Mahdy, G.A.; Nishikata, A.; Tsuru, T. Electrochemical corrosion monitoring of galvanized steel under cyclic wet-dry conditions. Corros. Sci. 2000, 42, 183–194. [Google Scholar] [CrossRef]
- Yadav, A.; Nishikata, A.; Tsuru, T. Electrochemical impedance study on galvanized steel corrosion under cyclic wet–dry conditions––influence of time of wetness. Corros. Sci. 2004, 46, 169–181. [Google Scholar] [CrossRef]
- Yadav, A.P.; Nishikata, A.; Tsuru, T. Degradation mechanism of galvanized steel in wet–dry cyclic environment containing chloride ions. Corros. Sci. 2004, 46, 361–376. [Google Scholar] [CrossRef]
- Somphotch, C.; Hayashibara, H.; Ooi, A.; Tada, E.; Nishikata, A. Corrosion behavior of zinc under thin solution films of different thicknesses. J. Electrochem. Soc. 2018, 165, C590. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, G.; Qiu, Y.; Chen, Z.; Guo, X.; Fu, C. The corrosion of tin under thin electrolyte layers containing chloride. Corros. Sci. 2013, 66, 14–25. [Google Scholar] [CrossRef]
- Liao, X.; Cao, F.; Zheng, L.; Liu, W.; Chen, A.; Zhang, J.; Cao, C. Corrosion behaviour of copper under chloride-containing thin electrolyte layer. Corros. Sci. 2011, 53, 3289–3298. [Google Scholar] [CrossRef]
- Wan, S.; Hou, J.; Zhang, Z.F.; Zhang, X.X.; Dong, Z.H. Monitoring of atmospheric corrosion and dewing process by interlacing copper electrode sensor. Corros. Sci. 2019, 150, 246–257. [Google Scholar] [CrossRef]
- El-Mahdy, G.; Kim, K.B. AC impedance study on the atmospheric corrosion of aluminum under periodic wet-dry conditions. Electrochim. Acta 2004, 49, 1937–1948. [Google Scholar] [CrossRef]
- Nishikata, A.; Van Tran, N.; Ooi, A.; Tada, E. (Keynote) EIS Study of Galvanic Corrosion of Al Alloy Coupled with High Strength Steel in Simulated Atmospheres. In Proceedings of the ECS Meeting Abstracts, PRiME 2020 (ECS, ECSJ & KECS Joint Meeting), Online, 4–9 October 2020; p. 1318. [Google Scholar]
- Van Tran, N.; Ooi, A.; Tada, E.; Nishikata, A. Galvanic Corrosion of Aluminum Alloy Coupled with Ultra High-Strength Steel Under Thin Chloride Solution Layers. In Proceedings of the ECS Meeting Abstracts, 236th ECS Meeting, Atlanta, GA, USA, 13–17 October 2019; p. 777. [Google Scholar]
- Van Tran, N.; Ooi, A.; Tada, E.; Nishikata, A. EIS Characteristics of Galvanic Couple of Aluminum Alloy and High-strength Steel under Thin Solution Films. J. Electrochem. Soc. 2020, 167, 131507. [Google Scholar] [CrossRef]
- Nishikata, A.; Zhu, Q.J.; Tada, E. Long-term monitoring of atmospheric corrosion at weathering steel bridges by an electrochemical impedance method. Corros. Sci. 2014, 87, 80–88. [Google Scholar] [CrossRef]
- Ito, M.; Ooi, A.; Tada, E.; Nishikata, A. In Situ Evaluation of Carbon Steel Corrosion under Salt Spray Test by Electrochemical Impedance Spectroscopy. J Electrochem. Soc. 2020, 167, 101508. [Google Scholar] [CrossRef]
- Li, C.L.; Ma, Y.T.; Li, Y.; Wang, F.H. EIS monitoring study of atmospheric corrosion under variable relative humidity. Corros. Sci. 2010, 52, 3677–3686. [Google Scholar] [CrossRef]
- Angelini, E.; Grassini, S.; Corbellini, S.; Ingo, G.M.; De Caro, T.; Plescia, P.; Riccucci, C.; Bianco, A.; Agostini, S. Potentialities of XRF and EIS portable instruments for the characterisation of ancient artefacts. Appl. Phys. A Mater 2006, 83, 643–649. [Google Scholar] [CrossRef]
- Angelini, E.; Corbellini, S.; Parvis, M.; Ferraris, F.; Grassini, S. An Arduino-based EIS with a Logarithmic Amplifier for Corrosion Monitoring. In Proceedings of the 2014 IEEE International Instrumentation and Measurement Technology Conference (I2mtc) Proceedings, Montevideo, Uruguay, 12–15 May 2014; pp. 905–910. [Google Scholar]
- Angelini, E.; Grassini, S.; Parvis, M.; Zucchi, F. Corrosion Prediction of Metallic Cultural Heritage Assets by EIS. Corros. Sci. Technol. Korea 2019, 18, 121–128. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.Z.; Wang, J.H.; Shi, J.B.; Bi, H.C.; Gao, Z.M. Determination of corrosion types from electrochemical noise by phase space reconstruction theory. Electrochem. Commun. 2012, 15, 88–92. [Google Scholar] [CrossRef]
- Han, L.; Xia, D.H.; Song, S.Z.; Zhang, Z.; Bi, H.C.; Gao, Z.M.; Wang, J.H.; Hu, W.B. Online Monitoring of the Atmospheric Corrosion of Aluminium Alloys Using Electrochemical Noise Technique. Russ. J. Electrochem. 2018, 54, 623–628. [Google Scholar] [CrossRef]
- Iverson, W.P. Transient Voltage Changes Produced in Corroding Metals and Alloys. J. Electrochem. Soc. 1968, 115, 617. [Google Scholar] [CrossRef]
- Stewart, J.; Williams, D. The initiation of pitting corrosion on austenitic stainless steel: On the role and importance of sulphide inclusions. Corros. Sci. 1992, 33, 457–474. [Google Scholar] [CrossRef]
- Eden, D.A.; John, D.G.; Dawson, J.L. Corrosion Monitoring. U.S. Patent 5,139,627, 18 August 1992. [Google Scholar]
- Mansfeld, F.; Xiao, H. Electrochemical Noise-Analysis of Iron Exposed to Nacl Solutions of Different Corrosivity. J. Electrochem. Soc. 1993, 140, 2205–2209. [Google Scholar] [CrossRef]
