Microstructural, Chemical, and Crystallographic Investigations of Dynamic Strain-Induced Ferrite in a Microalloyed QT Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigated Material
2.2. Dilatometric Experiments
2.2.1. Determination of Ae1, Ae3, and Ar3
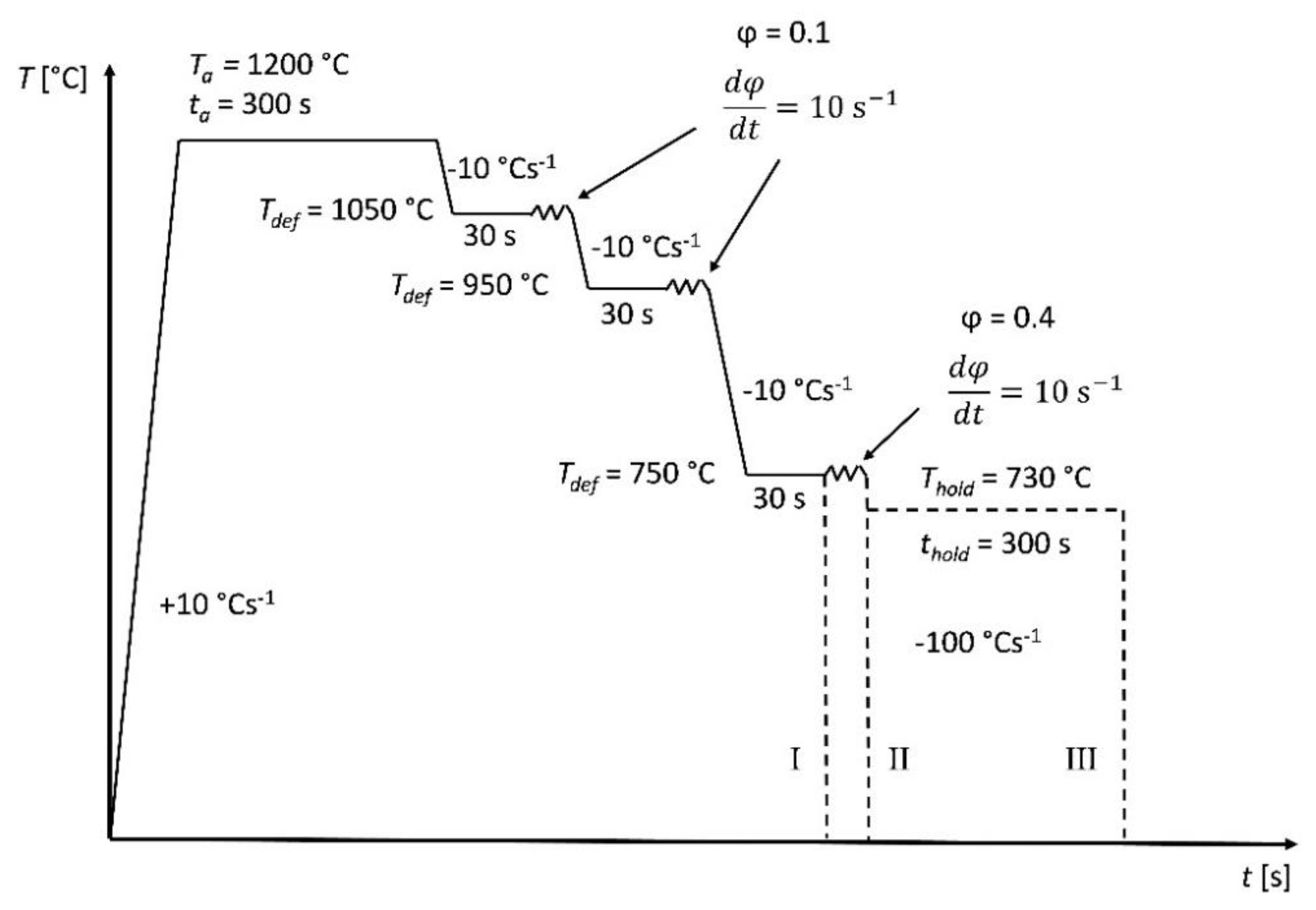
2.2.2. Compression Experiments
2.3. Microstructural and Crystallographic Characterization
3. Results
3.1. Determination of Ae1, Ae3 and Ar3
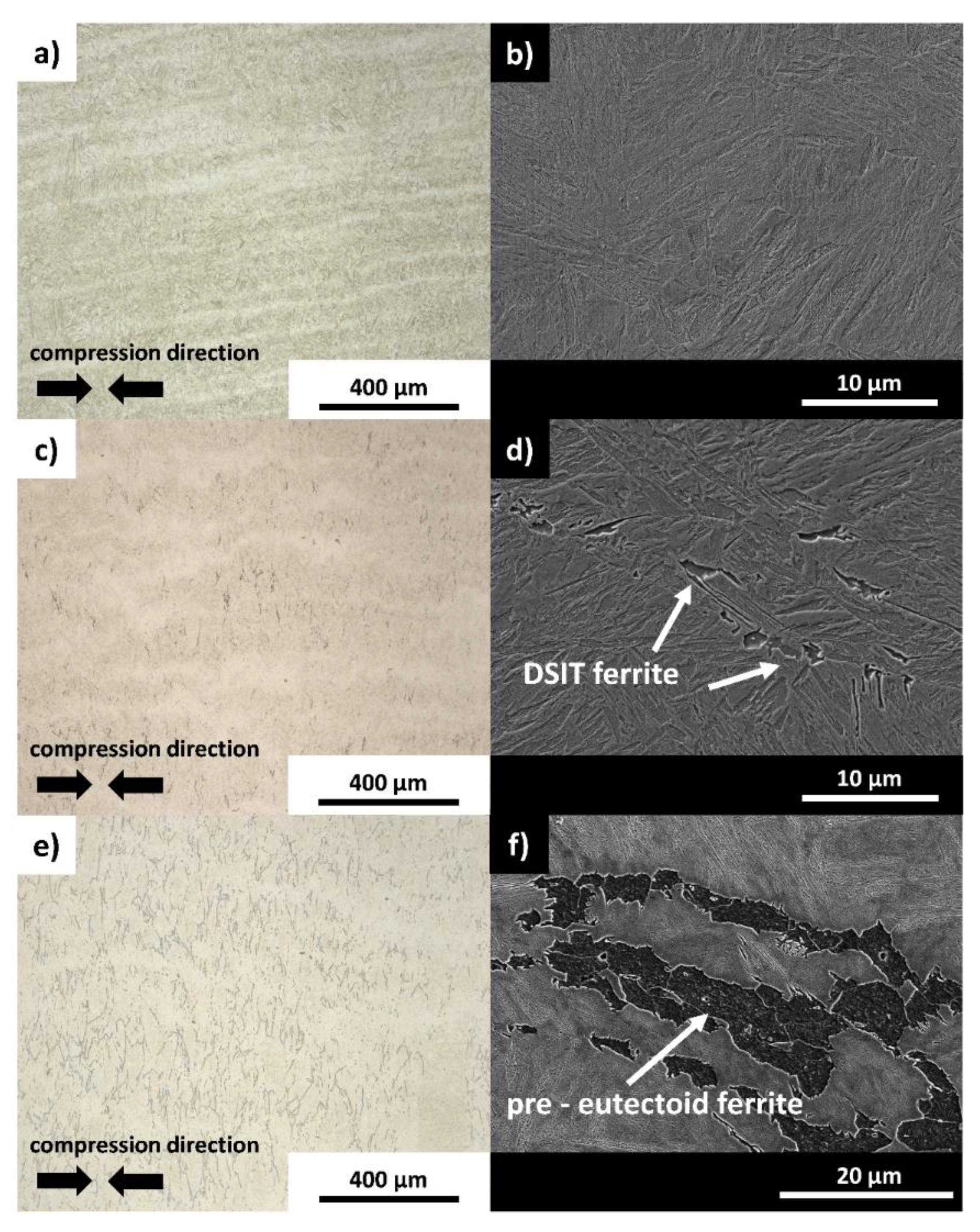