- Xia, D.-H.; Behnamian, Y. Electrochemical noise: A review of experimental setup, instrumentation and DC removal. Russ. J. Electrochem. 2015, 51, 593–601. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.Z.; Behnamian, Y.; Hu, W.B.; Cheng, Y.F.; Luo, J.L.; Huet, F. Review-Electrochemical Noise Applied in Corrosion Science: Theoretical and Mathematical Models towards Quantitative Analysis. J. Electrochem. Soc. 2020, 167, 081507. [Google Scholar] [CrossRef]
- Cottis, R.A. Interpretation of electrochemical noise data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]
- Uruchurtu, J.; Dawson, J. Noise analysis of pure aluminum under different pitting conditions. Corrosion 1987, 43, 19–26. [Google Scholar] [CrossRef]
- Hladky, K.; Dawson, J. The measurement of corrosion using electrochemical 1f noise. Corros. Sci. 1982, 22, 231–237. [Google Scholar] [CrossRef]
- Garcia-Ochoa, E.; Corvo, F. Copper patina corrosion evaluation by means of fractal geometry using electrochemical noise (EN) and image analysis. Electrochem. Commun. 2010, 12, 826–830. [Google Scholar] [CrossRef]
- Li, J.; Kong, W.K.; Shi, J.B.; Wang, K.; Wang, W.K.; Zhao, W.P.; Zeng, Z.M. Determination of Corrosion Types from Electrochemical Noise by Artificial Neural Networks. Int. J. Electrochem. Sci. 2013, 8, 2365–2377. [Google Scholar]
- Hei, M.; Xia, D.H.; Song, S.Z.; Gao, Z.M. Sensing Atmospheric Corrosion of Carbon Steel and Low-Alloy Steel Using the Electrochemical Noise Technique: Effects of Weather Conditions. Prot. Met. Phys. Chem. Surf. 2017, 53, 1100–1113. [Google Scholar] [CrossRef]
- Ma, C.; Song, S.Z.; Gao, Z.M.; Wang, J.H.; Hu, W.B.; Behnamian, Y.; Xia, D.H. Electrochemical noise monitoring of the atmospheric corrosion of steels: Identifying corrosion form using wavelet analysis. Corros. Eng. Sci. Technol. 2017, 52, 432–440. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.Z.; Jin, W.X.; Li, J.; Gao, Z.M.; Wang, J.H.; Hu, W.B. Atmospheric Corrosion Monitoring of Field-exposed Q235B and T91 Steels in Zhoushan Offshore Environment Using Electrochemical Probes. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 1433–1440. [Google Scholar] [CrossRef]
- Xia, D.-H.; Ma, C.; Song, S.; Xu, L. Detection of atmospheric corrosion of aluminum alloys by electrochemical probes: Theoretical analysis and experimental tests. J. Electrochem. Soc. 2019, 166, B1000. [Google Scholar] [CrossRef]
- ASTM G96-90(2008). Standard Guide for Online Monitoring of Corrosion in Plant Equipment (Electrical and Electrochemical Methods); ASTM: West Conshohocken, PA, USA, 2008. [Google Scholar] [CrossRef]
- Diler, E.; Lédan, F.; LeBozec, N.; Thierry, D. Real-time monitoring of the degradation of metallic and organic coatings using electrical resistance sensors. Mater. Corros. 2017, 68, 1365–1376. [Google Scholar] [CrossRef]
- Dubus, M.; Kouril, M.; Nguyen, T.P.; Prosek, T.; Saheb, M.; Tate, J. Monitoring Copper and Silver Corrosion in Different Museum Environments by Electrical Resistance Measurement. Stud. Conserv. 2010, 55, 121–133. [Google Scholar] [CrossRef]
- Yasri, M.; Lescop, B.; Diler, E.; Gallee, F.; Thierry, D.; Rioual, S. Fundamental basis of electromagnetic wave propagation in a zinc microstrip lines during its corrosion. Sens. Actuators B Chem. 2016, 223, 352–358. [Google Scholar] [CrossRef]
- Dyos, G. The Handbook of Electrical Resistivity: New materials and pressure effects; The Institution of Engineering and Technology: London, UK, 2012. [Google Scholar]
- Li, Z.L.; Fu, D.M.; Li, Y.; Wang, G.Y.; Meng, J.T.; Zhang, D.W.; Yang, Z.H.; Ding, G.Q.; Zhao, J.B. Application of An Electrical Resistance Sensor-Based Automated Corrosion Monitor in the Study of Atmospheric Corrosion. Materials 2019, 12, 1065. [Google Scholar] [CrossRef] [Green Version]
- Kouřil, M.; Prošek, T.; Scheffel, B.; Dubois, F. High sensitivity electrical resistance sensors for indoor corrosion monitoring. Corros. Eng. Sci. Technol. 2013, 48, 282–287. [Google Scholar] [CrossRef]
- Faifer, M.; Goidanich, S.; Laurano, C.; Petiti, C.; Toscani, S.; Zanoni, M. Measurement Setup for the Development of Pre-Corroded Sensors for Metal Artwork Monitoring. In Proceedings of the 2019 IMEKO TC-4, Florence, Italy, 4–6 December 2019; pp. 1–6. [Google Scholar]
- Zajec, B.; Leban, M.B.; Lenart, S.; Gavin, K.; Legat, A. Electrochemical impedance and electrical resistance sensors for the evaluation of anticorrosive coating degradation. Corros. Rev. 2017, 35, 65–74. [Google Scholar] [CrossRef]
- Prošek, T.; Le Bozec, N.; Thierry, D. Application of automated corrosion sensors for monitoring the rate of corrosion during accelerated corrosion tests. Mater. Corros. 2014, 65, 448–456. [Google Scholar] [CrossRef]
- Kouril, M.; Prosek, T.; Scheffel, B.; Degres, Y. Corrosion monitoring in archives by the electrical resistance technique. J. Cult. Herit. 2014, 15, 99–103. [Google Scholar] [CrossRef]
- Prosek, T.; Taube, M.; Dubois, F.; Thierry, D. Application of automated electrical resistance sensors for measurement of corrosion rate of copper, bronze and iron in model indoor atmospheres containing short-chain volatile carboxylic acids. Corros. Sci. 2014, 87, 376–382. [Google Scholar] [CrossRef]