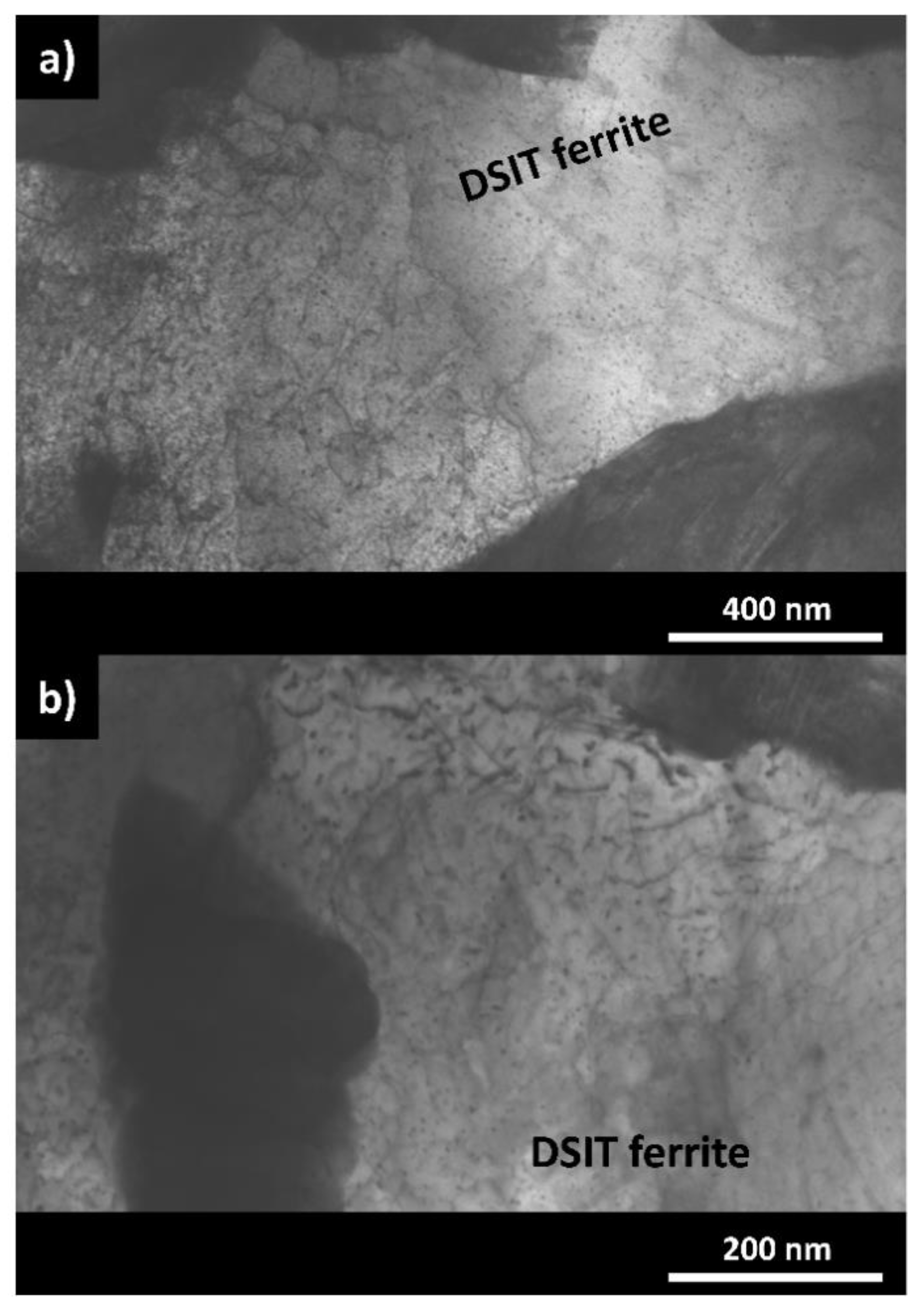
3.2. Microstructural and Chemical Characterization after the Deformation Experiments
3.3. Crystallographic Characterization
4. Discussion
5. Conclusions
- The LOM and SEM analysis of the quenched dilatometer samples did show that DSIT ferrite formed predominantly along the PAGB during the final compression step of route II. Investigations of the quenched dilatometer samples after route III indicate that DSIT ferrite serves as a nucleation site for the formation of pre-eutectoid ferrite.
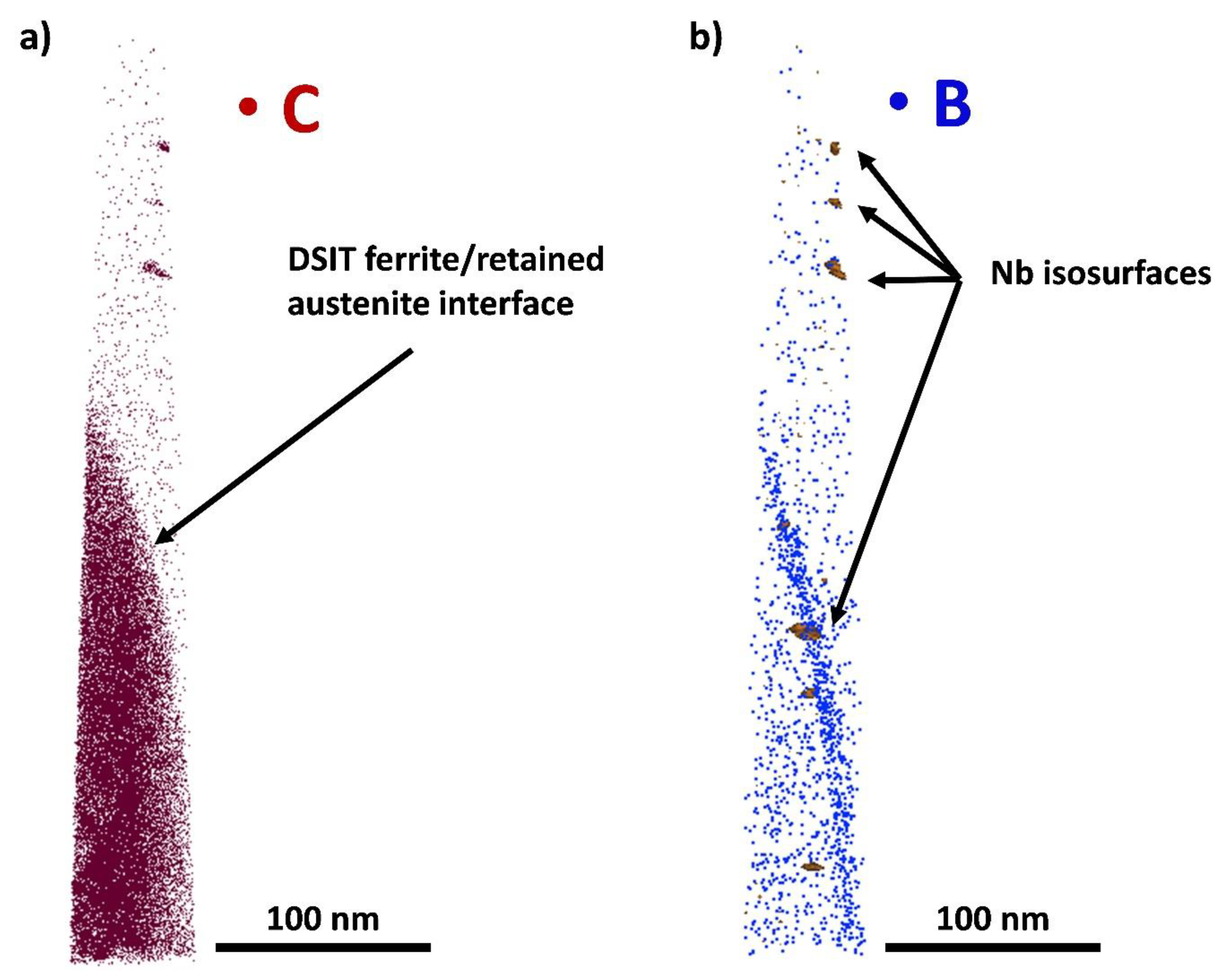
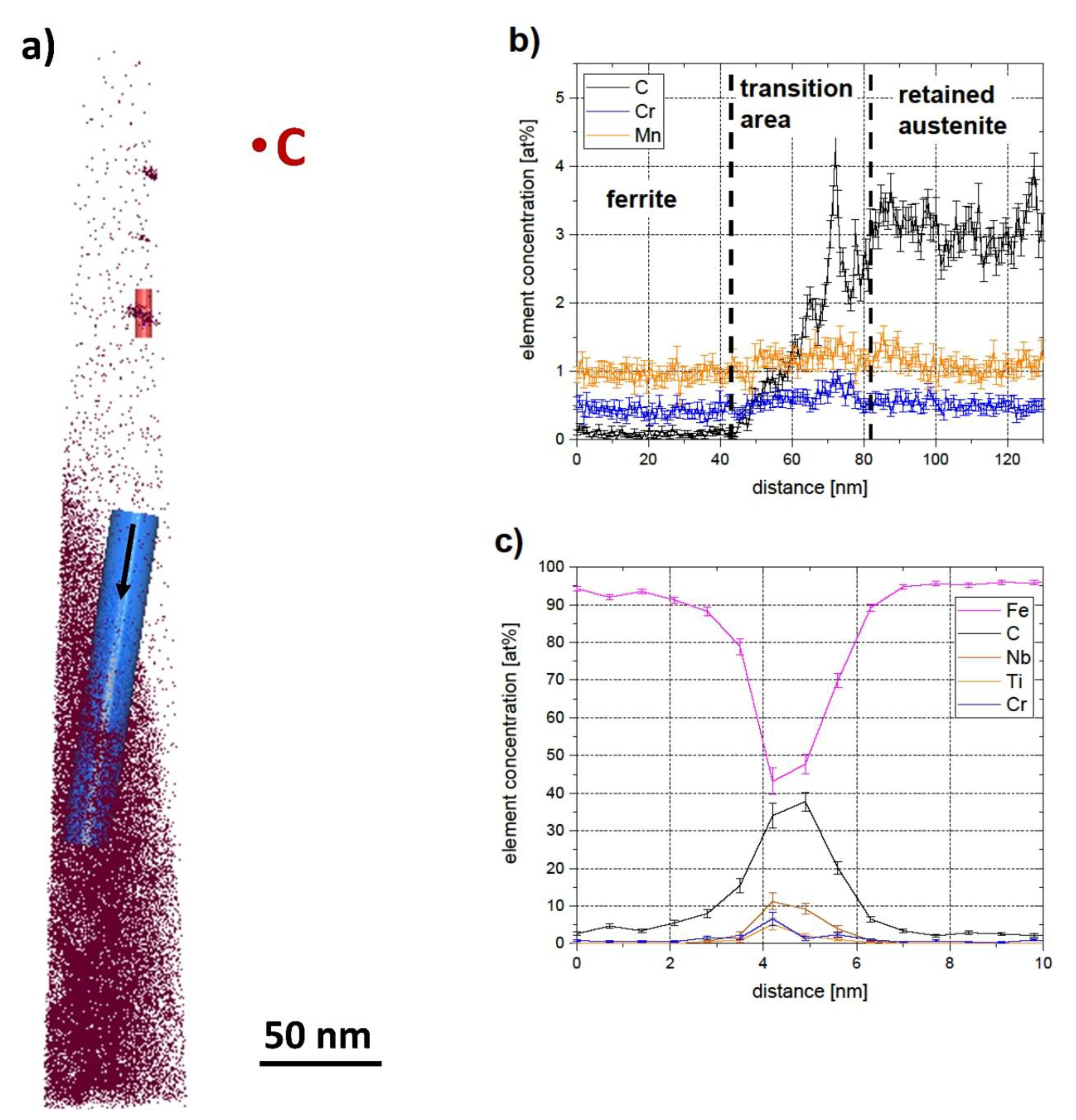
- APT measurements showed that the formation of DSIT ferrite was accompanied by the formation of retained austenite. This could be explained by the fact that C diffuses into neighboring areas during the formation of DSIT ferrite and stabilizes the austenite down to room temperature.