- Dubus, M.; Prosek, T. Standardized Assessment of Cultural Heritage Environments by Electrical Resistance Measurements. e-PRESERVATIONScience 2012, 9, 67–71. [Google Scholar]
- Kouřil, M.; Prošek, T.; Dubus, M.; Taube, M.; Hubert, V.; Scheffel, B.; Degres, Y.; Jouannic, M.; Thierry, D. Korozní monitoring v rukách restaurátorů a konzervátorů/Corrosion monitoring in the hands of restorers and conservators. Koroze A Ochr. Mater. 2012, 56, 67–75. [Google Scholar]
- Prosek, T.; Dubois, F.; Kouril, M.; Scheffel, B.; Degres, Y.; Jouannic, M.; Taube, M.; Dubus, M.; Hubert, V.; Thierry, D. Application of Automated Corrosion Sensors for Real-Time Monitoring in Atmospheres Polluted with Organic Acids. In Proceedings of the 18th International Corrosion Congress 2011, Perth, WA, USA, 20–24 November 2011; pp. 1477–1484. [Google Scholar]
- Vaissière, L.; Ragazzini, M.; Despert, G.; LeBozec, N.; Thierry, D. Cartography of corrosion aggressivity on vehicle in dynamic corrosion test in Renault. In Proceedings of the EUROCORR, Stockholm, Sweden, 4–8 September 2011. [Google Scholar]
- Masataka Omoda, D.M. Nobuyuki Ishikawa Analysis of Corrosion Behaviour of Iron and Zinc in Varioous Atmospheric Environments Using Electrical Resistance Technique. In Proceedings of the Galvatech, 11th International Conference on Zinc and Zinc Alloy Coated Steel Sheet, Tokio, Japan, 12–16 November 2017. [Google Scholar]
- Msallamova, S.; Kouril, M.; Strachotova, K.C.; Stoulil, J.; Popova, K.; Dvorakova, P. Historical lead seals and the influence of disinfectants on the lead corrosion rate. Herit. Sci. 2019, 7, 18. [Google Scholar] [CrossRef]
- Msallamova, S.; Kouril, M.; Strachotova, K.C.; Stoulil, J.; Popova, K.; Dvorakova, P.; Lhotka, M. Protection of lead in an environment containing acetic acid vapour by using adsorbents and their characterization. Herit. Sci. 2019, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Strachotova, K.C.; Kuchtakova, K.; Kouril, M.; Msallamova, S. Protection of Lead in Acetic Acid Containing Air by Means of Corrosion Inhibitors. In Proceedings of the 27th International Conference on Metallurgy and Materials (Metal 2018), Brno, Czech Republic, 23–25 May 2018; pp. 1045–1050. [Google Scholar]
- Strachotová, K.C.; Kouřil, M. Effect of the presence of corrosion products on the corrosion rate of lead during the exposition with paper packaging materials. Koroze A Ochr. Mater. 2018, 62, 87. [Google Scholar] [CrossRef] [Green Version]
- Van den Steen, N.; Simillion, H.; Thierry, D.; Deconinck, J. Modeling Film Thicknesses and Estimating Corrosion Depths Under Climate Control. In Proceedings of the ECS Meeting Abstracts, PRiME 2016/230th ECS Meeting, Honolulu, HI, USA, 2–7 October 2016; p. 1319. [Google Scholar]
- Van den Steen, N.; Simillion, H.; Thierry, D.; Terryn, H.; Deconinck, J. Comparing Modeled and Experimental Accelerated Corrosion Tests on Steel. J. Electrochem. Soc. 2017, 164, C554–C562. [Google Scholar] [CrossRef]
- Kreislova, K.; Fialova, P.; Bohackova, T. Indoor corrosivity in Klementinum baroque library hall. In Prague Structural Studies, Repairs and Maintenance of Heritage Architecture XVII & Earthquake Resistant Engineering Structures XIII; WIT Press: Ashurst Lodge, UK, 2021; p. 123. [Google Scholar]
- Bailey, G.; Brian, J.; Champion, C. An investigation into the impact of sealed wooden and acrylic showcases and storage cases on the corrosion of lead objects during long term storage and display. AICCM Bull. 2017, 38, 43–50. [Google Scholar] [CrossRef]
- Svadlena, J.; Voracova, E.; Stoulil, J. Corrosion of silver in environment containing halides, pseudohalides, or thiourea. Mater. Corros. Werkst. Und Korros. 2020, 71, 1721–1728. [Google Scholar] [CrossRef]
- Kosec, T.; Kuhar, V.; Kranjc, A.; Malnaric, V.; Belingar, B.; Legat, A. Development of an Electrical Resistance Sensor from High Strength Steel for Automotive Applications. Sensors 2019, 19, 1956. [Google Scholar] [CrossRef] [Green Version]
- Zajec, B.; Bajt Leban, M.; Kosec, T.; Kuhar, V.; Legat, A.; Lenart, S.; Fifer Bizjak, K.; Gavin, K. Corrosion monitoring of steel structure coating degradation. Teh. Vjesn. 2018, 25, 1348–1355. [Google Scholar]
- Shan, W.; LIAO, B.-k.; DONG, Z.-h.; GUO, X.-p. Comparative investigation on copper atmospheric corrosion by electrochemical impedance and electrical resistance sensors. Trans. Nonferrous Met. Soc. China 2021, 31, 3024–3038. [Google Scholar]
- AirCorr: Corrosion. Available online: https://nke-instrumentation.com/produit/aircorr-corrosion/ (accessed on 21 December 2021).
- ECM™ Environmental Condition Monitoring System. Available online: https://www.cosasco.com/product/ecm-environmental-condition-monitoring-system (accessed on 21 December 2021).
- Atmospheric Corrosion Sensor. Available online: https://www.cosasco.com/product/atmospheric-er-corrosion-sensor-610 (accessed on 21 December 2021).
- (ER) Electrical Resistance Probes. Available online: https://www.corrosionservice.com/wp-content/uploads/2017/03/COSASCO_ER-Electrical-Resistance-Probes.pdf (accessed on 21 December 2021).
- Light, E.; Little, B.; Barclay, M.; Manis, B.; Gay, R.; Bailey, J. Measuring the Corrosivity of Indoor Air. In Proceedings of the Indoor Air 2011, Austin, TX, USA, 5–10 June 2011. [Google Scholar]
- Morcillo, M.; Otero, E.; Chico, B.; de la Fuente, D. Atmospheric corrosion studies in a decommissioned nuclear power plant. In Nuclear Power; InTech: Rijeka, Croatia, 2010; pp. 243–265. [Google Scholar]
- Hart, L.; Storme, P.; Anaf, W.; Nuyts, G.; Vanmeert, F.; Dorrine, W.; Janssens, K.; de Wael, K.; Schalm, O. Monitoring the impact of the indoor air quality on silver cultural heritage objects using passive and continuous corrosion rate assessments. Appl. Phys. A Mater. 2016, 122, 923. [Google Scholar] [CrossRef]
- SURVEYOR PLUS™. Available online: https://circul-aire.com/corrosion-monitoring/surveyor-plus/ (accessed on 21 December 2021).
- Since 2015: Viledon ChemWatch. Available online: https://www.freudenberg-filter.com/en/innovations/chemwatch-online-monitoring/ (accessed on 21 December 2021).
- Bennett, A. Developments in air & gas filtration technology. Filtr. Separat. 2016, 53, 30–35. [Google Scholar]
- Marcus, P.; Mansfeld, F. Analytical Methods in Corrosion Science and Engineering; CRC Press: Boca Raton, FL, USA, 2006; 760p. [Google Scholar]
- England, W.G.; Osborne, M.W.; Xiuyi, Z. Applications of a Real-Time Electronic Contact Corrosion Monitor. Adv. Instrum. Control 1991, 46, 929–955. [Google Scholar]
- Forslund, M.; Leygraf, C. A quartz crystal microbalance probe developed for outdoor in situ atmospheric corrosivity monitoring. J. Electrochem. Soc. 1996, 143, 839–844. [Google Scholar] [CrossRef]
- Forslund, M.; Majoros, J.; Leygraf, C. A sensor system for high resolution in situ atmospheric corrosivity monitoring in field environment. J. Electrochem. Soc. 1997, 144, 2637–2642. [Google Scholar] [CrossRef]
- ASTM B808-97(2003). Standard Test Method for Monitoring of Atmospheric Corrosion Chambers by Quartz Crystal Microbalances. ASTM: West Conshohocken, PA, USA, 2003. [CrossRef]
- Petrunin, M.A.; Gladkikh, N.A.; Maleeva, M.A.; Yurasova, T.A.; Terekhova, E.V.; Maksaeva, L.B. Application of the quartz crystal microbalance technique in corrosion studies. A review. Int. J. Corros. Scale Inhib. 2020, 9, 92–117. [Google Scholar] [CrossRef]
- Baek, Y.; Frankel, G.S. Electrochemical quartz crystal microbalance study of corrosion of phases in AA2024-T3. Corros. Corros. Prot. 2001, 2001, 814–821. [Google Scholar]
- Fujii, K.; Ohashi, K.; Hashimoto, T.; Hara, N. Atmospheric Corrosivity Estimation at Electrical Control Unit Room by Multichannel Quartz Crystal Microbalance Corrosion Sensors. Mater. Trans. 2012, 53, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Hosseinpour, S.; Schwind, M.; Kasemo, B.; Leygraf, C.; Johnson, C.M. Integration of Quartz Crystal Microbalance with Vibrational Sum Frequency Spectroscopy-Quantification of the Initial Oxidation of Alkanethiol-Covered Copper. J. Phys. Chem. C 2012, 116, 24549–24557. [Google Scholar] [CrossRef]
- Ehahoun, H.; Gabrielli, C.; Keddam, M.; Perrot, H.; Cetre, Y.; Diguet, L. Electrochemical quartz crystal microbalance corrosion sensor for solid metals and metal alloys—Application to the dissolution of 304 stainless steel. J. Electrochem. Soc. 2001, 148, B333–B336. [Google Scholar] [CrossRef]
- Kleber, C.; Hilfrich, U.; Schreiner, M. In situ QCM and TM-AFM investigations of the early stages of degradation of silver and copper surfaces. Appl. Surf. Sci. 2007, 253, 3712–3721. [Google Scholar] [CrossRef]
- Kleber, C.; Wiesinger, R.; Schnoller, J.; Hilfrich, U.; Hutter, H.; Schreiner, M. Initial oxidation of silver surfaces by S2- and S4+ species. Corros. Sci. 2008, 50, 1112–1121. [Google Scholar] [CrossRef]
- Wiesinger, R.; Kleber, C.; Frank, J.; Schreiner, M. A New Experimental Setup for in Situ Infrared Reflection Absorption Spectroscopy Studies of Atmospheric Corrosion on Metal Surfaces Considering the Influence of Ultraviolet Light. Appl. Spectrosc. 2009, 63, 465–470. [Google Scholar] [CrossRef]
- Wiesinger, R.; Schreiner, M.; Kleber, C. Investigations of the interactions of CO2, O3 and UV light with silver surfaces by in situ IRRAS/QCM and ex situ TOF-SIMS. Appl. Surf. Sci. 2010, 256, 2735–2741. [Google Scholar] [CrossRef]
- Wiesinger, R.; Schade, U.; Kleber, C.; Schreiner, M. An experimental set-up to apply polarization modulation to infrared reflection absorption spectroscopy for improved in situ studies of atmospheric corrosion processes. Rev. Sci. Instrum. 2014, 85, 064102. [Google Scholar] [CrossRef]
- Wiesinger, R.; Martina, I.; Kleber, C.; Schreiner, M. Influence of relative humidity and ozone on atmospheric silver corrosion. Corros. Sci. 2013, 77, 69–76. [Google Scholar] [CrossRef]
- Wan, S.; Ma, X.Z.; Miao, C.H.; Zhang, X.X.; Dong, Z.H. Inhibition of 2-phenyl imidazoline on chloride-induced initial atmospheric corrosion of copper by quartz crystal microbalance and electrochemical impedance. Corros. Sci. 2020, 170, 108692. [Google Scholar] [CrossRef]