- STEM and APT measurements revealed the presence of fine NbC precipitates (around 5–10 nm) within DSIT ferrite, as well as at the interface between DSIT ferrite and retained austenite.
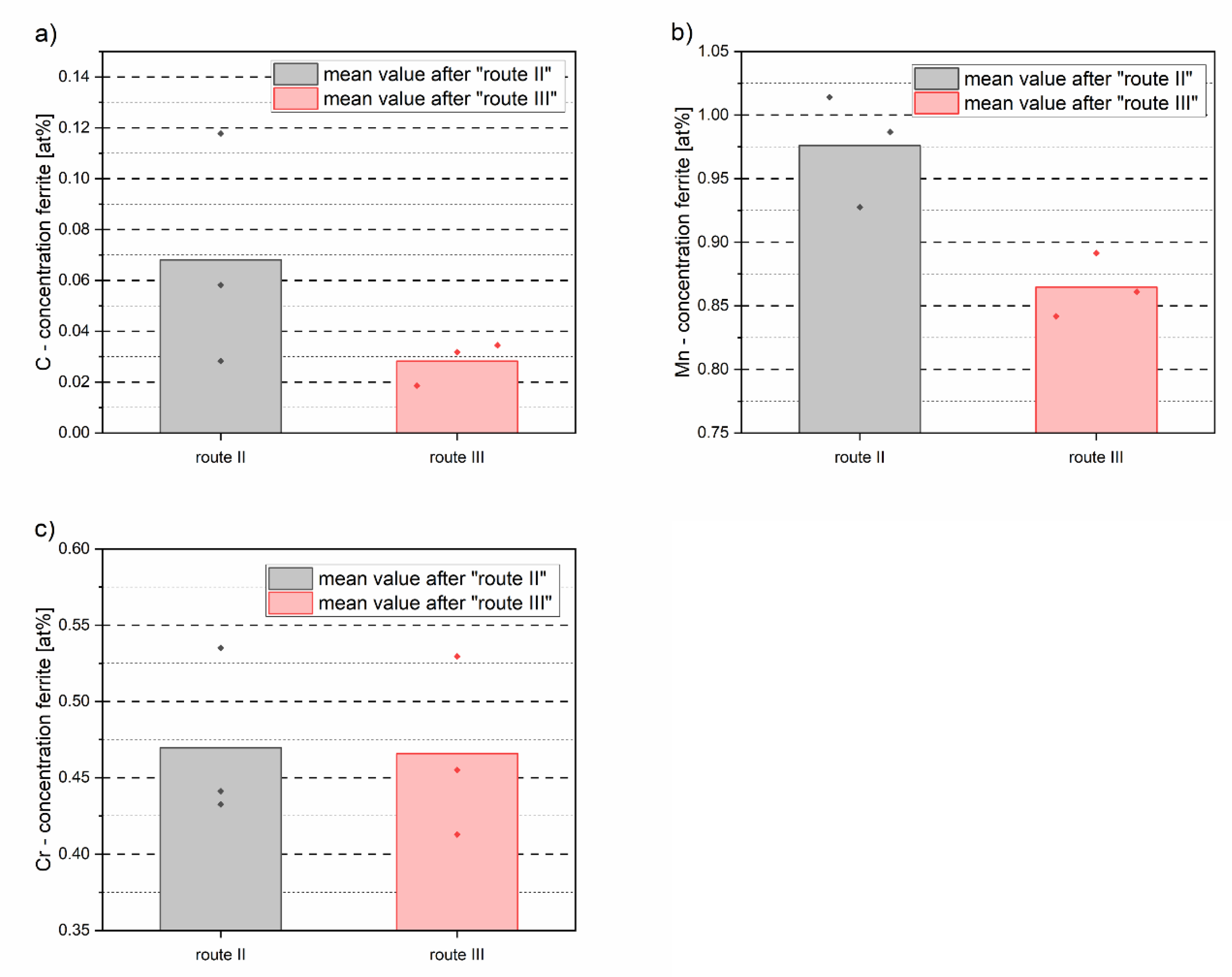
- During the formation of DSIT ferrite, only a diffusion of C into neighboring areas was observed. Both the Mn and Cr concentration were equal within DSIT ferrite and the neighboring retained austenite right after the formation of DSIT ferrite. The reason for this is assumed to be that the heavier elements, such as Mn and Cr, did not have enough time to diffuse during the formation of DSIT ferrite.
- The C and Mn concentrations within DSIT ferrite after route II were higher than the concentrations in the pre-eutectoid ferrite after route III. This leads to the assumption that C and Mn did not have enough time to diffuse during the formation of DSIT ferrite and that DSIT ferrite is therefore oversaturated with C and Mn right after the formation. To verify this, further APT measurements of retained austenite after route III would have to be carried out to determine the chemical composition in the equilibrium state.
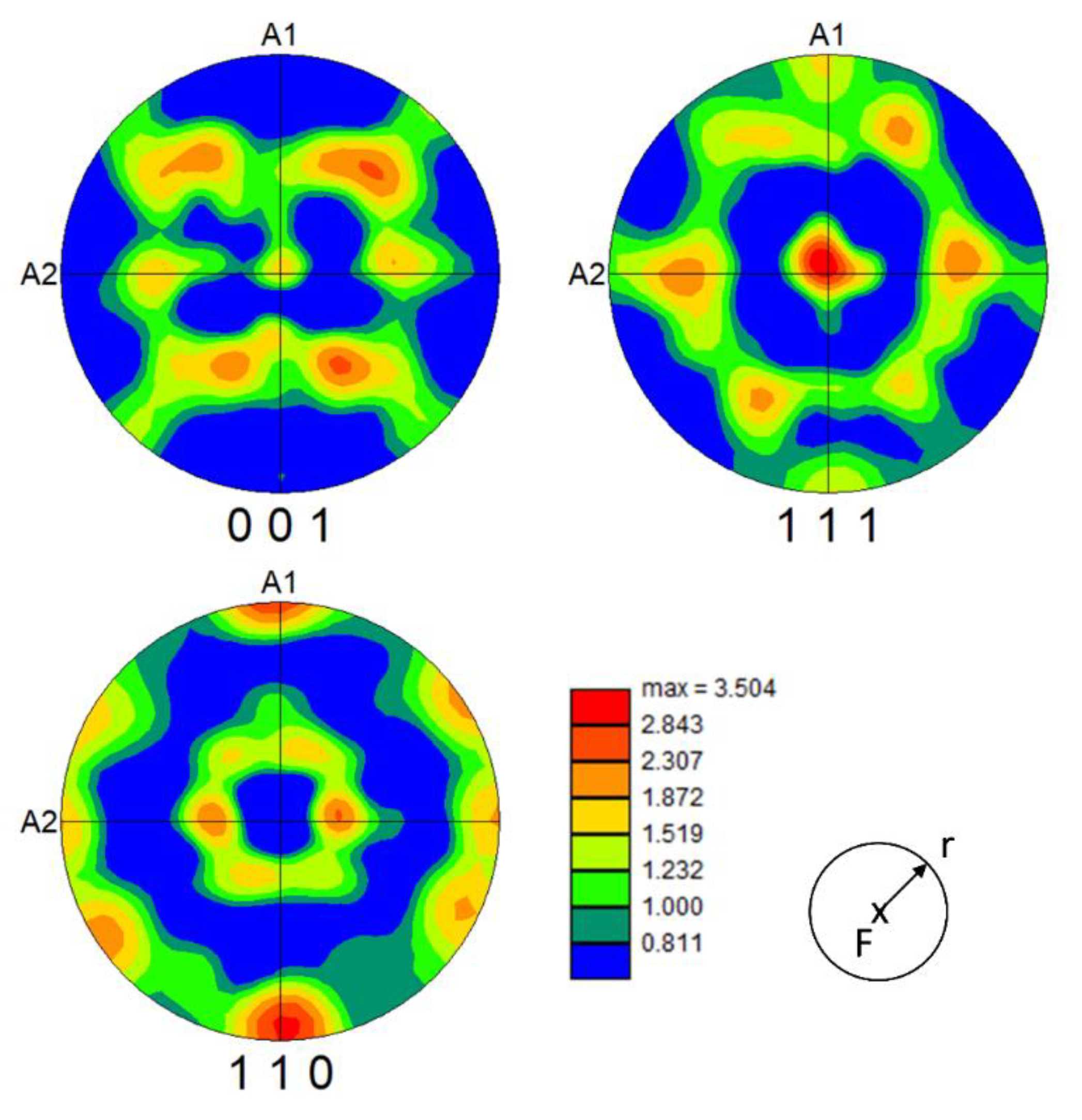
- An EBSD measurement of the crystallographic orientation of DSIT ferrite after route II showed that the <111> plane normals were parallel to the compression direction, with the <110> directions pointing towards the radial direction of the compressed sample.