- Schwind, M.; Langhammer, C.; Kasemo, B.; Zoric, I. Nanoplasmonic sensing and QCM-D as ultrasensitive complementary techniques for kinetic corrosion studies of aluminum nanoparticles. Appl. Surf. Sci. 2011, 257, 5679–5687. [Google Scholar] [CrossRef]
- Larsson, E.M.; Edvardsson, M.E.M.; Langhammer, C.; Zoric, I.; Kasemo, B. A combined nanoplasmonic and electrodeless quartz crystal microbalance setup. Rev. Sci. Instrum. 2009, 80, 125105. [Google Scholar] [CrossRef]
- Schwind, M.; Hosseinpour, S.; Johnson, C.M.; Langhammer, C.; Zoric, I.; Leygraf, C.; Kasemo, B. Combined in Situ Quartz Crystal Microbalance with Dissipation Monitoring, Indirect Nanoplasmonic Sensing, and Vibrational Sum Frequency Spectroscopic Monitoring of Alkanethiol-Protected Copper Corrosion. Langmuir 2013, 29, 7151–7161. [Google Scholar] [CrossRef] [PubMed]
- Odlyha, M.; Jakiela, S.; Bergsten, C.J.; Slater, J.M.; Niklasson, A.; Svensson, J.; Cavicchioli, A.; de Faria, D.; Thickett, D.; Grøntoft, T. Dosimetry for monitoring in organ pipes and in microclimate frames for paintings. In Metal 2010: Proceedings of the interim meeting of the ICOM-CC Metal Working Group, Charleston, CA, USA, 11-15 October 2010; Metal Working Group: Fairfield, OH, USA, 15 October 2010; pp. 321–326. [Google Scholar]
- Odlyha, M.; Slater, J.M.; Grøntoft, T.; Jakiela, S.; Obarzanowski, M.; Thickett, D.; Hackney, S.; Andrade, G.; Wadum, J.; Christensen, A.H. A Portable Tool for the Evaluation of Microclimate Conditions within Museum Enclosures, Transit Frames, and Transport Cases. Stud. Conserv. 2018, 63, 407–410. [Google Scholar] [CrossRef]
- Agbota, H.; Mitchell, J.E.; Odlyha, M.; Strlič, M. Remote assessment of cultural heritage environments with wireless sensor array networks. Sensors 2014, 14, 8779–8793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OnGuard Smart. Available online: https://www.purafil.com/products/monitoring/active-monitoring/onguard-smart/ (accessed on 21 December 2021).
- OnGuard Lite. Available online: https://www.purafil.com/products/monitoring/active-monitoring/onguard-lite/ (accessed on 21 December 2021).
- ACM—Atmospheric Corrosivity Monitor. Available online: https://www.bryair.com/products-solutions/gas-phase-filtration-systems/acm-atmospheric-corrosivity-monitor/ (accessed on 21 December 2021).
- He, Y.L.; McLaughlin, S.; Lo, J.S.H.; Shi, C.; Lenos, J.; Vincelli, A. Radio frequency identification (RFID) based corrosion monitoring sensors Part 1-Component selection and testing. Corros. Eng. Sci. Technol. 2015, 50, 63–71. [Google Scholar] [CrossRef]
- Alamin, M.; Tian, G.Y.; Andrews, A.; Jackson, P. Corrosion detection using low-frequency RFID technology. Insight 2012, 54, 72–75. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, G.Y.; Marindra, A.M.J.; Sunny, A.I.; Zhao, A.B. A Review of Passive RFID Tag Antenna-Based Sensors and Systems for Structural Health Monitoring Applications. Sensors 2017, 17, 265. [Google Scholar] [CrossRef]
- Dante, J.F.; Steiner, A.; Friedersdorf, F. RF system for corrosivity monitoring. In Proceedings of the CORROSION, New Orleans, LO, USA, 16 March 2008. [Google Scholar]
- Dante, J.F.; Friedersdorf, F.; Innovations, L. Low-Cost Wireless Corrosivity Sensors. In Proceedings of the 2007 Tri-Service Corrosion Conference, Denver, CO, USA, 3–7 December 2007. [Google Scholar]
- He, Y.L.; McLaughlin, S.; Lo, J.S.H.; Shi, C.; Lenos, J.; Vincelli, A. Radio frequency identification (RFID) based corrosion monitoring sensors Part 2-Application and testing of coating materials. Corros. Eng. Sci. Technol. 2014, 49, 695–704. [Google Scholar] [CrossRef]
- He, Y.L. Wireless Corrosion Monitoring Sensors Based on Electromagnetic Interference Shielding of RFID Transponders. Corrosion 2020, 76, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Yasri, M.; Gallee, F.; Leseop, B.; Diler, E.; Thierry, D.; Rioual, S. Passive Wireless Sensor for Atmospheric Corrosion Monitoring. In Proceedings of the 8th Eurntennas and Opean Conference on Antennas and Propagation (EuCAP 2014), The Hague, The Netherlands, 6–11 April 2014; pp. 2945–2949. [Google Scholar]
- Yasri, M.; Khalifeh, R.; Lescop, B.; Gallée, F.; Diler, E.; Thierry, D.; Rioual, S. Development of environmental sensors for monitoring of corrosion in marine offshore and wind energy industries. In Proceedings of the 2014 IEEE Sensor Systems for a Changing Ocean (SSCO), Brest, France, 13–17 October 2014; pp. 1–4. [Google Scholar]
- Khalifeh, R.; Yasri, M.S.; Lescop, B.; Gallee, F.; Diler, E.; Thierry, D.; Rioual, S. Development of Wireless and Passive Corrosion Sensors for Material Degradation Monitoring in Coastal Zones and Immersed Environment. IEEE J. Ocean. Eng. 2016, 41, 776–782. [Google Scholar] [CrossRef]
- Yasri, M.; Lescop, B.; Diler, E.; Gallee, F.; Thierry, D.; Rioual, S. Monitoring uniform and localised corrosion by a radiofrequency sensing method. Sens. Actuators B Chem. 2018, 257, 988–992. [Google Scholar] [CrossRef]