- It was found that during the formation of DSIT ferrite, only diffusion from C into neighboring areas occurred. At the same time a preferred orientation of DSIT ferrite in relation to the compression direction exists. This suggests that the formation of DSIT ferrite is a displacive mechanism, accompanied with C diffusion and the formation of retained austenite.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buchmayr, B.; Hatzenbichler, T.; Kienreich, R.; Beyer, S. Werkstoff- und verfahrenstechnische Optimierung bei der Herstellung hochfester Schrauben. Berg Huettenmaennische Mon. 2008, 153, 423–429. [Google Scholar] [CrossRef]
- Beladi, H.; Kelly, G.L.; Hodgson, P.D. Ultrafine grained structure formation in steels using dynamic strain induced transformation processing. Int. Mater. Rev. 2007, 52, 14–28. [Google Scholar] [CrossRef]
- Zhao, L.; Park, N.; Tian, Y.; Shibata, A.; Tsuji, N. Dynamic Transformation Mechanism for Producing Ultrafine Grained Steels. Adv. Eng. Mater. 2018, 20, 1701016. [Google Scholar] [CrossRef]
- Ghosh, C.; Aranas, C.; Jonas, J.J. Dynamic transformation of deformed austenite at temperatures above the Ae3. Prog. Mater. Sci. 2016, 82, 151–233. [Google Scholar] [CrossRef]
- Priestner, R.; de los Rios, E. Ferrite grain refinement by controlled rolling of lovv-carbon and microalloyed steel. Met. Technol. 1980, 7, 309–316. [Google Scholar] [CrossRef]
- Yada, H.; Li, C.-M.; Yamagata, H. Dynamic γ→α Transformation during Hot Deformation in Iron-Nickel-Carbon Alloys. ISIJ Int. 2000, 40, 200–206. [Google Scholar] [CrossRef]
- Poliak, E.I.; Jonas, J.J. A one-parameter approach to determining the critical conditions for the initiation of dynamic recrystallization. Acta Mater. 1996, 44, 127–136. [Google Scholar] [CrossRef]
- Poliak, E.I.; Jonas, J.J. Initiation of Dynamic Recrystallization in Constant Strain Rate Hot Deformation. ISIJ Int. 2003, 43, 684–691. [Google Scholar] [CrossRef]
- Sun, L.; Muszka, K.; Wynne, B.P.; Palmiere, E.J. Effect of strain path on dynamic strain-induced transformation in a microalloyed steel. Acta Mater. 2014, 66, 132–149. [Google Scholar] [CrossRef]
- Choi, J.-K.; Seo, D.-H.; Lee, J.-S.; Um, K.-K.; Choo, W.-Y. Formation of Ultrafine Ferrite by Strain-induced Dynamic Transformation in Plain Low Carbon Steel. ISIJ Int. 2003, 43, 746–754. [Google Scholar] [CrossRef]
- Beladi, H.; Kelly, G.L.; Shokouhi, A.; Hodgson, P.D. The evolution of ultrafine ferrite formation through dynamic strain-induced transformation. Mater. Sci. Eng. A 2004, 371, 343–352. [Google Scholar] [CrossRef]
- Bae, Y.H.; Lee, J.S.; Choi, J.-K.; Choo, W.-Y.; Hong, S.H. Effects of Austenite Conditioning on Austenite/Ferrite Phase Transformation of HSLA Steel. Mater. Trans. 2004, 45, 137–142. [Google Scholar] [CrossRef]
- Hong, S.C.; Lim, S.H.; Hong, H.S.; Lee, K.J.; Shin, D.H.; Lee, K.S. Effect of Nb on grain growth of ferrite in C-Mn steel during isothermal holding after severe deformation. Mater. Sci. Technol. 2004, 20, 207–212. [Google Scholar] [CrossRef]
- Gourdet, S.; Montheillet, F. A model of continuous dynamic recrystallization. Acta Mater. 2003, 51, 2685–2699. [Google Scholar] [CrossRef]