- Alamin, M. Passive Low Frequencey RFID for Detection and Monitoring of Corrosion under Paint and Insulation; Newcastle University: Newcastle upon Tyne, UK, 2014. [Google Scholar]
- Sunny, A.I.; Tian, G.Y.; Zhang, J.; Pal, M. Low frequency (LF) RFID sensors and selective transient feature extraction for corrosion characterisation. Sens. Actuators A Phys. 2016, 241, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tian, G.Y.; Simm, A.; Alamin, M. Electromagnetic Methods for Corrosion under Paint Coating Measurement. In Proceedings of the Eighth International Symposium on Precision Engineering Measurements and Instrumentation, Chengdu, China, 8–11 August 2012; Volume 2013, p. 8759. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, R.Z.; He, Y.Z.; Tian, G.Y.; Xu, L.X.; Wu, R.K. Identification and characterisation of steel corrosion using passive high frequency RFID sensors. Measurement 2016, 92, 421–427. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, G.Y. UHF RFID Tag Antenna-Based Sensing for Corrosion Detection & Characterization Using Principal Component Analysis. IEEE Trans. Antennas Propag. 2016, 64, 4405–4414. [Google Scholar] [CrossRef]
- Zhao, A.B.; Zhang, J.; Tian, G.Y. Miniaturization of UHF RFID Tag Antenna Sensors for Corrosion Characterization. IEEE Sens. J. 2017, 17, 7908–7916. [Google Scholar] [CrossRef]
- Annamdas, K.K.K.; Annamdas, V.G.M. Review on Developments in Fiber Optical Sensors and Applications. Int. J. Mater. Eng. 2011, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sabri, N.; Aljunid, S.A.; Salim, M.S.; Ahmad, R.B.; Kamaruddin, R. Toward Optical Sensors: Review and Applications. In Proceedings of the 2013 International Conference on Science & Engineering in Mathematics, Chemistry and Physics (Scietech 2013), Jakarta, Indonesia, 24–25 January 2013; p. 423. [Google Scholar] [CrossRef]
- Luo, D.; Li, J.N.; Li, Y.Y. A Review of Fiber-optic Corrosion Sensor in Civil Engineering. In Proceedings of the 6th International Conference on Computer-Aided Design, Manufacturing, Modeling and Simulation (Cdmms 2018), Busan, South Korea, 14–15 April 2018; p. 1967. [Google Scholar] [CrossRef]
- Qi, X.; J Gelling, V. A review of different sensors applied to corrosion detection and monitoring. Recent Pat. Corros. Sci. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Leung, C.K.Y.; Wan, K.T.; Chen, L. A novel optical fiber sensor for steel corrosion in concrete structures. Sensors 2008, 8, 1960–1976. [Google Scholar] [CrossRef] [Green Version]
- Wan, K.T.; Leung, C.K.Y. Durability Tests of a Fiber Optic Corrosion Sensor. Sensors 2012, 12, 3656–3668. [Google Scholar] [CrossRef]
- Dong, S.Y.; Liao, Y.B.; Tian, Q.; Luo, Y.N.; Qiu, Z.G.; Song, S.Z. Optical and electrochemical measurements for optical fibre corrosion sensing techniques. Corros. Sci. 2006, 48, 1746–1756. [Google Scholar] [CrossRef]
- Dong, S.Y.; Peng, G.D.; Luo, Y.N. Preparation techniques of metal clad fibres for corrosion monitoring of steel materials. Smart Mater. Struct. 2007, 16, 733–738. [Google Scholar] [CrossRef]
- Qiao, G.F.; Zhou, Z.; Ou, J.P. Thin Fe-C alloy solid film based fiber optic corrosion sensor. In Proceedings of the 2006 1st Ieee International Conference on Nano/Micro Engineered and Molecular Systems, Vols 1–3, Zhuhai, China, 18–21 June 2006; p. 541. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H. Optical fiber corrosion sensor based on laser light reflection. Smart Mater. Struct. 2011, 20, 085003. [Google Scholar] [CrossRef]
- Khorsandi, A.; Shojaei, S.; Hosseinibalam, F. Second-harmonic laser-coupled optical fiber sensor for pH measurement and corrosion detection based on evanescent field absorption. Opt. Laser Technol. 2012, 44, 1564–1569. [Google Scholar] [CrossRef]
- Deng, F.; Huang, Y.; Azarmi, F. Corrosion Behavior Evaluation of Coated Steel Using Fiber Bragg Grating Sensors. Coatings 2019, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Geng, J.; Wu, J.; Zhao, X.M. Application of Fiber Bragg Grating Sensor for Rebar Corrosion. Second Int. Conf. Smart Mater. Nanotechnol. Eng. 2009, 7493, 749333. [Google Scholar] [CrossRef]
- Hu, W.B.; Cai, H.L.; Yang, M.H.; Tong, X.L.; Zhou, C.M.; Chen, W. Fe-C-coated fibre Bragg grating sensor for steel corrosion monitoring. Corros. Sci. 2011, 53, 1933–1938. [Google Scholar] [CrossRef]
- Tan, C.H.; Shee, Y.G.; Yap, B.K.; Adikan, F.R.M. Fiber Bragg grating based sensing system: Early corrosion detection for structural health monitoring. Sens. Actuators A Phys. 2016, 246, 123–128. [Google Scholar] [CrossRef]