- Pandi, R.; Yue, S. Dynamic Transformation of Austenite to Ferrite in Low Carbon Steel. ISIJ Int. 1994, 34, 270–279. [Google Scholar] [CrossRef][Green Version]
- Zheng, C.; Xiao, N.; Hao, L.; Li, D.; Li, Y. Numerical simulation of dynamic strain-induced austenite–ferrite transformation in a low carbon steel. Acta Mater. 2009, 57, 2956–2968. [Google Scholar] [CrossRef]
- Hao, L.; Sun, M.; Xiao, N.; Li, D. Characterizations of Dynamic Strain-induced Transformation in Low Carbon Steel. J. Mater. Sci. Technol. 2012, 28, 1095–1101. [Google Scholar] [CrossRef]
- Hurley, P.J.; Hodgson, P.D.; Muddle, B.C. Analysis and characterisation of ultra-fine ferrite produced during a new steel strip rolling process. Scr. Mater. 1999, 40, 433–438. [Google Scholar] [CrossRef]
- Irvine, K.J.; Pickering, F.B.; Gladman, T. Grain-refined C-Mn steels. J. Iron Steel Res. Int. 1967, 205, 161–182. [Google Scholar]
- Miller, M.K.; Russell, K.F.; Thompson, G.B. Strategies for fabricating atom probe specimens with a dual beam FIB. Ultramicroscopy 2005, 102, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre-Ulrikson, W.; Vurpillot, F.; Sauvage, X. (Eds.) Atom Probe Tomography: Put Theory into Practice; Academic Press: London, UK, 2016; ISBN 0128046473. [Google Scholar]
- Lerchbacher, C.; Zinner, S.; Leitner, H. Atom probe study of the carbon distribution in a hardened martensitic hot-work tool steel X38CrMoV5-1. Micron 2012, 43, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dong, H.; Liu, Q.; Weng, Y. Dynamically transformed ferrite fraction inferred from dilatometry measurements after deformation. Mater. Sci. Eng. A 2008, 487, 93–98. [Google Scholar] [CrossRef]
- He, X.L.; Chu, Y.Y.; Jonas, J.J. Grain boundary segregation of boron during continuous cooling. Acta Metall. 1989, 37, 147–161. [Google Scholar] [CrossRef]


| Fe | C | N | Mn | Si | Cr | B | Nb | Ti | |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | bal. | 0.32 | 0.004 | 0.98 | 0.10 | 0.43 | 0.0020 | 0.0110 | 0.0220 |
| at.% | bal. | 1.45 | 0.016 | 0.98 | 0.19 | 0.46 | 0.0100 | 0.0060 | 0.0250 |
| λ [°Cs−1] | Ar3 [°C] |
|---|---|
| 0.1 | 751 |
| 0.3 | 728 |
| 1.0 | 717 |
| 3.0 | 690 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monschein, S.; Ragger, K.S.; Fasching, J.; Zügner, D.; Schnitzer, R. Microstructural, Chemical, and Crystallographic Investigations of Dynamic Strain-Induced Ferrite in a Microalloyed QT Steel. Metals 2022, 12, 313. https://doi.org/10.3390/met12020313
Monschein S, Ragger KS, Fasching J, Zügner D, Schnitzer R. Microstructural, Chemical, and Crystallographic Investigations of Dynamic Strain-Induced Ferrite in a Microalloyed QT Steel. Metals. 2022; 12(2):313. https://doi.org/10.3390/met12020313
Chicago/Turabian StyleMonschein, Stefan, Katharina S. Ragger, Josef Fasching, Dominik Zügner, and Ronald Schnitzer. 2022. "Microstructural, Chemical, and Crystallographic Investigations of Dynamic Strain-Induced Ferrite in a Microalloyed QT Steel" Metals 12, no. 2: 313. https://doi.org/10.3390/met12020313
APA StyleMonschein, S., Ragger, K. S., Fasching, J., Zügner, D., & Schnitzer, R. (2022). Microstructural, Chemical, and Crystallographic Investigations of Dynamic Strain-Induced Ferrite in a Microalloyed QT Steel. Metals, 12(2), 313. https://doi.org/10.3390/met12020313