- Tan, C.H.; Adikan, F.R.M.; Shee, Y.G.; Yap, B.K. Non-destructive fiber Bragg grating based sensing system: Early corrosion detection for structural health monitoring. Sens. Actuators A Phys. 2017, 268, 61–67. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Tang, F.J.; Bao, Y.; Tang, Y.; Chen, G.D. A Fe-C coated long-period fiber grating sensor for corrosion-induced mass loss measurement. Opt. Lett. 2016, 41, 2306–2309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Tang, F.J.; Tang, Y.; O'Keefe, M.J.; Chen, G.D. Mechanism and sensitivity of Fe-C coated long period fiber grating sensors for steel corrosion monitoring of RC structures. Corros. Sci. 2017, 127, 70–81. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, Z.; Chen, G.D.; Xiao, H. Long period fiber grating sensors coated with nano iron/silica particles for corrosion monitoring. Smart Mater. Struct. 2013, 22, 075018. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, F.J.; Liang, X.; Chen, G.D.; Xiao, H.; Azarmi, F. Steel bar corrosion monitoring with long-period fiber grating sensors coated with nano iron/silica particles and polyurethane. Struct. Health Monit. Int. J. 2015, 14, 178–189. [Google Scholar] [CrossRef]
- Ahmed, R.; Rifat, A.A.; Yetisen, A.K.; Salem, M.S.; Yun, S.H.; Butt, H. Optical microring resonator based corrosion sensing. Rsc. Adv. 2016, 6, 56127–56133. [Google Scholar] [CrossRef]
- Strebl, M.; Bruns, M.; Virtanen, S. Editors' Choice-Respirometric in Situ Methods for Real-Time Monitoring of Corrosion Rates: Part I. Atmospheric Corrosion. J. Electrochem. Soc. 2020, 167, 021510. [Google Scholar] [CrossRef]
- Stratmann, M.; Streckel, H. On the atmospheric corrosion of metals which are covered with thin electrolyte layers—II. Experimental results. Corros. Sci. 1990, 30, 697–714. [Google Scholar] [CrossRef]
- Strebl, M.; Virtanen, S. Real-Time Monitoring of Atmospheric Magnesium Alloy Corrosion. J. Electrochem. Soc. 2018, 166, C3001–C3009. [Google Scholar] [CrossRef]
- Strebl, M.; Virtanen, S. New Method for Monitoring Hydrogen Evolution of Magnesium Alloys Under Atmospheric Exposure. In Proceedings of the ECS Meeting Abstracts, 232nd ECS Meeting, National Harbor, MD, USA, 1–5 October 2017; p. 721. [Google Scholar]
- Matthiesen, H. A novel method to determine oxidation rates of heritage materials in vitro and in situ. Stud. Conserv. 2007, 52, 271–280. [Google Scholar] [CrossRef]
- Matthiesen, H.; Wonsyld, K. In situ measurement of oxygen consumption to estimate corrosion rates. In Corrosion of Archaeological and Heritage Artefacts EFC 45; Routledge: London, UK, 2017; pp. 350–356. [Google Scholar]
- Matthiesen, H.; Stemann-Petersen, K. A fast and non-destructive method to document and quantify the efficiency of metals conservation. In Proceedings of the Metal 2013: Interim Meeting of the ICOM-CC Metal Working Group, Edinburgh, Scotland, 16–20 September 2013; pp. 16–20. [Google Scholar]
- Watkinson, D.; Rimmer, M. Quantifying Effectiveness of Chloride Desalination Treatments for Archaeological Iron Using Oxygen Measurement. In Proceedings of the Metal 2013: Interim Meeting of the ICOM-CC Metal Working Group, Edinburg, Schotland, 16–20 September 2013; pp. 95–102. [Google Scholar]
- Watkinson, D.; Emmerson, N.; Seifert, J. Matching Display Relative Humidity to Corrosion Rate: Quantitative Evidence for Marine Cast Iron Cannon Balls. In Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, New Delhi, India, 26–30 September 2016; pp. 195–202. [Google Scholar]
- Watkinson, D.E.; Rimmer, M.B.; Emmerson, N.J. The influence of relative humidity and intrinsic chloride on post-excavation corrosion rates of archaeological wrought iron. Stud. Conserv. 2019, 64, 456–471. [Google Scholar] [CrossRef]
- Emmerson, N.J.; Watkinson, D.E. Surface preparation of historic wrought iron: Evidencing the requirement for standardisation. Mater. Corros. 2016, 67, 176–189. [Google Scholar] [CrossRef]
- Watkinson, D.; Emmerson, N. The impact of aqueous washing on the ability of βFeOOH to corrode iron. Environ. Sci. Pollut. Res. 2017, 24, 2138–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmerson, N.; Seifert, J.; Watkinson, D. Refining the use of oxygen consumption as a proxy corrosion rate measure for archaeological and historic iron. Eur. Phys. J. Plus 2021, 136, 546. [Google Scholar] [CrossRef]
- Virtanen, S. Novel in-Situ Methods for Real-Time Monitoring of Corrosion. In Proceedings of the ECS Meeting Abstracts, PRiME 2020 (ECS, ECSJ & KECS Joint Meeting, Online, 4–9 October 2020; p. 1279. [Google Scholar]
- Garcia-Ochoa, E.; Gonzalez-Sanchez, J.; Corvo, F.; Usagawa, Z.; Dzib-Peerez, L.; Castaneda, A. Application of electrochemical noise to evaluate outdoor atmospheric corrosion of copper after relatively short exposure periods. J. Appl. Electrochem. 2008, 38, 1363–1368. [Google Scholar] [CrossRef]
- Jamali, S.S.; Zhao, Y.; Gao, Z.M.; Li, H.J.; Hee, A.C. In situ evaluation of corrosion damage using non-destructive electrochemical measurements - A case study. J. Ind. Eng. Chem. 2016, 43, 36–43. [Google Scholar] [CrossRef]




| Technique | Environment * | Sensing Metal ** | Range of Measured Corrosion Rates ***, [µm·a−1] | References | Localised Corrosion Detection |
|---|---|---|---|---|---|
| ACM 1 | Outdoor exposures | Fe | 1 × 10−1–1 × 102 | [16,21,22,23,24,25,26,27,30] | – |
| Zn | Not calculated | [32] | |||
| ACTs | Fe | 1 × 102 | [20] | ||
| Laboratory tests | Fe | 1 × 101–1 × 103 | [19,31] | ||
| Zn | 1 × 101–1 × 103 | [19,31] | |||
| Cu | 1 × 101–1 × 103 | [19] | |||
| Al | 1 × 101–1 × 103 | [19] | |||
| ER | Outdoor exposures | Fe | 1 × 10−1–1 × 103 | [97,108,118] | [3,29,100] |
| Zn | 1 × 10−1–1 × 101 | [108] | |||
| Cu | 1 × 10−1–1 × 100 | [120] | |||
| ACTs | Fe | 1 × 101–1 × 103 | [2,93,101,108,113,114,119] | ||
| Zn | 1 × 100–1 × 103 | [2,93,101] | |||
| Cu | 1 × 103 | [2] | |||
| Al | 1 × 10−1–1 × 101 | [29] | |||
| Laboratory tests | Fe | 1 × 10−3–1 × 101 | [2,3,103] | ||
| Cu | 1 × 10−3–1 × 10−1 | [2,4,98,103] | |||
| Ag | 1 × 10−3–1 × 101 | [4,98,117] | |||
| Zn | 1 × 100–1 × 102 | [2] | |||
| Pb | 1 × 10−3–1 × 102 | [109,110,112] | |||
| Indoor exposures | Cu | 1 × 10−3–1 × 10−1 | [4,94,102,104,115,125] | ||
| Ag | 1 × 10−3–1 × 10−1 | [4,94,102,115,127] | |||
| Pb | 1 × 10−2–1 × 101 | [102,115,116] | |||
| EIS 2 | Outdoor exposures | Fe | 1 × 10−1–1 × 101 | [47,69] | [89,91] |
| Cu | 1 × 102–1 × 103 | [120] | |||
| ACTs | Fe | 1 × 102–1 × 103 | [70] | ||
| Laboratory tests | Fe | 1 × 10−1–1 × 104 | [45,48,50,52,71] | ||
| Zn-coated steel | 1 × 100–1 × 103 | [56,57,58,59] | |||
| Zn | 1 × 101 | [44] | |||
| Cu | 1 × 10−1–1 × 101 | [43,64,120] | |||
| EN 3 | Outdoor exposures | Fe | 1 × 10−1–1 × 101 | [89,90] | [89,90,91,209] |
| Cu | 1 × 10−2–1 × 102 | [86,209] | |||
| QCM 4 | Laboratory tests | Cu | 1 × 10−1–1 × 100 | [147] | – |
| Ag | 1 × 10−3–1 × 10−2 | [141,142,143,144,145,146] | |||
| Indoor exposures | Cu | 1 × 10−3–1 × 10−1 | [102,138] | ||
| Ag | 1 × 10−2–1 × 10−1 | [102,127,138] | |||
| Co | 1 × 10−2–1 × 10−1 | [138] | |||
| RFID | ACTs | Fe | 1 × 102–1 × 103 | [157,163] | [95,167] |
| Laboratory tests | Zn | 1 × 101 | [95,166,167] | ||
| FOCS | Fe | No data for atmospheric corrosion | – | ||
| Respirometry 5 | Laboratory tests | Fe | 1 × 10−1–1 × 102 | [200,205] | [195,197] |
| Cu | 1 × 10−2–1 × 10−1 | [200] | |||
| Al | 1 × 10−1–1 × 100 | [195] | |||
| Mg | 1 × 101–1 × 103 | [195,197] | |||
| Technique | Current Applications | Potential Fields of Application | Sensitivity * | Commercial Suppliers | Main Advantages | Main Drawbacks |
|---|---|---|---|---|---|---|
| Coupons | Indoor and outdoor corrosivity classification according to standards Verification of other techniques | Applicable in any environment | High at long exposure times, otherwise medium | Several | Standardised technique Easy data interpretation | No real-time data Time-consuming |
| ACM | Outdoor monitoring TOW assessment | Outdoor and indoor at higher RH | Medium | 1 | Not sensitive to temperature fluctuations Suitable for harsh outdoor environments | Corrosion acceleration due to galvanic coupling Unclear data interpretation during rainfall Electrolyte presence required |
| EIS | Laboratory tests at higher RH and under thin electrolyte layers Assessment of protective coatings | Outdoor and indoor at higher RH | Medium | 0 | Information about corrosion mechanism Non-destructive assessment of coatings | Knowledge about investigated system needed for correct data interpretation Electrolyte presence required Unclear results under very thin electrolyte layers and in presence of thick corrosion products |
| EN | Outdoor corrosion monitoring | Outdoor and indoor at higher RH | Medium | 0 | Localised corrosion detection Corrosion mechanism determination | Complex and unclear interpretation Electrolyte presence required |
| ER | Indoor and outdoor corrosion monitoring, laboratory studies Corrosivity classification | Applicable in any environment | High | 4 | Universal technique High sensitivity Easy operation and data interpretation Optimal for uniform corrosion monitoring | Sensitive to temperature fluctuations Limited possibilities in monitoring of non-uniform corrosion |
| QCM | Indoor corrosivity classification Laboratory tests | Indoor at lower corrosivity | High | 2 | High sensitivity and short response time Electrolyte presence not required | Sensitive to temperature fluctuations, moisture and pollutants presence Not suitable for harsh environments |
| RFID | Laboratory tests | Outdoor and indoor at higher corrosivity | Low | 0 | Compact and wireless Electrolyte presence not required | Further development needed |
| FOCS | None for atmospheric corrosion | Not clear yet, as the technique is at the development stage | Not available | 0 | Not known for atmospheric corrosion yet | |
| Respirometry | Laboratory tests | Not clear yet, as the technique is at the development stage | High | 0 | High sensitivity Information about corrosion mechanism Electrolyte presence not required | Sensitivity to RH, temperature and pressure fluctuations Further development needed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, K.; Prošek, T. Corrosion Monitoring in Atmospheric Conditions: A Review. Metals 2022, 12, 171. https://doi.org/10.3390/met12020171
Popova K, Prošek T. Corrosion Monitoring in Atmospheric Conditions: A Review. Metals. 2022; 12(2):171. https://doi.org/10.3390/met12020171
Chicago/Turabian StylePopova, Kateryna, and Tomáš Prošek. 2022. "Corrosion Monitoring in Atmospheric Conditions: A Review" Metals 12, no. 2: 171. https://doi.org/10.3390/met12020171
APA StylePopova, K., & Prošek, T. (2022). Corrosion Monitoring in Atmospheric Conditions: A Review. Metals, 12(2), 171. https://doi.org/10.3390/met12020171







